-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoo Kosuge, Takahiro Kiuchi, Kiyoshi Mukai, Tadao Kakizoe, for the Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer (JSAP), A Multicenter Randomized Controlled Trial to Evaluate the Effect of Adjuvant Cisplatin and 5-Fluorouracil Therapy after Curative Resection in Cases of Pancreatic Cancer, Japanese Journal of Clinical Oncology, Volume 36, Issue 3, March 2006, Pages 159–165, https://doi.org/10.1093/jjco/hyi234
Close - Share Icon Share
Abstract
Background: There have been few randomized controlled clinical trials until now to determine the effectiveness of adjuvant treatments for pancreatic cancer, and the results reported so far are inconsistent.
Methods: Patients with invasive ductal pancreatic cancer who underwent radical surgery with clear histological margins at 11 Japanese institutions were enrolled and randomly assigned to one of two groups: surgery-alone group (no further treatment after surgery) and the surgery + chemotherapy group [two courses of postoperative adjuvant systemic chemotherapy with cisplatin (80 mg/m2, Day 1) and 5-fluorouracil (500 mg/m2/day, Days 1–5)]. Patients with a positive resectional margin or with resected distant metastases were excluded from the trial in order to minimize the influence of residual cancer.
Results: Between 1992 and 2000, 89 patients were randomized into the two arms of the trial (45 patients to the surgery + chemotherapy arm and 44 patients to the surgery-alone arm). Four patients in total were found to be ineligible (three in the surgery + chemotherapy group and one in the surgery-alone group). The baseline characteristics were comparable between the two groups. In the surgery + chemotherapy group, four patients did not receive the adjuvant treatment because of patient refusal. Toxicity was minor and acceptable among the eligible patients in the surgery + chemotherapy group. The estimated 5-year survival rates were 26.4% in the surgery + chemotherapy group and 14.9% in the surgery-alone group, and the median duration of survival was 12.5 months and 15.8 months, respectively. The recurrence rates at 5 years were 73.6 and 80.8%, respectively, in the surgery + chemotherapy and the surgery-alone groups. The differences in the survival and recurrence rates between the two groups were not statistically significant.
Conclusions: Postoperative adjuvant chemotherapy using cisplatin and 5-fluorouracil was safe and well tolerated; however, no clear survival benefit could be demonstrated.
INTRODUCTION
Pancreatic cancer is the fifth most common cause of death from cancer in Japan (1) and the United States (2), and its incidence is rising. Although radical resection appears to be the only means to obtain a cure, the 5-year survival rate after potentially curative resection remains extremely low, in the range of 5–30% (3–5). Therefore, effective adjuvant therapy is currently being sought. The Gastrointestinal Tumor Study Group (GITSG) performed the first multicenter randomized controlled trial to evaluate the efficacy of adjuvant treatment (6), and they concluded that adjuvant chemoradiotherapy prolonged the postoperative survival of patients with pancreatic cancer. However, the results of a few subsequent randomized controlled trials (7–10) have been inconsistent, and further evidence concerning the effectiveness of adjuvant treatments for pancreatic cancer is awaited. Combination chemotherapy with 5-fluorouracil (5-FU) and cisplatin was considered to be a promising regimen for pancreatic cancer in the early 1990s (11,12). Based on our experience of using this regimen in patients with unresectable pancreatic cancer, we expected that it might prove to be suitable for postoperative adjuvant treatment (13). In 1992, we initiated a multicenter randomized controlled trial to evaluate the efficacy of adjuvant chemotherapy with cisplatin and 5-FU after margin-negative resection in patients with pancreatic cancer.
METHODS
Patients and Design
Patients with ductal pancreatic cancer who underwent resectional surgery with histologically clear margins between April 1992 and March 2000 in 11 Japanese institutions were enrolled for the present study. Patients with other pancreatic and periampullary neoplasms, such as intraductal papillary mucinous neoplasm, cystadenocarcinoma and endocrine tumor, were excluded. Presence of distant metastases, even if they were resected, and presence of peritoneal seeding were regarded as criteria for exclusion from the study. After we obtained their written informed consent, the patients were registered with the randomization center by fax within 10 weeks of surgery and were then randomly assigned to one of two groups: the surgery-alone group and the surgery + chemotherapy group. They were stratified according to the institution and tumor stage using the minimization technique. The tumor stage was determined according to either the fourth or fifth edition of the UICC TNM classification, depending on the time of patient registration, and the patients were divided into two categories for stratification as follows: those with tumor in stage I or stage II according to the fifth edition (14) [equivalent to stage I of the fourth edition (15)] were assigned to one group and the remaining patients were included in the other group. Resection procedures and the range of dissection were determined according to institutional policy. Handling and histological examination of the resected specimens were carried out according to the recommendations of the Japan Pancreatic Society (16). Patients in the surgery-alone arm and those in the surgery + chemotherapy arm were followed up at 3 month intervals. Blood tests and imaging by computed tomography or ultrasound were carried out. Diagnosis of recurrence was made based on the imaging findings. Treatment after recurrence was not defined. All data were collected at a central registration office. Three pathologists performed the pathology reviews for the first 18 cases; thereafter, institutional histological diagnoses were relied upon. No external beam radiation was given to any of the patients. Intraoperative irradiation was administered on an institution-by-institution basis, and this was given to all candidates of any institution who opted to use it. The trial was conducted with the approval of the local ethics committee at each institution.
Adjuvant Chemotherapy
Chemotherapy was started within 1 week of randomization. Two courses of treatment with a combination regimen of 5-FU and cisplatin were administered. Cisplatin was administered at a dose of 80 mg/m2 on the first day of the treatment course; 5-FU was given at a daily dose of 500 mg/m2 as a continuous infusion for the first 5 days of the treatment course. The second course was repeated 4–8 weeks after the start of the first course. Toxicity was assessed according to the World Health Organization (WHO) guidelines (17). The second course was withheld if toxicity of grade 3 or above severity was observed or if the patient's condition did not improve sufficiently to fit the eligibility criteria for registration within 8 weeks of the start of the initial course.
Statistics
The primary endpoint of the study was the duration of survival. Duration of survival was calculated from the date of registration to the date of death due to any cause or was censored at the latest follow-up. The two treatment arms were also compared for recurrence rate. Safety analyses were performed based on data obtained from all the eligible patients who had started chemotherapy. Efficacy analyses were performed according to the intention-to-treat principle. Survival curves were drawn using the Kaplan–Meier technique. Differences in the duration of survival were compared using a two-sided log-rank test, with the significance level set at 5%. The prognostic value of the variables was tested by multivariate analysis using the Cox proportional hazards model. Assuming an overall 2-year survival rate of 15% in the surgery-alone arm, the present study was designed to enroll more than 86 patients in order to detect an absolute increase by 25% (i.e. 40% survival rate for 2 years) in the surgery + chemotherapy arm, at a significance level of 5% with 80% power.
RESULTS
Patient Characteristics
Between April 1992 and March 2000, 89 patients were randomized: 45 patients to the surgery + chemotherapy arm and 44 patients to the surgery-alone arm. Three patients in the surgery + chemotherapy group and one in the surgery-alone group were rated ineligible, resulting in 95.5% compliance. The reasons for ineligibility included resected distant metastases (two cases), histologically positive resection margin (one case) and severe postoperative complication (one case). The baseline characteristics of the patients in the two groups were comparable (Table 1).
Demographic and clinical data for the patients
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Gender (M:F) | 29:16 | 21:23 |
| Age (mean ± SD) | 60.8 ± 8.1 | 60.1 ± 8.9 |
| Operative procedure (pancreaticoduodenectomy:others) | 37:8 | 34:10 |
| Intraoperative irradiation (30:0 Gy) | 30:15 | 27:17 |
| Location of the tumor (head:body/tail) | 35:9 | 34:8 |
| Size of the tumor (<4:≥4 cm) | 35:10 | 36:8 |
| Histological type (papillary and well-differentiated:others) | 24:21 | 21:23 |
| Nodal involvement (present:absent) | 12:23 | 9:35 |
| pT (pT1–3:pT4) | 36:9 | 33:11 |
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Gender (M:F) | 29:16 | 21:23 |
| Age (mean ± SD) | 60.8 ± 8.1 | 60.1 ± 8.9 |
| Operative procedure (pancreaticoduodenectomy:others) | 37:8 | 34:10 |
| Intraoperative irradiation (30:0 Gy) | 30:15 | 27:17 |
| Location of the tumor (head:body/tail) | 35:9 | 34:8 |
| Size of the tumor (<4:≥4 cm) | 35:10 | 36:8 |
| Histological type (papillary and well-differentiated:others) | 24:21 | 21:23 |
| Nodal involvement (present:absent) | 12:23 | 9:35 |
| pT (pT1–3:pT4) | 36:9 | 33:11 |
Demographic and clinical data for the patients
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Gender (M:F) | 29:16 | 21:23 |
| Age (mean ± SD) | 60.8 ± 8.1 | 60.1 ± 8.9 |
| Operative procedure (pancreaticoduodenectomy:others) | 37:8 | 34:10 |
| Intraoperative irradiation (30:0 Gy) | 30:15 | 27:17 |
| Location of the tumor (head:body/tail) | 35:9 | 34:8 |
| Size of the tumor (<4:≥4 cm) | 35:10 | 36:8 |
| Histological type (papillary and well-differentiated:others) | 24:21 | 21:23 |
| Nodal involvement (present:absent) | 12:23 | 9:35 |
| pT (pT1–3:pT4) | 36:9 | 33:11 |
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Gender (M:F) | 29:16 | 21:23 |
| Age (mean ± SD) | 60.8 ± 8.1 | 60.1 ± 8.9 |
| Operative procedure (pancreaticoduodenectomy:others) | 37:8 | 34:10 |
| Intraoperative irradiation (30:0 Gy) | 30:15 | 27:17 |
| Location of the tumor (head:body/tail) | 35:9 | 34:8 |
| Size of the tumor (<4:≥4 cm) | 35:10 | 36:8 |
| Histological type (papillary and well-differentiated:others) | 24:21 | 21:23 |
| Nodal involvement (present:absent) | 12:23 | 9:35 |
| pT (pT1–3:pT4) | 36:9 | 33:11 |
Treatment Data
Four patients assigned to the surgery + chemotherapy arm refused treatment after randomization, and the detailed data for three ineligible patients were not available. As a result, a total of 38 patients were evaluated for treatment toxicity. Of these, 31 patients (81.6% of the patients who received chemotherapy) received two courses of chemotherapy, and 7 patients received only one course of chemotherapy. The reasons for treatment discontinuation were patients' withdrawal from the trial (four cases), development of recurrent disease (two cases) and unresolving leucopenia (one case).
Toxicity
One ineligible patient who was suffering from a severe postoperative complication that was not documented at the time of registration died of sepsis after one course of chemotherapy. Minor toxicity was commonly observed, especially nausea and vomiting, among the 38 eligible patients who actually received the adjuvant chemotherapy. In a few patients, toxicities of grade 3 or higher severity were encountered (Table 2). However, the toxicities were reversible and resolved with conservative treatment alone in all patients.
Summary of toxicities according to WHO criteria (n = 38)
. | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Nausea/vomiting | 15 | 12 | 5 | 0 |
| Leukopenia | 14 | 6 | 2 | 0 |
| Granulocytopenia | 6 | 5 | 3 | 1 |
| Thrombocytopenia | 7 | 2 | 0 | 0 |
| Mucositis | 1 | 1 | 2 | 0 |
| Cardiac | 0 | 0 | 0 | 0 |
| Hepatic | 17 | 9 | 3 | 0 |
| Renal | 3 | 1 | 0 | 0 |
. | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Nausea/vomiting | 15 | 12 | 5 | 0 |
| Leukopenia | 14 | 6 | 2 | 0 |
| Granulocytopenia | 6 | 5 | 3 | 1 |
| Thrombocytopenia | 7 | 2 | 0 | 0 |
| Mucositis | 1 | 1 | 2 | 0 |
| Cardiac | 0 | 0 | 0 | 0 |
| Hepatic | 17 | 9 | 3 | 0 |
| Renal | 3 | 1 | 0 | 0 |
Summary of toxicities according to WHO criteria (n = 38)
. | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Nausea/vomiting | 15 | 12 | 5 | 0 |
| Leukopenia | 14 | 6 | 2 | 0 |
| Granulocytopenia | 6 | 5 | 3 | 1 |
| Thrombocytopenia | 7 | 2 | 0 | 0 |
| Mucositis | 1 | 1 | 2 | 0 |
| Cardiac | 0 | 0 | 0 | 0 |
| Hepatic | 17 | 9 | 3 | 0 |
| Renal | 3 | 1 | 0 | 0 |
. | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Nausea/vomiting | 15 | 12 | 5 | 0 |
| Leukopenia | 14 | 6 | 2 | 0 |
| Granulocytopenia | 6 | 5 | 3 | 1 |
| Thrombocytopenia | 7 | 2 | 0 | 0 |
| Mucositis | 1 | 1 | 2 | 0 |
| Cardiac | 0 | 0 | 0 | 0 |
| Hepatic | 17 | 9 | 3 | 0 |
| Renal | 3 | 1 | 0 | 0 |
Recurrence
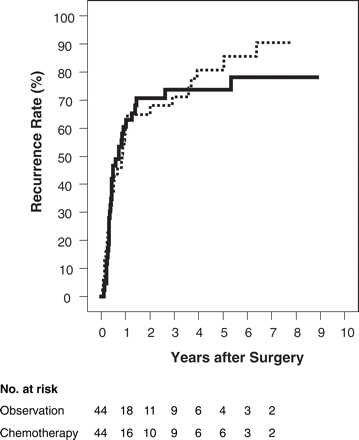
Seventy-one patients died and 18 patients were alive at the end of the follow-up period. The median follow-up duration for the survivors was 44.8 months. The recurrence status remained unknown in one ineligible patient. Among the remaining 88 randomized patients, 34 (77.3%) in the surgery-alone group and 32 (71.1%) in the surgery + chemotherapy group developed recurrence. The recorded sites of recurrence are shown in Table 3. The liver was the most frequent site of recurrence for metastasis, followed by peritoneal seeding and local recurrence, in both groups. There was no significant advantage of adjuvant chemotherapy in terms of the recurrence rate (Figure 1). The median time to recurrence was 10.2 months in the 44 patients in the surgery-alone group and 8.6 months in the 44 patients in the surgery + chemotherapy group. The 5-year recurrence rates were 80.8 and 73.6%, respectively, in the two groups (P = 0.80).
Cumulative recurrence rate. Solid line: surgery + chemotherapy group; dotted line: surgery-alone group.
Sites of recurrence
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Liver | 20 | 22 |
| Peritoneum | 10 | 9 |
| Pleura | 1 | 1 |
| Local recurrence | 6 | 7 |
| Lymph node | 3 | 1 |
| Lung | 1 | 1 |
| Bone | 1 | 1 |
| Skin | 0 | 2 |
| Brain | 1 | 0 |
| Number of patients with recurrence | 32 | 34 |
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Liver | 20 | 22 |
| Peritoneum | 10 | 9 |
| Pleura | 1 | 1 |
| Local recurrence | 6 | 7 |
| Lymph node | 3 | 1 |
| Lung | 1 | 1 |
| Bone | 1 | 1 |
| Skin | 0 | 2 |
| Brain | 1 | 0 |
| Number of patients with recurrence | 32 | 34 |
Sites of recurrence
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Liver | 20 | 22 |
| Peritoneum | 10 | 9 |
| Pleura | 1 | 1 |
| Local recurrence | 6 | 7 |
| Lymph node | 3 | 1 |
| Lung | 1 | 1 |
| Bone | 1 | 1 |
| Skin | 0 | 2 |
| Brain | 1 | 0 |
| Number of patients with recurrence | 32 | 34 |
. | Surgery + chemotherapy . | Surgery alone . |
|---|---|---|
| Liver | 20 | 22 |
| Peritoneum | 10 | 9 |
| Pleura | 1 | 1 |
| Local recurrence | 6 | 7 |
| Lymph node | 3 | 1 |
| Lung | 1 | 1 |
| Bone | 1 | 1 |
| Skin | 0 | 2 |
| Brain | 1 | 0 |
| Number of patients with recurrence | 32 | 34 |
Duration of Survival
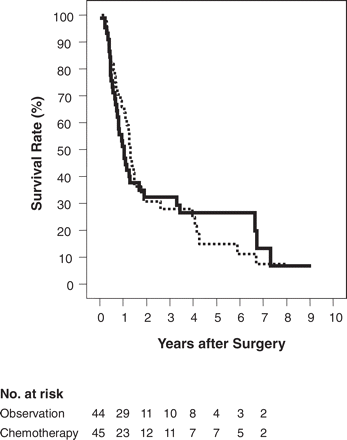
In the randomized patients, the cause of death was recurrent disease in 63 patients (32 from the surgery-alone group and 31 from the surgery + chemotherapy group), in-hospital death in 1 patient (from the surgery + chemotherapy group), non-malignant/non-toxicity death in 5 patients (3 from the surgery-alone group and 2 from the surgery + chemotherapy group) and unknown in 2 patients (1 from each group). The duration of survival was not influenced by adjuvant chemotherapy in either the randomized or the eligible patients. The survival curves of all the randomized patients are shown in Figure 2. The median survival was 15.8 months in the surgery-alone group and 12.5 months in the surgery + chemotherapy group, and the 5-year survival rate was 14.9% in the surgery-alone group and 26.4% in the surgery + chemotherapy group (P = 0.94).
Cumulative survival rate. Solid line: surgery + chemotherapy group; dotted line: surgery-alone group.
Prognostic Factors
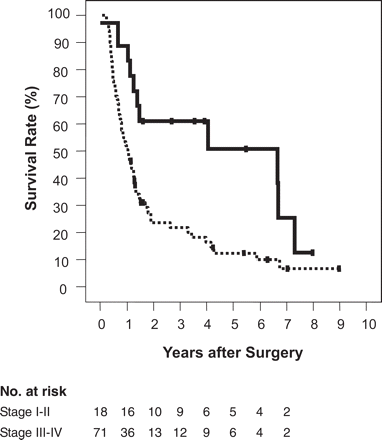
In order to assess the influence of prognostic factors, the relationship of the outcomes to the following variables were investigated: gender, age, histological type, size of tumor, tumor location, pT factor, nodal involvement, type of operative procedure and administration of intraoperative radiotherapy. Calculation of the correlation coefficients (r) of pairs of variables revealed a close correlation for the tumor location and the type of operative procedure (r = 0.96), whereas the coefficients for all the other pairs were less than 0.5. Consequently, ‘operative procedure’ was excluded from the subsequent multivariate analysis. The prognostic value of the remaining variables together with the assigned treatment arm as an additional variable was tested using multivariate analysis. The significant factors determined from this analysis were nodal involvement and the histological type of the tumor (Table 4); the effect of the pT factor was marginal. Consequently, the stage of the disease, the major determinants of which were the nodal status and the pT factor, was determined to be a good prognostic indicator. Patients with tumor in stage I or in stage II according to fifth edition of the UICC TNM classification survived significantly longer than those with more advanced disease (Figure 3). The median survival time in the two groups was 79.7 and 12.6 months, respectively (P = 0.004).
Cumulative survival rate categorized by the disease stage according to the fifth edition of the UICC TNM classification. Solid line: stages I and II; dotted line: stages III, IVa and IVb.
Multivariate analysis
| Variable . | β . | SE . | P . | HR . | 95% CI . |
|---|---|---|---|---|---|
| Nodal involvement (absent versus present) | 1.167 | 0.348 | 0.001 | 3.213 | (1.626–6.350) |
| Histological type (papillary or well-differentiated tubular versus moderately or poorly differentiated tubular) | 0.791 | 0.273 | 0.004 | 2.206 | (1.291–3.769) |
| pT factor (pT1–3 versus pT4) | 0.528 | 0.300 | 0.078 | 1.695 | (0.942–3.050) |
| Gender (female versus male) | 0.393 | 0.272 | 0.148 | 1.482 | (0.869–2.526) |
| Size of tumor (<4 cm versus ≥4 cm) | 0.166 | 0.172 | 0.334 | 1.181 | (0.843–1.654) |
| Age | 0.017 | 0.016 | 0.282 | 1.017 | (0.986–1.049) |
| Chemotherapy | −0.053 | 0.254 | 0.835 | 0.948 | (0.576–1.561) |
| Intraoperative radiotherapy | −0.280 | 0.299 | 0.349 | 0.756 | (0.421–1.357) |
| Location of the lesion (head versus body or tail of the pancreas) | −0.354 | 0.291 | 0.224 | 0.702 | (0.397–1.241) |
| Variable . | β . | SE . | P . | HR . | 95% CI . |
|---|---|---|---|---|---|
| Nodal involvement (absent versus present) | 1.167 | 0.348 | 0.001 | 3.213 | (1.626–6.350) |
| Histological type (papillary or well-differentiated tubular versus moderately or poorly differentiated tubular) | 0.791 | 0.273 | 0.004 | 2.206 | (1.291–3.769) |
| pT factor (pT1–3 versus pT4) | 0.528 | 0.300 | 0.078 | 1.695 | (0.942–3.050) |
| Gender (female versus male) | 0.393 | 0.272 | 0.148 | 1.482 | (0.869–2.526) |
| Size of tumor (<4 cm versus ≥4 cm) | 0.166 | 0.172 | 0.334 | 1.181 | (0.843–1.654) |
| Age | 0.017 | 0.016 | 0.282 | 1.017 | (0.986–1.049) |
| Chemotherapy | −0.053 | 0.254 | 0.835 | 0.948 | (0.576–1.561) |
| Intraoperative radiotherapy | −0.280 | 0.299 | 0.349 | 0.756 | (0.421–1.357) |
| Location of the lesion (head versus body or tail of the pancreas) | −0.354 | 0.291 | 0.224 | 0.702 | (0.397–1.241) |
SE, standard error; P, significance; HR, hazard ratio; CI, confidence interval.
Multivariate analysis
| Variable . | β . | SE . | P . | HR . | 95% CI . |
|---|---|---|---|---|---|
| Nodal involvement (absent versus present) | 1.167 | 0.348 | 0.001 | 3.213 | (1.626–6.350) |
| Histological type (papillary or well-differentiated tubular versus moderately or poorly differentiated tubular) | 0.791 | 0.273 | 0.004 | 2.206 | (1.291–3.769) |
| pT factor (pT1–3 versus pT4) | 0.528 | 0.300 | 0.078 | 1.695 | (0.942–3.050) |
| Gender (female versus male) | 0.393 | 0.272 | 0.148 | 1.482 | (0.869–2.526) |
| Size of tumor (<4 cm versus ≥4 cm) | 0.166 | 0.172 | 0.334 | 1.181 | (0.843–1.654) |
| Age | 0.017 | 0.016 | 0.282 | 1.017 | (0.986–1.049) |
| Chemotherapy | −0.053 | 0.254 | 0.835 | 0.948 | (0.576–1.561) |
| Intraoperative radiotherapy | −0.280 | 0.299 | 0.349 | 0.756 | (0.421–1.357) |
| Location of the lesion (head versus body or tail of the pancreas) | −0.354 | 0.291 | 0.224 | 0.702 | (0.397–1.241) |
| Variable . | β . | SE . | P . | HR . | 95% CI . |
|---|---|---|---|---|---|
| Nodal involvement (absent versus present) | 1.167 | 0.348 | 0.001 | 3.213 | (1.626–6.350) |
| Histological type (papillary or well-differentiated tubular versus moderately or poorly differentiated tubular) | 0.791 | 0.273 | 0.004 | 2.206 | (1.291–3.769) |
| pT factor (pT1–3 versus pT4) | 0.528 | 0.300 | 0.078 | 1.695 | (0.942–3.050) |
| Gender (female versus male) | 0.393 | 0.272 | 0.148 | 1.482 | (0.869–2.526) |
| Size of tumor (<4 cm versus ≥4 cm) | 0.166 | 0.172 | 0.334 | 1.181 | (0.843–1.654) |
| Age | 0.017 | 0.016 | 0.282 | 1.017 | (0.986–1.049) |
| Chemotherapy | −0.053 | 0.254 | 0.835 | 0.948 | (0.576–1.561) |
| Intraoperative radiotherapy | −0.280 | 0.299 | 0.349 | 0.756 | (0.421–1.357) |
| Location of the lesion (head versus body or tail of the pancreas) | −0.354 | 0.291 | 0.224 | 0.702 | (0.397–1.241) |
SE, standard error; P, significance; HR, hazard ratio; CI, confidence interval.
DISCUSSION
Resectional surgery provides the only chance of cure for patients with pancreatic cancer. However, in most cases the operation is non-curative, and an extremely high recurrence rate is observed. Consequently, a number of adjuvant treatments have been tried in the hope of prolonging survival. GITSG performed the first randomized controlled trial to evaluate the effect of adjuvant treatment in a group of 43 patients with pancreatic cancer. They concluded that adjuvant chemoradiotherapy after curative resection prolonged patient survival (6). However, subsequent larger studies carried out in Europe failed to show any evident benefit of adjuvant chemoradiotherapy (8–10). Similarly, the value of systemic chemotherapy in an adjuvant setting also remains controversial owing to the scarcity of convincing evidence. Only three randomized controlled trials have been reported to assess adjuvant chemotherapy for pancreatic cancer in the literature (Table 5). Bakkevold et al. (7) claimed that adjuvant combination chemotherapy using 5-FU, doxorubicin and mitomycin C (AMF) prolonged the median survival time in a cohort of postoperative patients with pancreatic or ampullary cancer. However, their results were not definitive, because there was no detailed documentation of the data. Takada et al. found no advantage of adjuvant treatment using mitomycin C and 5-FU in a larger number of patients (18). Neoptolemos et al. (9,10) conducted a prospective randomized trial (ESPAC-1) to assess the effects of two types of adjuvant treatments, namely, chemotherapy and chemoradiotherapy. They concluded that chemotherapy alone improved the survival rate and that chemoradiotherapy may even have had an adverse effect on survival. However, their conclusion remains a subject of debate because of the unorthodox and complex design of the study.
Randomized controlled trials of adjuvant chemotherapy for pancreatic cancer
| Author . | Year of publication . | Disorder . | Chemotherapy . | Number of cases . | MST (months) . | 5-year SR (%) . | Significance . |
|---|---|---|---|---|---|---|---|
| Bakkevold et al. | 1993 | PC and AMP (R0) | AMF | 31 | 23 | 4 | NS with generalized Wilcoxon's test |
| Observation | 30 | 11 | 8 | ||||
| Takada et al. | 2002 | PC (R1) | MF | 81 | NA | 11.5 | NS with the log-rank test |
| Observation | 77 | NA | 18.0 | ||||
| ESPAC | 2004 | PC (R1) | 5-FU + LV | 147 | 20.1 | 21 | P = 0.009 the with log-rank test |
| No chemotherapy | 142 | 15.5 | 8 | ||||
| Present study | PC (R0) | FP | 45 | 12.5 | 26.4 | NS with the log-rank test | |
| Observation | 44 | 15.8 | 14.9 |
| Author . | Year of publication . | Disorder . | Chemotherapy . | Number of cases . | MST (months) . | 5-year SR (%) . | Significance . |
|---|---|---|---|---|---|---|---|
| Bakkevold et al. | 1993 | PC and AMP (R0) | AMF | 31 | 23 | 4 | NS with generalized Wilcoxon's test |
| Observation | 30 | 11 | 8 | ||||
| Takada et al. | 2002 | PC (R1) | MF | 81 | NA | 11.5 | NS with the log-rank test |
| Observation | 77 | NA | 18.0 | ||||
| ESPAC | 2004 | PC (R1) | 5-FU + LV | 147 | 20.1 | 21 | P = 0.009 the with log-rank test |
| No chemotherapy | 142 | 15.5 | 8 | ||||
| Present study | PC (R0) | FP | 45 | 12.5 | 26.4 | NS with the log-rank test | |
| Observation | 44 | 15.8 | 14.9 |
AMF, doxorubicin + mitomycin C + 5-FU; AMP, ampullary carcinoma; LV, folinic acid; MF, mitomycin C + 5-FU; MST, median survival time; NA, not available; NS, not significant; PC, pancreatic cancer; SR, survival rate.
Randomized controlled trials of adjuvant chemotherapy for pancreatic cancer
| Author . | Year of publication . | Disorder . | Chemotherapy . | Number of cases . | MST (months) . | 5-year SR (%) . | Significance . |
|---|---|---|---|---|---|---|---|
| Bakkevold et al. | 1993 | PC and AMP (R0) | AMF | 31 | 23 | 4 | NS with generalized Wilcoxon's test |
| Observation | 30 | 11 | 8 | ||||
| Takada et al. | 2002 | PC (R1) | MF | 81 | NA | 11.5 | NS with the log-rank test |
| Observation | 77 | NA | 18.0 | ||||
| ESPAC | 2004 | PC (R1) | 5-FU + LV | 147 | 20.1 | 21 | P = 0.009 the with log-rank test |
| No chemotherapy | 142 | 15.5 | 8 | ||||
| Present study | PC (R0) | FP | 45 | 12.5 | 26.4 | NS with the log-rank test | |
| Observation | 44 | 15.8 | 14.9 |
| Author . | Year of publication . | Disorder . | Chemotherapy . | Number of cases . | MST (months) . | 5-year SR (%) . | Significance . |
|---|---|---|---|---|---|---|---|
| Bakkevold et al. | 1993 | PC and AMP (R0) | AMF | 31 | 23 | 4 | NS with generalized Wilcoxon's test |
| Observation | 30 | 11 | 8 | ||||
| Takada et al. | 2002 | PC (R1) | MF | 81 | NA | 11.5 | NS with the log-rank test |
| Observation | 77 | NA | 18.0 | ||||
| ESPAC | 2004 | PC (R1) | 5-FU + LV | 147 | 20.1 | 21 | P = 0.009 the with log-rank test |
| No chemotherapy | 142 | 15.5 | 8 | ||||
| Present study | PC (R0) | FP | 45 | 12.5 | 26.4 | NS with the log-rank test | |
| Observation | 44 | 15.8 | 14.9 |
AMF, doxorubicin + mitomycin C + 5-FU; AMP, ampullary carcinoma; LV, folinic acid; MF, mitomycin C + 5-FU; MST, median survival time; NA, not available; NS, not significant; PC, pancreatic cancer; SR, survival rate.
In contrast to the ESPAC-1 trial, our study was simple in design, allowing the comparison of survival between two patient groups: one group with adjuvant chemotherapy and the other group without adjuvant chemotherapy. Patients were stratified according to the institution and stage of the disease in order to minimize the influence of possible prognostic factors. Furthermore, patients with a positive histological margin were excluded from the study with the objective of excluding possible bias introduced by one of the strongest prognostic factors, the status of the resectional margin (19). However, this last criterion did interfere with the rapid recruitment of patients. It took almost 8 years to carry out the registration and randomization of 89 patients. Fortunately, there have been no remarkable changes in the diagnosis or treatment of pancreatic cancer during this period, and the trial could be continued without any major revisions of the protocol. Only two previous trials have evaluated adjuvant treatment for patients with R0 resection (6,7). Both encountered similar difficulties and a smaller number of patients were enrolled than in the present study. The patient characteristics, especially the stage distribution and proportion of patients with nodal involvement, were comparable among these trials.
The present study showed that adjuvant combination chemotherapy using 5-FU and cisplatin could be carried out with acceptable safety, as long as the patients met the eligibility criteria, although one patient died of sepsis after a single course of chemotherapy. The monitoring committee, after analysis of the case data, judged that this particular patient was unsuitable for enrollment into the trial. According to the recommendation of the committee, part of the study protocol was modified to clarify some requirements regarding the postoperative condition of the patients. No serious complications were encountered after the modification was carried out.
On the other hand, this trial failed to show any significant benefit of adjuvant chemotherapy in terms of either survival or recurrence, even though the absolute values of the survival rate and recurrence rate at 5 years were slightly better in the patients to whom adjuvant chemotherapy was administered. It is possible that a larger number of patients must be examined to appreciate the statistical significance of the treatment effect. However, a significant influence of the typical prognostic factors on survival was confirmed in the present trial. It is possible that the influence of adjuvant chemotherapy on survival is much weaker than that of these prognostic factors. Another possibility is that further courses of chemotherapy might reinforce the effectiveness of the treatment and allow it to become evident. However, it must also be considered that the life expectancy of patients with pancreatic cancer is extremely short. Adjuvant treatment for pancreatic cancer would be practical only when its beneficial effect can compensate for the compromised quality of life of the patient resulting from the treatment. Therefore, a distinct effect with a short treatment period, besides minimum toxicity, would seem to be the essential prerequisite of effective adjuvant chemotherapy. Otherwise, the lifetime spent with low quality of life can cancel out or even reverse the potentially beneficial effects of adjuvant treatment.
To conclude, the present trial did not prove that the regimen can be recommended as adjuvant treatment for pancreatic cancer.
Contributors
Leading members: Tomoo Kosuge, National Cancer Center Hospital, Tokyo, surgery; Shuichi Okada, National Cancer Center Hospital, Tokyo, chemotherapy; Takahiro Kiuchi, University of Tokyo, Tokyo, statistics and randomization; Kiyoshi Mukai, National Cancer Center Hospital East, Kashiwa, pathology; Tadao Kakizoe, National Cancer Center Hospital, Tokyo, supervisor.
Specialists responsible for patient treatment at each of the participating institutions: Tomoo Kosuge, National Cancer Center Hospital, Tokyo; Makoto Seki, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo; Tomoe Beppu, Juntendo University Hospital, Tokyo; Masaru Konishi, National Cancer Center Hospital East, Kashiwa; Nobutaka Umekita, Tokyo Metropolitan Bokuto Hospital, Tokyo; Hitonobu Moriya, St Marianna University Hospital, Kawasaki; Hitoshi Kanamaru, Fujieda Municipal General Hospital, Fujieda; Seiji Kawasaki, Shinshu University Hospital, Matsumoto; Yasushi Harihara, Tokyo University Hospital, Tokyo; Tatsuro Wakayama, Tokyo Metropolitan Hiroo Hospital, Tokyo; Taizo Kimura, Hamamatsu University Hospital, Hamamatsu.
Monitoring Committee: Satoshi Ebihara, National Cancer Center Hospital East, Kashiwa; Masayoshi Yoshimori, National Cancer Center Hospital, Tokyo; Toshiya Sato, Institute of Statistical Mathematic, Tokyo.
(The affiliation of each member is the affiliation at the time of the patient recruitment.)
This research was supported by a grant-in-aid for cancer research from the Ministry of Health and Welfare (currently the Ministry of Health, Labor and Welfare) of Japan. This work was presented at the 19th World Congress of International Society for Digestive Surgery, Yokohama, Japan, December 8–11, 2004. The authors would like to convey their special thanks to Dr Shuichi Okada, a medical oncologist at the National Cancer Center Hospital, who made an enormous contribution to the planning and execution of this trial; to our grief Shuichi Okada passed away prematurely.
References
The Editorial Board of the Cancer Statistics in Japan. Number of deaths and proportional mortality rates from malignant neoplasms by site in Japan (2001). In: Cancer Statistics in Japan 2003. Tokyo: Foundation for Promotion of Cancer Research
Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic Cancer Registry in Japan: 20 years of experience.
Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection.
Bakkevold KE, Amesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater: results of a controlled, prospective, randomized multicentre study.
Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group.
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomized controlled trial.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.
Rothman H, Cantrell JE Jr, Lokich J, et al. Continuous infusion 5-fluorouracil plus weekly cisplatin for pancreatic carcinoma.
Rougier P, Zarba JJ, Ducreux M, et al. Phase II study of cisplatin and 120-hour continuous infusion of 5-fluorouracil in patients with advanced pancreatic adenocarcinoma.
Okusaka T, Okada S, Ishii H, et al. Clinical response to systemic combined chemotherapy with 5-fluorouracil and cisplatin (FP therapy) in patients with advanced pancreatic cancer.
Sobin LH, Wittekind CH, editors. International Union Against Cancer (UICC). TNM Classification of Malignant Tumors. 5th edn. New York: John Wiley & Sons,
Hermanek P, Sobin LH, editors. International Union Against Cancer (UICC). TNM classification of malignant tumors. 4th edn. Berlin: Springer,
Japan Pancreatic Society. Classification of pancreatic carcinoma (1st English edition). Tokyo: Kanehara & Co,
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment.
Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma.
Author notes
1Division of Hepatobiliary and Pancreatic Surgery, National Cancer Center Hospital, Tokyo, 2University Hospital Medical Information Network Center, the University of Tokyo Hospital, Tokyo, 3Department of Diagnostic Pathology, Tokyo Medical University, Tokyo and 4National Cancer Center, Tokyo, Japan