-
PDF
- Split View
-
Views
-
Cite
Cite
Suchitra Sumitran‐Holgersson, HLA‐specific alloantibodies and renal graft outcome, Nephrology Dialysis Transplantation, Volume 16, Issue 5, May 2001, Pages 897–904, https://doi.org/10.1093/ndt/16.5.897
Close - Share Icon Share
Abstract
HLA‐specific humoral immunity, as a result of recipient allosensitization, induces hyperacute rejection of allogenic kidney grafts. Cross‐match tests are performed to avoid this complication. However, current techniques do not allow determination of HLA‐specificity of donor‐reactive antibodies in the acute cadaver‐donor situation. New methods are described and discussed in this report as well as the alloantibody specificities that are of clinical importance. Alloantibodies not only mediate hyperacute rejection but may also participate in the acute rejection of organ grafts. Clinical associations between early immunological complications, such as acute rejection, in heart, liver and kidney allografted patients and pre‐transplantation humoral alloimmunity emphasize the need for proper determination of donor‐specific humoral immunity prior to transplantation.
Introduction
This review will discuss the importance of humoral immunity in renal allograft rejection and the necessity to critically evaluate recipient HLA‐specific donor immunity prior to organ transplantation. Donor‐specific humoral alloimmunity exists in individuals who have been alloimmunized as a result of whole blood transfusion, pregnancies or earlier transplants [1–3]. In such patients, cellular alloimmunity would also exist. Immunological cross‐match tests reveal humoral donor‐specific alloimmunity prior to organ transplantation, but there are no tests available for cellular alloimmunity. However, it is important to note that tissue damage in vivo is often caused by immunological mechanisms where antibodies and lymphocytes act in concert. Furthermore, the immunological specificity for hyperacute and acute rejection is believed to be due to allogenic HLA molecules.
Induction of alloimmunity
HLA antibodies are mainly found in alloimmunized recipients. It is uncommon to find HLA‐specific antibodies in non‐immunized individuals, but such cases have been reported [4–7]. The specificities of these antibodies have been mainly anti‐A2 and anti‐B8. Whether the immunogens for these antibodies are cross‐reactive microbial determinants or not is unknown. In our study on kidney transplanted patients, who were grafted in spite of a positive B‐cell cross‐match, six male patients had HLA class I or II antibodies without prior sensitization. Of these, three lost their kidney grafts within 1 year [7].
Why the need for determination of antibody specificity in alloimmunized patients
Immunological cross‐matches are performed to avoid transplantation to recipients who have already formed donor‐reactive humoral antibodies, which might induce hyperacute rejection. The frequency of such complications is low nowadays, indicating that conventional procedures for cross‐matches are adequate. However, there is still a problem, since false‐positive cross‐matches, caused by non‐HLA antibodies, are frequent. A certain fraction of patients might therefore be denied a transplant even when there is no increased risk of rejection. Furthermore, a long series of publications have shown that better selection of organ recipients, based on improved cross‐match testing, might not only reduce the risk for hyperacute rejection, but also decrease the frequency of acute rejection episodes and even improve graft survival [8–16]. Summarized here are our efforts to evaluate different cross‐match methods, aiming at finding conditions that will provide optimal chances for graft survival and reduced frequencies of immunological complications.
Cross‐matches were introduced in the routine clinical practice during the late 1960s. Terasaki and McClelland [17] as well as Kissmeyer‐Nielsen and Kjerbye [18] developed microlymphocytotoxicity assays for the determination of HLA antibodies. Earlier, HLA antigens were being defined using serological reagents, usually with antisera procured from women immunized following several pregnancies. Only in the beginning of the 1970s were HLA class II molecules, the first being HLA‐DR (D Related), defined with antibodies. HLA class I molecules are expressed on most nucleated cells, whereas class II molecules are constitutively expressed on blood monocytes and B lymphocytes. Before it was understood that lymphocytotoxic methods have limited sensitivity, it was believed that a positive cross‐match, demonstrated on both donor T and B lymphocytes, indicated the presence of class I antibodies, whereas reactivity on B lymphocytes indicated the presence of class II antibodies. It is now well established that B lymphocytes are more sensitive to the cytotoxic effects of HLA class I antibodies than T lymphocytes; therefore reactivity with only B cells as targets is often caused by class I antibodies [19]. To distinguish between class I and class II antibodies, absorptions with thrombocytes became a frequently used procedure. Absorption of all antibodies in the sample was taken as evidence for the sole presence of class I antibodies. However, this test had several problems. First, thrombocyte absorption is time‐consuming, and secondly, lack of complete absorption could be due either to very high titres of antibodies, or the combined presence of both HLA class I antibodies, and HLA class II and/or non‐HLA specific antibodies. Ozturk and Terasaki [20] reported that rheumatoid factor (RF)‐like antibodies could cause a false positive reaction on target B lymphocytes, and also that patients with certain autoimmune diseases, such as systemic lupus erythematosus (SLE), often had T‐cell cytotoxic antibodies even without previous alloimmunization.
Only certain Ig classes of antibodies can cause cytotoxicity by fixing complement. Not all IgG subclasses are complement fixing, nor is IgA. In addition, some patients have only non‐complement fixing antibodies. These antibodies can only be detected with other serological tests, such as antibody binding to target cells using the augmented anti‐globulin reaction or flow cytometric (FACS) analysis using fluorescent antibodies, which permits the detection of all Ig classes [21,22]. Patients who are alloimmunized might at one time point be panel reactive antibody (PRA)‐positive using the conventional cytotoxicity assay, but negative using other serum samples. These transient antibodies are often non‐HLA specific and could be the result of polyclonal activation during certain viral infections. However, even HLA class I‐specific PRA reactivities can disappear. It is important to note that these patients might not have lost their antibodies; they could have developed a new class of antibodies that is not complement binding. Such antibodies in a current serum sample could be shown to compete with antibodies from a past sample and completely block cytotoxicity. This situation is not necessarily rare. Therefore, it is erroneous to conclude that a patient has developed cytotoxicity blocking antibodies of presumed anti‐idiotypic specificity, if these conclusions are based only on such tests. Furthermore, such ‘blocking’ antibodies may be harmful in kidney transplantation.
We have carefully investigated the clinical outcome of kidney allografts in a series of patients who had been transplanted earlier during a time period when a weakly positive B‐cell cross‐match was not considered to be a contraindication to transplantation. We found that the presence of HLA class I antibodies in the cross‐match sample was associated with a significantly lower graft survival at 1 year compared with patients without donor‐reactive antibodies. Antibody class was of no clinical significance [9].
A similar study was conducted in patients with liver allografts. A statistically significant correlation between graft survival at 1 year and pre‐transplant donor‐reactive HLA antibodies was found. In this study, we also found that a substantial fraction of liver patients had cytotoxic non‐HLA specific antibodies in their pre‐transplant sera. This high fraction could explain why no significant correlation has been observed between liver allograft survival and pre‐transplant cross‐match reactivity [12,15].
It became obvious that a more sensitive and specific cross‐match method was required. Garovoy et al. developed flow cytometry for cross‐matches [23], a method that we evaluated for sensitivity and specificity in a retrospective study of 76 consecutive kidney allografts. We studied microlymphocytotoxicity and flow cytometric reactivity against donor cells, Ig class, reactivity against T and B lymphocytes, as well as antibody specificity using monoclonal blocking reagents [9]. Of 76 patients, 46 developed an acute rejection and 26 did not. There was no clear statistical correlation between acute rejection episodes and a weakly positive B‐cell cytotoxicity cross‐match. However, using flow cytometry the association was clear (Table 1). Furthermore, specificity assessment showed that all patients with a weakly positive cytotoxic cross‐match and without rejection episodes lacked HLA‐specific donor‐reactive antibodies, hence the cytotoxic reaction was falsely positive. In contrast, in 25 out of 46 patients who experienced acute rejection, 22 had HLA‐specific antibodies against donor cells. Thus, with a better cross‐match, with greater specificity and following definition of HLA specificity of donor antibodies, it would be possible to reduce the frequency of rejection episodes by 50%. The P value for differences between HLA antibodies detected using flow cytometry pre‐transplantation or no HLA antibodies and graft survival at 1 year was 0.0003. Consequently, we have since decided always to perform flow cytometric cross‐matches prior to kidney allografting, both with living and cadaver donors. The flow cytometric cross‐match is more sensitive than microcytotoxicity and in our hands has a higher specificity for HLA class I antibodies.
In a follow‐up study, we evaluated further 11 patients with weakly positive B cell reactivity and added 14 new patients, who had experienced early acute rejections but had completely negative cytotoxicity cross‐match tests. We also included 12 controls without any complications and five patients with early graft losses due to non‐immunological causes. Using flow cytometry, HLA‐specific antibodies were confirmed in the first group of 11 patients, but also demonstrated in 11 out of 14 additional patients with early acute rejections. Of these, all but one had class I specific antibodies, and one had class II as well as autoantibodies. None of the 12 controls, nor any of the additional five patients with non‐immunological reasons for graft failure had any demonstrable antibodies. IgG subclass determinations were performed on all sera. Most positive sera contained IgG1 and IgG3 antibodies, or IgG3 antibodies alone. Occasional sera contained HLA‐specific antibodies of other classes, including IgA [22].
Our studies thus suggest that the number of episodes of acute rejections would be reduced and graft survival at 1 year improved if adequate cross‐matches could be performed prior to kidney transplantation. Flow cytometric assays should be used in all instances prior to recipient selection, even if the B‐cell microlymphocytotoxicity cross‐match is completely negative. Implementing these strategies at our centre has considerably reduced the frequency of acute rejections, and increased graft survival at 1 year by ∼10% in our alloimmunized group of patients.
HLA alloimmunity and acute rejections in 72 consecutive patients
| Acute rejections | No rejections | |
| Possible B cytotoxic cross‐match | 32/46 | 11/26 |
| HLA antibodies | 21/32 | 0/11 |
| Possible T/B FACS cross‐match | 25/46 | 4/26 |
| HLA antibodies | 21/25 | 0/4 |
| Acute rejections | No rejections | |
| Possible B cytotoxic cross‐match | 32/46 | 11/26 |
| HLA antibodies | 21/32 | 0/11 |
| Possible T/B FACS cross‐match | 25/46 | 4/26 |
| HLA antibodies | 21/25 | 0/4 |
HLA alloimmunity and acute rejections in 72 consecutive patients
| Acute rejections | No rejections | |
| Possible B cytotoxic cross‐match | 32/46 | 11/26 |
| HLA antibodies | 21/32 | 0/11 |
| Possible T/B FACS cross‐match | 25/46 | 4/26 |
| HLA antibodies | 21/25 | 0/4 |
| Acute rejections | No rejections | |
| Possible B cytotoxic cross‐match | 32/46 | 11/26 |
| HLA antibodies | 21/32 | 0/11 |
| Possible T/B FACS cross‐match | 25/46 | 4/26 |
| HLA antibodies | 21/25 | 0/4 |
Specificity characterization of platelet absorbable antisera in 49/101 patients using HLA antigen‐coated microbeads
| HLA class I antibodies | Non‐HLA class I antibodies | |
| No. of patients | 41 | 8 |
| Class I and II antibodies | 8 | |
| Graft loss | 16 | 2 |
| Early acute rejection | 30 |
| HLA class I antibodies | Non‐HLA class I antibodies | |
| No. of patients | 41 | 8 |
| Class I and II antibodies | 8 | |
| Graft loss | 16 | 2 |
| Early acute rejection | 30 |
Specificity characterization of platelet absorbable antisera in 49/101 patients using HLA antigen‐coated microbeads
| HLA class I antibodies | Non‐HLA class I antibodies | |
| No. of patients | 41 | 8 |
| Class I and II antibodies | 8 | |
| Graft loss | 16 | 2 |
| Early acute rejection | 30 |
| HLA class I antibodies | Non‐HLA class I antibodies | |
| No. of patients | 41 | 8 |
| Class I and II antibodies | 8 | |
| Graft loss | 16 | 2 |
| Early acute rejection | 30 |
Specificity characterization of B‐cell cytotoxic antisera not blocked by HLA class I/II monoclonal antibodies
| Total no. of samples | 30 |
| FACS T‐cell positive | 15 |
| Microbeads positive | 9 |
| Total no. of samples | 30 |
| FACS T‐cell positive | 15 |
| Microbeads positive | 9 |
4/9 anti‐HLA class I, 2/9 anti‐HLA class II, 3/9 anti‐HLA class I+II, all also non‐HLA.
Graft loss in 4/7 with anti‐HLA class I.
Specificity characterization of B‐cell cytotoxic antisera not blocked by HLA class I/II monoclonal antibodies
| Total no. of samples | 30 |
| FACS T‐cell positive | 15 |
| Microbeads positive | 9 |
| Total no. of samples | 30 |
| FACS T‐cell positive | 15 |
| Microbeads positive | 9 |
4/9 anti‐HLA class I, 2/9 anti‐HLA class II, 3/9 anti‐HLA class I+II, all also non‐HLA.
Graft loss in 4/7 with anti‐HLA class I.
HLA antibodies in B‐cell cytotoxicity negative sera
| Total number of samples | 95 |
| FACS T‐cell positive | 24 |
| Microbeads positive | 17 |
| Total number of samples | 95 |
| FACS T‐cell positive | 24 |
| Microbeads positive | 17 |
10/17 anti‐HLA class I, 2/17 anti‐HLA class II, 5/17 anti‐HLA class I+II.
Graft loss in 11/15 with anti‐HLA class I.
HLA antibodies in B‐cell cytotoxicity negative sera
| Total number of samples | 95 |
| FACS T‐cell positive | 24 |
| Microbeads positive | 17 |
| Total number of samples | 95 |
| FACS T‐cell positive | 24 |
| Microbeads positive | 17 |
10/17 anti‐HLA class I, 2/17 anti‐HLA class II, 5/17 anti‐HLA class I+II.
Graft loss in 11/15 with anti‐HLA class I.
Description of various methods to determine HLA specificity of alloreactive antibodies
The importance of correctly assessing humoral alloimmunity in patients awaiting kidney allografts is now well documented. Immunological complications in kidney, liver and heart allograft recipients can be reduced with proper cross‐matching procedures. However, as mentioned above, early immunological complications still occur in a sizeable fraction of transplant recipients and it is conceivable that more sensitive cross‐match tests might reduce such events. Furthermore, currently employed cross‐match tests often give rise to false‐positive reactions and patients on the waiting list for allogenic kidneys may be denied transplantation for the wrong reason. So far, only donor‐specific HLA class I antibodies have been found to cause hyperacute rejection and facilitate early immunological complications.
Unfortunately, as yet, there are no tests available to determine the immunological specificity of a positive cross‐match. However, ELISA‐based methods as well as flow cytometric assays using non‐autofluorescent microbeads coupled with HLA class I and class II antigens are available for panel screening of patients on the waiting list for transplantation [24–27]. The first publication concerning the effectiveness of ELISA methods for determination of HLA antibodies was published by Kao et al. [25]. They purified HLA antigens from pools of platelets, and coupled antigens directly onto ELISA plates. This assay seems to have high sensitivity as well as specificity, a finding that we have confirmed. Commercially available kits, such as PRA‐STAT, are based on a capture ELISA method, which unfortunately we do not find to be very sensitive. In addition, Harmer et al. [28] have shown that this test, for unknown reasons, also detects HLA class II antibodies. The possible deleterious effect of HLA class II antibodies in clinical kidney transplantations has not yet been documented in a large series of patients, although anecdotal case reports have been published [29,30]. It should be noted, however, that it is impossible to attribute rejections to the presence of class II antibodies as such, since all these patients who have formed HLA antibodies also have cellular alloimmunity against donor cells.
In our laboratory, we have tested several assays to determine HLA‐specific antibodies, with the ultimate goal of developing a simple and efficient method for use in the acute organ donor situation. The first method we employed was blocking of cytotoxicity or antigen binding by non‐complement binding monoclonal antibodies against monomorphic determinants on HLA class I or class II antigens [7]. Unfortunately, there were some allele‐specific reactions that were not efficiently blocked by the available monoclonal antibodies, so this method was eventually abandoned. Instead, we purified soluble HLA class I antigens from pools of outdated thrombocytes (∼100–150 samples for each batch) and HLA class II antigens from pools of donor splenic tissue. Purified material could be used for blocking of antibody reactivity in vitro and could be used in the acute donor situation [31]. However, substantial quantities of material had to be used for efficient neutralization; therefore this method was not cost‐effective. Furthermore, each antigen batch would have to be carefully tested for efficiency at blocking specific reactions directed against the most common HLA antigens.
A simple method to identify donor reactivity against class I antigens was discovered when we accidentally observed that HLA class I antigens were labile at low pH. A short treatment of target cells with citrate at pH 3 temporarily led to loss of HLA class I surface antigens on lymphocytes. A positive cross‐match reaction, which was completely negative in the presence of citrate, suggested that this was caused by HLA class I antibodies. HLA class II antibody reactivity, or reactions against other cell surface molecules on lymphocytes, was not affected by citrate treatment [32]. One disadvantage of this method was that it was not useful if patient samples contained mixtures of class I and other donor‐reactive antibodies.
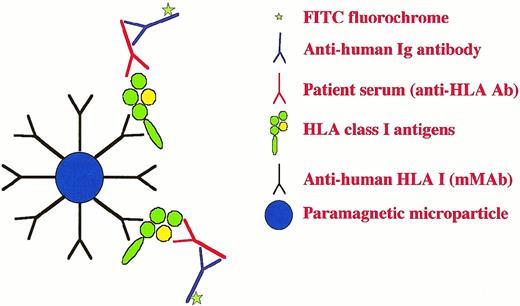
Another quick and adequate method for the purification of HLA antigens was developed, which was based on paramagnetic non‐autofluorescent beads coupled with HLA class I or class II specific monoclonal antibodies for capture of soluble HLA antigens, prepared by detergent treatment of donor lymphocytes or thrombocytes. Such antigen‐coated beads could be used directly for flow cytometry to detect HLA‐specific immunity [33] (see also Figure 1). Solubilized material was first pre‐cleared with two sets of control beads to reduce non‐specific absorption. The first step was to absorb the crude cell lysate with mini‐Macs beads coated with goat anti‐mouse IgG, followed by a second step with Dyna beads coupled with sheep anti‐mouse IgG. After incubation and magnet separation, the specific Macs beads coupled with HLA monoclonal antibodies were added. The purity of HLA antigens bound to beads was tested by elution at low pH and polyacrylamide gel electrophoresis using a Phast gel.
Antigen‐coupled microbeads have been very useful for the identification of antibody specificity in sera from patients on the transplantation waiting list. Pools of antigen from several donors are used, which minimizes laboratory work. The use of pools of antigen adequately permits the identification of HLA class I humoral immunity, but does not permit determination of specific donor‐reactivity and therefore has certain drawbacks. However, the usefulness of antigen‐coated microbeads to assess the presence of HLA‐specific antibodies in sera from patients on the waiting list for organ transplantation cannot be overestimated. An application of such bead technology is FlowPRA, now available commercially [24].
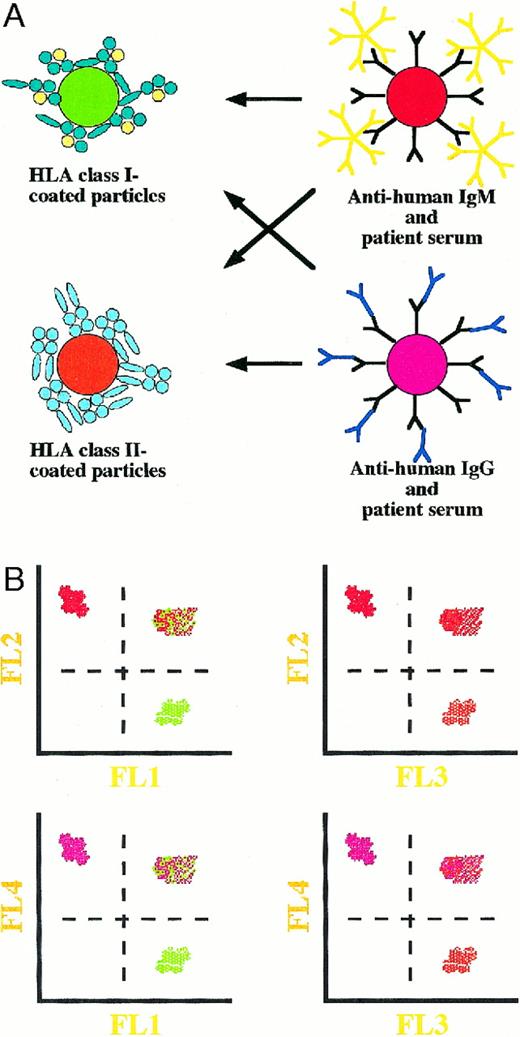
A further development would be the use of a single diagnostic step to detect the presence of IgG and IgM antibodies that bind to HLA class I and/or class II antigens coated on magnetic microbeads and analysed by means of flow cytometry. We plan to use paramagnetic particles containing different fluorochromes emitting light of wavelengths that can be distinguished using a four‐colour, flow cytometric analysis (see Figure 2A and B). Employing such a set‐up, it may be possible to detect the presence of, for example, anti‐HLA class I and II antibodies of both IgG and IgM classes in a patient's serum prior to transplantation using a single‐step assay. Obviously, the assay could be used for any analyses of multi‐component protein–protein interactions. It is important that the particles are compatible with flow cytometry, and they must be paramagnetic in order to facilitate gentle washing and to avoid non‐specific interactions caused by centrifugation. Such an assay is currently being investigated. Commercial availability of such a test would be beneficial to all transplantation laboratories.
A schematic drawing of the paramagnetic microparticle‐based, flow cytometric assay used to identify the presence of anti‐HLA class I antibodies (IgG and IgM) in kidney transplanted patients.
A proposed single‐step assay for the simultaneous detection of IgG and IgM antibodies directed against either HLA class I or II antigens using paramagnetic microparticles carrying four different fluorochromes together with flow cytometry. The possible reactions that may occur are shown in (A) and the corresponding flow cytometric results are shown in (B). Interactions between particles carrying different fluorochromes will result in aggregates that can be distinguished by virtue of their fluorescence (B).
Advantages and disadvantages of the various methods used to detect HLA antibodies
Microlymphocytotoxicity tests
These have low sensitivity and only detect complement‐binding antibodies. False‐positive reactions are frequent and can be caused by autoantibodies and by immune complexes. Cytotoxic autoantibodies are not limited to the IgM class, as a fraction of patients with autoimmune diseases, such as SLE, produce lymphocytotoxic IgG antibodies. Finally, the test is not HLA specific.
Flow cytometry analysis using donor lymphocytes
This method has a higher sensitivity than microlymphocytotoxicity but is associated with 5–10% false‐positive reactions. The advantage is that antibodies of all immunoglobulin isotypes can be determined. Implementation of flow cytometry cross‐match tests has led to improved kidney graft survival in alloimmunized patients. The test is not HLA specific.
ELISA or flow cytometric methods using purified HLA class I and class II antigens coupled directly to the plates or beads
These tests are useful for HLA antibody screening using pools of antigen, to reduce costs. The commercially available FlowPRA test can be used with mixtures of beads, where each bead preparation is coupled with antigens from one donor. A disadvantage is that these methods cannot be used for cross‐matching in the acute necrodonor situation. The test is HLA specific.
Cross‐matching techniques that permit specificity determination
For cross‐match testing, quick antigen purification methods are required. Lysates from donor spleen tissue can be produced and antigen semi‐purified by capture using monoclonal antibodies on ELISA plates or non‐autofluorescent beads for flow cytometric assays. These methods have high sensitivity but, in our experience, are associated with certain specificity problems. False‐positive reactions may be caused by natural antibodies against polysaccharide epitopes expressed on xenogenic capture antibodies or by antibodies, such as rheumatoid factor, that may bind directly to the Fc portion of monoclonal antibodies used for capture of HLA antigens. Therefore, control plates or beads without HLA antigens must be included in all tests.
The PRA‐STAT test employs a capture technique with mouse monoclonal antibodies. In spite of the fact that monoclonal antibodies specific for HLA class I molecules are used for capture, HLA class II antibodies are also detected in this assay. The reason for this is unknown and in our opinion it is highly unlikely that all reactions using this commercially available test are caused by HLA‐specific antibodies; reactivity against capture antibodies would result in false‐positives.
We have performed a careful retrospective study on sera from 196 patients. Prior donor‐reactivity was assessed by microlymphocytotoxicity and flow cytometry. Antibody specificity determinations were performed using either platelet absorptions, blocking with monoclonal antibodies, or with microbeads coated with antigens captured on beads derived from donor splenic lymphocytes. A strong correlation was found between donor‐specific HLA class I antibodies in the cross‐match serum and early acute rejection episodes, as well as with graft loss at 1 year [34]. These data form the basis for our opinion that efforts should be spent on the careful characterization of the immunological specificity of donor‐reactive pre‐transplantation antibodies.
We recommend that a future strategy for patients on the waiting list for allogenic organ transplantation include a careful screening of sera from transplant candidates using direct or capture ELISA, or flow cytometry using microbeads coated with soluble antigens prepared from pools of platelets or spleens. Sera from patients who have access to a living donor should be tested with donor antigens, prepared from lysates of blood lymphocytes. Using capture antibodies on microbeads that can be directly tested in flow cytometry, the test for donor‐specific HLA antibodies against class I antigens could be performed within 1 day. The test could be even used for an acute cadaver‐kidney situation. Positive sera should be tested also for the presence of autoantibodies. In addition, capture methods for preparation of soluble antigens from individual donors should be controlled for reactivity against beads/plates coated with capture antibody only, to evaluate the contribution of natural xenoreactive antibodies binding directly to the monoclonal antibodies against HLA antigens as well as of RF‐like antibodies. It is often stated that autoantibodies, which give false positive reactions in the microlymphocytotoxicity assay, are primarily of IgM class; however, even if most natural xenoreactive antibodies as well as RF are of the IgM class, IgG antibodies also contribute to reactivity.
If patient sera have been subjected to careful screening tests and have been found to contain only class I specific antibodies, it might be sufficient to conclude that a subsequent positive cross‐match is due to HLA antibodies. Our idea of using HLA antigenic material from pools of donors has many advantages over tests using antigen preparations from individual donors. Such a pre‐testing procedure avoids using costly panels with several donors to assess allele‐specificity of antibodies. If there are doubts as to specificity, absorptions using HLA class I or class II antigen‐pool coated magnetic microbeads can be included. All cross‐match tests should be performed using methods with a minimum of false‐positive reactions, such as flow cytometry. Using these measures, we are convinced that a substantial proportion of early immunological complications associated with organ transplantation can be prevented.
Mechanisms by which alloantibodies facilitate early acute rejections
Humoral antibodies may cause tissue injury or dysfunction by themselves or in collaboration with inflammatory/immunocompetent cells. As stated above, hyperacute rejection occurs in recipients with donor‐specific HLA class I antibodies. The mechanism for tissue injury is thought to be a direct effect by antibodies, either as complement‐dependent cytotoxicity or complement‐dependent or ‐independent endothelial cell activation, which could induce a procoagulant state with resulting thrombosis. It is not envisaged that direct complement‐mediated cytotoxicity is of central importance for hyperacute rejection, since most endothelial cells are well equipped with regulators of complement activity molecules such as decay accelerating factor, membrane co‐factor protein and homologous restriction factor. However, it is possible that endothelial cells damaged by ischaemia or reperfusion could be more sensitive than normal cells. Antibody‐induced endothelial cell activation could also contribute to increased extravasation of alloimmune immunocompetent cells to facilitate early rejection.
Antibodies cooperating with Fc‐receptor‐bearing killer cells could induce target cell damage, a phenomenon referred to as ADCC. Whether this type of cytotoxicity contributes to kidney allograft rejection is yet unknown.
Alloactivation can occur directly, by host T‐cell recognition of allogenic major histocompatibility complex (MHC) allopeptide complexes present on professional antigen‐presenting cells, which migrate to the regional lymph nodes to induce alloactivation. However, activation can also occur indirectly, i.e. against donor cell‐derived proteins, which have been taken up, processed and presented by host antigen‐presenting cells. Thus, if graft‐derived HLA molecules are first taken up by host cells, such as dendritic cells or macrophages, they will probably be presented as self‐HLA restricted allopeptides. If B cells react with soluble alloantigenic molecules, they will internalize the antigens and present them as self‐HLA class II restricted allopeptides. Since T cells in this situation will only specifically recognize allopeptides associated with self‐HLA molecules, the reacting effector cells would not find the equivalent target structures on the cells in the graft. Here, it is implied that the self‐MHC–allopeptide complex would probably not exist on the grafted cells, unless this particular MHC restriction molecule is shared by or cross‐reacts with MHC–peptide complex on cells in the graft. Furthermore, it implies that cells normally express MHC peptides in their endogenously produced MHC molecules. Therefore, antigen presentation performed by host cells might contribute more to the induction of humoral antibodies, which could cause harmful effects on the grafted cells, and less to the induction of a cellular response involving cytotoxic T lymphocytes with activity against cells in the transplant.
The uptake of alloantigens is believed to be greatly enhanced by the presence of specific antibodies. But, since the immune response would need to be reinduced in T cells, rejection would not occur immediately upon grafting. Therefore, even acute rejections, in a sense, could be antibody dependent. These ideas are compatible with our findings of a strong correlation between the presence of donor‐reactive HLA class I reactive antibodies and the risk of acute rejection. T helper cells are thought to be essential for activation of both cytotoxic effector T cells and B cells. Thus, in the absence of class II incompatibilities on the grafted tissues, which interact with specific T helper cells, rejection will not be induced. However, a class II compatible, class I incompatible graft could still induce a specific response in the host, either as complexes of allo‐class II with incompatible endogenous class I peptides or via class I peptides presented in a host HLA restricted manner. The demonstration of a class I‐specific class II‐restricted T cell clone supports this hypothesis [35]. Thus, pre‐formed donor‐specific HLA antibodies may facilitate indirect HLA‐specific alloimmunity.
Finally, the binding of antibodies on target cells in the grafts might recruit Fc receptor‐bearing inflammatory cells to the graft, thereby contributing to acute inflammation, such as delayed hypersensitivity.
A regulatory role of non‐cytotoxic antibodies can also be envisaged, since efferent inhibition of cytotoxic T lymphocytes can be demonstrated by MHC‐specific antibodies. Only certain antibody isotypes bind complement, and only certain immunoglobulin isotypes bind efficiently to Fc receptors. The relative importance of donor‐reactive antibodies of different immunoglobulin classes for potentiation or inhibition of cellular alloimmunity is yet to be completely understood.
Summary
Recipient sensitization to HLA antigens via blood transfusions, multiple pregnancies and earlier lost grafts is one of the most critical problems in clinical organ transplantation. However, current methods for detecting such sensitization and continued efforts to define better and correctly assess the specificity of donor‐reactive antibodies will help to improve our knowledge of antibodies that can be potentially harmful to allografts. The improved chances of correct recipient allocation of a given donor organ might decrease the risk of early complications, reduce post‐transplant costs such as hospitalization, expensive treatments with monoclonal antibodies and plasmapheresis, and above all improve the quality of life for transplant patients.
Correspondence and offprint requests to: Dr Suchitra Sumitran‐Holgersson, Division of Clinical Immunology, F‐79, Huddinge University Hospital AB, S‐141 86 Stockholm, Sweden.
Financed by grants from the Swedish Medical Research Council (00793 and K98‐16X‐12629‐01A), Magnus Bergwall, Åke Wiberg and Tore Nilsson Foundations.
References
Walford RL, Carter PK, Anderson RE. Leukocyte antibodies following skin homografting in the human.
Collins ZV, Arnold PF, Peetoom F, Smith G‐S, Walford RL. A naturally occurring monospecific anti‐HLA‐A8 isoantibody.
Lepage V, Degos L, Dasset J. A natural anti‐HLA‐A2 antibody reacting with homozygous cells.
Sumitran‐Karuppan S, Lindholm A, Möller E. Characterization and significance of donor‐reactive B cell antibodies in current sera of kidney transplant patients.
Gordon RD, Fung JS, Markus BM et al. The antibody crossmatch in liver transplantation.
Sumitran‐Karuppan S, Lindholm A, Möller E. Fewer acute rejection episodes and improved outcome in kidney transplanted patients with changed criteria based on cross‐matching.
Talbot D, White M, Sheriton BU et al. Flow cytometric crossmatching in renal transplantation—the long term outcome.
Goggins WC, Fisher RA, Kimball PM et al. The impact of a positive crossmatch upon outcome after liver transplantation.
Karuppan S, Ericzon BG, Möller E. Relevance of a positive cross‐match in liver transplantation.
Kobashigawa JA, Sabad A, Drinkwater D et al. Pretransplant panel reactive‐antibody screens. Are they truly a marker for poor outcome after cardiac transplantation?
Donnaldson PT, Thomson LJ, Heads A et al. IgG donor‐specific crossmatches are not associated with graft rejection or poor graft survival after liver transplantation.
Hathaway M, Gunson BK, Keogh AC, Briggs D, McMaster P, Neuberger JM. A positive crossmatch in liver transplantation —no effect or inappropriate analysis? A prospective study.
Kerman RH, Orosz CG, Lorber MI. Clinical relevance of anti‐HLA antibodies pre and post transplant.
Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins.
Kissmeyer‐Nielsen F, Kjerbye KE. Lymphocytotoxic microtechnique. Purification of lymphocytes by flotation. In:
D'Apice AJF, Tait BD. Most positive B cell crossmatches are not caused by anti‐HLA DR antibodies.
Ozturk G, Terasaki PI. Cytotoxic antibodies against surface immunoglobulin.
Al‐Hussein KA, Shenton BK, Bell A et al. Characterization of donor‐directed antibody class in the post‐transplant period using flow cytometry in renal transplantation.
Sumitran‐Karuppan S, Ohlman S, Möller E. The occurrence of cytotoxic and non‐complement fixing antibodies in the cross‐match serum of patients with early acute rejection episodes.
Garavoy MR, Reinsschmidt MA, Bigos M et al. Flow cytometry analysis. A high technology crossmatch technique facilitating transplantation.
Pei R et al. Simultaneous HLA class I and class II antibodies screening with flow cytometry.
Kao K‐J, Scornik JC, Small SJ. Enzyme linked immunoassay for anti‐HLA antibodies—an alternative to panel studies by lymphocytotoxicity.
Bryan CF, Baier KA, Flora‐Ginter G et al. Detection of HLA IgG antibodies by two enzyme‐linked immunoassays, solubilized HLA class I and PRA‐STAT comparison with the AHG PRA.
Zachary AA, Griffin J, Lucas DP, Hart JM, Leffell MS. Evaluation of HLA antibodies with the PRA‐STAT test. An ELISA test using soluble HLA class I molecules.
Harmer AW, Heads AJ, Vaughan RW. Detection of HLA class I‐ and class II specific antibodies by flow cytometry and PRA‐STAT screening in renal transplant recipients.
Taylor CJ, Chapman JR, Fuggle SV, Ting A, Morris PJ. A positive B cell cross‐match due to IgG anti‐HLA‐DQ antibody present at the time of transplantation in a successful renal allograft.
Mohanakumar T, Rhodes C, Mendez‐Picon G, Goldman M, Moncure C, Lee H. Renal allograft rejection associated with presensitization to HLA‐DR antigens.
Sumitran‐Karuppan S, Möller E. Specific inhibition of HLA class I and II antibodies by soluble antigens: a method for the identification of antibody specificity in sera from alloimmunized individuals.
Sumitran‐Karuppan S, Möller E. Acid treatment of lymphocytes selectively decreases the expression of HLA class I antigens: a method for rapid identification of HLA class I antibodies for use in clinical transplantation.
Sumitran‐Karuppan S, Möller E. The use of magnetic beads coated with soluble HLA class I or class II antigens for use in antibody screening and for specificity determination of donor‐reactive antibodies.
Sumitran S. The clinical importance of choosing the right assay for detection of HLA‐specific donor‐reactive antibodies.





Comments