-
PDF
- Split View
-
Views
-
Cite
Cite
Meritxell Jodar, Sellappan Selvaraju, Edward Sendler, Michael P. Diamond, Stephen A. Krawetz, for the Reproductive Medicine Network, The presence, role and clinical use of spermatozoal RNAs, Human Reproduction Update, Volume 19, Issue 6, November/December 2013, Pages 604–624, https://doi.org/10.1093/humupd/dmt031
Close - Share Icon Share
Abstract
Spermatozoa are highly differentiated, transcriptionally inert cells characterized by a compact nucleus with minimal cytoplasm. Nevertheless they contain a suite of unique RNAs that are delivered to oocyte upon fertilization. They are likely integrated as part of many different processes including genome recognition, consolidation-confrontation, early embryonic development and epigenetic transgenerational inherence. Spermatozoal RNAs also provide a window into the developmental history of each sperm thereby providing biomarkers of fertility and pregnancy outcome which are being intensely studied.
Literature searches were performed to review the majority of spermatozoal RNA studies that described potential functions and clinical applications with emphasis on Next-Generation Sequencing. Human, mouse, bovine and stallion were compared as their distribution and composition of spermatozoal RNAs, using these techniques, have been described.
Comparisons highlighted the complexity of the population of spermatozoal RNAs that comprises rRNA, mRNA and both large and small non-coding RNAs. RNA-seq analysis has revealed that only a fraction of the larger RNAs retain their structure. While rRNAs are the most abundant and are highly fragmented, ensuring a translationally quiescent state, other RNAs including some mRNAs retain their functional potential, thereby increasing the opportunity for regulatory interactions. Abundant small non-coding RNAs retained in spermatozoa include miRNAs and piRNAs. Some, like miR-34c are essential to the early embryo development required for the first cellular division. Others like the piRNAs are likely part of the genomic dance of confrontation and consolidation. Other non-coding spermatozoal RNAs include transposable elements, annotated lnc-RNAs, intronic retained elements, exonic elements, chromatin-associated RNAs, small-nuclear ILF3/NF30 associated RNAs, quiescent RNAs, mse-tRNAs and YRNAs. Some non-coding RNAs are known to act as epigenetic modifiers, inducing histone modifications and DNA methylation, perhaps playing a role in transgenerational epigenetic inherence. Transcript profiling holds considerable potential for the discovery of fertility biomarkers for both agriculture and human medicine. Comparing the differential RNA profiles of infertile and fertile individuals as well as assessing species similarities, should resolve the regulatory pathways contributing to male factor infertility.
Dad delivers a complex population of RNAs to the oocyte at fertilization that likely influences fertilization, embryo development, the phenotype of the offspring and possibly future generations. Development is continuing on the use of spermatozoal RNA profiles as phenotypic markers of male factor status for use as clinical diagnostics of the father's contribution to the birth of a healthy child.
Introduction
Spermatogenesis is a highly regulated transcriptional, translational and posttranslational process. Transcription continues through the initial stages of spermiogenesis until development of the round spermatids. Those transcripts that are required to complete the transition to the spermatozoa are protected and maintained as ribonucleoproteins (RNPs). During this time, the majority of the cytoplasm with its RNA component is depleted as a cytoplasmic droplet, residual body (reviewed in Cooper, 2005) and phagocytosed by the Sertoli cells. At this point, nuclear compaction can be cytologically observed as the majority of sperm histones are replaced by protamines and the sperm nucleus assumes a highly condensed structure. This yields a cell which is transcriptionally inert and devoid of translational activity as ensured by the absence of intact rRNAs (Betlach and Erickson, 1976; Johnson et al., 2011b). The transcripts that do remain within the spermatozoa provide a select source of both coding and non-coding RNAs that include both fragmented and preferentially non-degraded mRNAs, si (small interfering), mi (micro), pi (Piwi-interacting) and lnc (long non-coding)-RNAs.
The very existence of spermatozoal RNAs was originally questioned based on the assumption that since transcription ceases in the round spermatid stage, with the cytoplasm destined to be expunged and thus void of the components necessary for translational activity, any remaining male haploid RNA would be inconsequential. This view was supported by the observed heterogeneity of the ejaculate, the presence of somatic cell contaminants which accounted for the majority of large RNAs in most samples and the absence of intact ribosomal RNAs. These caveats partly reflected the inadequacy of the methods that were available to purify spermatozoa and to detect low abundance RNAs (reviewed in Krawetz, 2005). The controversy was resolved when several laboratories independently identified specific sperm RNAs in plants (Rejon et al., 1988) and in mammals, including rat (Pessot et al., 1989), mouse (Wykes et al., 2000) and human (Kumar et al., 1993; Miller et al., 1994; Wykes et al., 1997) using RT–PCR and in situ hybridization.
To date the population of spermatozoal transcripts from the human, are the best characterized amongst all mammals. The RNA profile of human spermatozoa was initially attempted using a cDNA cloning and sequencing strategy (Miller et al., 1999) that was followed by select RT–PCR (Lambard et al., 2004; Wang et al., 2004). Both of these methods were able to survey only a small fraction of all potential transcripts. The first general spermatozoal RNA profiles obtained using microarrays suggested that human spermatozoa contain ∼3000–7000 different coding transcripts (Ostermeier et al., 2002). This was subsequently extended to the clinic with the assessment of specific transcripts in cases of asthenozoospermia (Jodar et al., 2012), teratozoospermia (Platts et al., 2007), oligozoospermia (Montjean et al., 2012) and idiopathic infertile males (Garrido et al., 2009). Their potential as biomarkers of fertility was highlighted. Transcript profiling of coding RNAs using microarrays in conjunction with RT–PCR has now broadly defined the abundance of known sperm transcripts in other mammals (Gilbert et al., 2007; Bissonnette et al., 2009; Yang et al., 2010) and non-mammalian species like plants (Borges et al., 2008) and Drosophila Melanogaster (Fischer et al., 2012).
In comparison to the aforementioned, RNA-seq has provided a much more complete picture of the population of human sperm transcripts, allowing for the identification, quantification and characterization of both known and previously unknown RNAs (Krawetz et al., 2011; Sendler et al., 2013). These studies highlighted the selective retention of a cadre of both coding RNAs and small non-coding RNAs in all individuals studied. Recently others have begun employing RNA-seq to examine the distribution of sperm RNAs in bovine (Card et al., 2013) and stallion (Das et al., 2013) and the small RNA population of mouse (Kawano et al., 2012; Peng et al., 2012).
The overall functional significance of many spermatozoal RNAs remains to be understood and their individual importance remains to be elucidated. Using the zona-free hamster oocyte/human sperm penetration assay, it has been established that sperm-specific transcripts (not present in the unfertilized oocyte) are transmitted to the oocyte upon fertilization (Ostermeier et al., 2004). They can also be translated into a functional protein as shown by the injection of the sperm borne PLCζ (1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase zeta) transcript into the mouse oocyte, yielding a functional calcium oscillator (Sone et al., 2005). The distinctive landscape of non-coding RNAs that appears during the final stages of sperm maturation also strongly hints at their potential role in early post-fertilization and embryo development (Ostermeier et al., 2004; Liu et al., 2012). This has now been extended to the position that spermatozoal RNAs may epigenetically and transgenerationally affect phenotype (reviewed in Rando, 2012). These avenues remain to be explored.
A substantial number of spermatozoal transcripts appear compromised (Sendler et al., 2013), suggesting that they may simply be remnants echoing prior roles (Ostermeier et al., 2002). Even if only existing in mature sperm in this form, comparison of the differential transcript profiles between fertile and infertile patients has shown their utility as markers of fertility (reviewed in Waclawska and Kurpisz, 2012).
The primary focus of this review is to examine what a spermatozoal transcript profile may reveal with regard to the integrity of the spermatogenic pathway, characteristics of the mature sperm and their potential epigenetic and post-fertilization developmental functions. The potential use of spermatozoal RNAs as biomarkers impacting human clinical diagnosis and agriculture will also be discussed.
Methods
Previously published human RNA-seq results (Krawetz et al., 2011; Sendler et al., 2013) and recent results from RNA sequencing of four representative sperm and two testes samples from a larger dataset were utilized. This set of sperm samples (D1–D4) were from four of the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Reproductive Medicine Network for Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation dataset, whose partner withdrew from the study. Sperm and testes libraries were prepared for sequencing using the SEQR RNA Amplification kit (Sigma-Aldrich) and ENCORE® NGS Library System (NUGEN Technologies, Inc.). Amplification begins using 2 ng of total RNA for both sperm and testes samples. Library construction continues using 200 ng of amplified material. All libraries were paired-end sequenced using Illumina Hi-Seq 2500 and aligned using Novoalign (V2.08.01, NovoCraft Technologies Sdn Bhd), with distribution of reads shown as UCSC genome browser tracks, similar to that described (Sendler et al., 2013). The main figures in this review show sperm transcript characteristics of interest as portrayed by a single sample, while the supplementary figures show similarities among the four sequenced donors for each of these characteristics.
Genomic distribution (exonic, intronic and intergenic) of the large sperm RNA fraction was obtained from total sperm sample AS062 (GEO accession GSM721696) using Genomatix RegionMiner (Genomatix, v 3.0426). RT–PCR of specific intronic RNAs was performed using primer pairs that spanned the complete intron length in both sense and anti-sense orientations and were separately performed on both sperm and testes RNA in order to determine both the direction and relative abundance of these elements in sperm. Distribution of snc-RNA reads across transposable elements LINE1 (Genbank accession M80343), variant forms of ERVL-MaLR (RepBase 16.09) and tRNAs were obtained from results of small RNA sequencing of sperm sample AS062 (GSM530234). The NCBI HomoloGene database (Coordinators, 2013) was mined to identify homologous human, stallion and bovine sequences. Gene abundance from stallion RNA-seq was based on the distribution the most abundant exon of mapped RNA sequence tags (Das et al., 2013). Literature was mined through i-HOP, PubMed, GoPubMed and GEPS (Dietze et al., 2009; Epple and Sherf, 2009; Leitner et al., 2009; Reavie, 2009).
Spermatozoal RNAs-characterization and potential roles
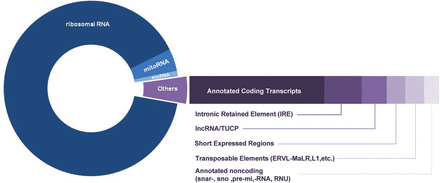
The most extensive sperm transcript profiling is available from human. RNA-seq has been performed for both small and large RNA fractions, with the latter examined as both total RNA and separate A+ and A− fractions. The landscape of sperm RNAs evident from multiple normal individual samples has revealed a wealth of different classes of coding and non-coding transcripts (Fig. 1). Many relatively abundant RNAs that appear in all normal sperm samples are unannotated or of unknown function and appear uniquely in sperm, emphasizing the high level of complexity of the population of spermatozoal RNAs.
Composition of spermatozoal RNAs. The distribution of the various classes of RNAs as determined by RNA-seq is shown. The most abundant class is ribosomal RNAs followed by mitochondrial RNA (mitoRNAs), annotated coding transcripts, small non-coding RNAs (snc-RNAs), intronic retained elements, lnc-RNAs and Transcribed regions of Unknown Coding Potential (TUCP), short expressed regions, transposable elements and annotated non-coding RNAs, including snars, sno, pri-mir and RNU.
Considerations
A single spermatozoon contains ∼50 fg of long RNA (>200 nt) and 0.3 fg of small non-coding (snc) RNA (<200 nt; Goodrich et al., 2013). This amount is ∼200 times less than the quantity of RNA found in other cells (10 pg of long RNA and 1–3 pg of snc-RNAs), making somatic cell removal essential to resolve the unique pool of sperm transcripts. The absence of intact 28S and 18S sperm rRNA is often used to confirm the absence of somatic contamination (Johnson et al., 2011b; Goodrich et al., 2013). This was previously thought to be reflective of complete removal of rRNA with consequent enrichment of mRNA, but in fact was later resolved as the selective fragmentation of the majority of sperm rRNAs (Ostermeier et al., 2002; Johnson et al., 2011b). To overcome these challenges the protocol to isolate spermatozoal RNA has undergone several revisions (Goodrich et al., 2013).
Resolution of the RNA population has been optimized with the use of Next Generation Sequencing (NGS) strategies. Typical RNA-seq library construction uses poly(A+) selection to provide an enriched population of mRNAs excluding the otherwise overwhelming contribution of ribosomal and mitochondrial RNAs. While this provides an effective strategy to enrich for coding transcripts, it will exclude RNAs with short poly(A) tails and those that are not polyadenylated. In contrast, total RNA libraries are not subject to this limitation. This is an important consideration when characterizing sperm RNAs that have been observed to possess a large population of non-coding RNAs. However, sequencing the total population of RNAs comes at the cost of the increased representation of ribosomal and mitochondrial RNAs. Specific fractions of snc-RNAs (<200 nt) are typically lost during library construction but can be recovered and sequenced with modified protocols and size selection (Krawetz et al., 2011).
Coding RNAs
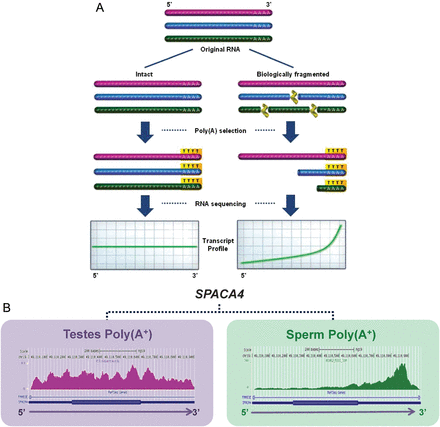
Both RNA-seq analysis in the human (Sendler et al., 2013) and RT–PCR of select bovine transcripts (Gilbert et al., 2007) suggests that the majority of coding RNA observed in sperm exists in a fragmented, or at the very least, atypical state. In contrast to equivalently selected testes RNA, RNA-seq profiles of the majority of poly(A+) selected sperm transcripts exhibit a marked 3′ bias. As illustrated in Fig. 2, this characteristic is indicative of transcript fragmentation in spermatozoal RNA. Ontological analysis of the relatively minor fraction of intact coding transcripts retained in sperm shows an enrichment of genes associated with male infertility, fertilization and early embryo development (Sendler et al., 2013). This is strongly suggestive of a functional role for these preferentially retained transcripts during the final stages of spermatogenesis or upon delivery. For example, INTS1 (Integrator complex subunit 1), involved in the transcription and processing of small-nuclear RNAs (snRNA) U1 and U2, is retained and appears by microarray analysis to increase after fertilization prior to zygotic genome activation (Vassena et al., 2011). INTS1 knockouts are embryonic lethal at the blastocyst stage (Hata and Nakayama, 2007). This is congruent with a potential role of this complex in the first steps of embryogenesis which could reflect the paternal contribution.
The degree of transcript fragmentation can influence the sequencing profile after Poly(A+) selection. (A) Intact RNA shows minimal 3′ bias, even with poly(A+) selection. However, transcripts which are biologically fragmented (i.e. prior to the typical fragmentation step of most RNA-seq protocols) show significant 3′ end profile bias as selection preferentially retains the 3′ poly(A+) containing ends. (B) For example, SPACA4, exhibits fairly even coverage across transcript length in testes, and marked 3′ bias in sperm.
As in the above, RNA-seq also affords the ability to identify novel transcripts or isoforms. The production of transcript variants through the use of alternative promoters and splicing has been described in testes (Freiman, 2009). Interestingly, mature sperm display isoforms that are distinct in a variety of ways from those found in whole testes, indicating that these modifications arise only in the final transcriptionally active stages of spermatogenesis. This includes the sperm-specific isoform of PKM2 (Pyruvate kinase isozymes M1/M2), a key enzyme regulating glucose metabolism (Sendler et al., 2013). Further, approximately one quarter of the sperm transcripts show alternative sites of polyadenylation (APA), which maintain the integrity of the coding region, but exhibit an abbreviated 3′ untranslated region (UTR) (Fig. 3). This trait is common in testes (Liu et al., 2007) and may serve to modulate transcript stability, localization and/or transport. Additionally, this modification may impact translation by affecting the ability of different regulatory proteins and miRNAs to bind to the alternative UTR (Di Giammartino et al., 2011). It has recently been reported that Bromodomain testis-specific protein (BRDT) actively modulates APA in testes (Berkovits et al., 2012). Male mice lacking the first bromodomain of BRDT are infertile (Shang et al., 2007) and spermatozoal transcripts from such knockouts possess longer 3′UTRs (Berkovits et al., 2012). This likely emphasizes the critical nature of APA observed in human sperm. Lastly, sperm RNA-seq has identified many examples of abundant predicted transcripts (such as ORFs) that are not observed in somatic cells and are of low abundance in testes. Together, variations in expression and form of coding RNAs found in sperm likely have a significant impact on both the regulation and function of this class of RNAs.
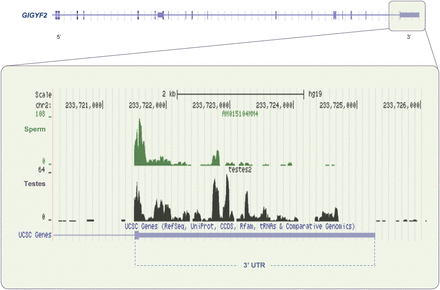
Alternative polyadenlation of sperm transcripts. GIGYF2 encodes a protein that interacts with GRB10 and may be involved in the regulation of tyrosine kinase receptor signaling. The 3′UTR region of GIGYF2 gene is highlighted (upper panel). RNA-seq (lower panel) of this specific region exhibits a truncated 3′UTR in sperm (green). This contrasts with coverage extending over most of the UTR observed in testes (black). (See Supplementary data, Fig. S1 for more details.)
Small non-coding RNAs
It has been proposed that the germline genome is protected through paternal small non-coding miRNAs, siRNAs, piRNAs, qRNAs and repeat associated RNA mechanisms (reviewed in O'Donnell and Boeke, 2007; Bourc'his and Voinnet, 2010; Krawetz et al., 2011; Siomi et al., 2011). For function, the parental RNAs must be of sufficient quantity and quality to interact with their target for successful embryo development (Bourc'his and Voinnet, 2010). Recent sequencing of the small (18–24 nt) RNA population from multiple normal human donors has also shed light on the complexity of the snc-RNA population present in spermatozoa. The majority of sperm snc-RNAs correspond to four major classes: repetitive elements, transcription start sites (TSS)/promoter associated, piRNAs, and miRNAs, with other classes such as snRNAs, snoRNAs, mse-tsRNA and YRNAs representing a relatively minor portion (Krawetz et al., 2011). Additional snc-RNA sequencing reads correspond to unannotated regions of the genome and portions of coding and non-coding transcripts. Whether these short fragments serve a particular role, e.g. regulating their longer precursor elements, or are merely end-points of fragmentation is not yet known.
miRNAs
The most well-characterized non-coding sperm RNAs are miRNAs, which have been shown to modulate various stages of spermatogenesis (reviewed in Moazed, 2009). Along with siRNAs, these RNAs typically function to regulate expression by inhibiting or activating translation or targeting mRNAs for degradation usually by binding to a 3′UTR target sequence (reviewed in Gangaraju and Lin, 2009). They are typically transcribed by polymerase II as larger precursors that are then processed to an intermediate form by DROSHA (Ribonuclease 3) and DGCR8 (DiGeorge syndrome critical region 8). These precursors are subsequently transported to the cytoplasm and further matured by DICER, an RNase III endonuclease, to their mature 20–24 nt functional form. They are then incorporated into an Argonaute containing RNP forming RISC, RNA-induced silencing complex. Spermatogenic-specific DICER or DROSHA knockouts arrest spermatogenesis (Hayashi et al., 2008; Korhonen et al., 2011; Wu et al., 2012), confirming their essential role.
While many miRNAs are conserved among different species, some are species-specific (Curry et al., 2009; Krawetz et al., 2011; Govindaraju et al., 2012; Peng et al., 2012; Das et al., 2013). The majority of mature spermatozoal miRNAs are also observed in testes (Landgraf et al., 2007), but most of their computationally predicted 3′UTR targets are absent in mature sperm (Krawetz et al., 2011). Recent studies suggest that some miRNAs act as transcriptional regulators by targeting other regions, e.g. promoters (Kim et al., 2008; Place et al., 2008). Perhaps in the transcriptionally quiescent sperm, they provide a signal for early embryonic histone replacement (Johnson et al., 2011a) or transcriptionally poise the genome for early embryonic expression or affect epigenetic modification (Khraiwesh et al., 2010). Support for this notion has been gained from the observation that more than 10% of all snc-RNAs map to histone-enriched TSS and promoters. These novel RNAs, termed quiescent RNAs (qRNAs), are similar to tiny RNAs (tiRNAs). They are associated with the TSS region but not enriched in GC regions or correlated with histone modifications (Krawetz et al., 2011). The tiRNAs derived from regions adjacent to TSS may indirectly modulate local chromatin states through other binding factors (Taft et al., 2011). However, the function of qRNAs remains to be established (Krawetz et al., 2011).
The most abundant sperm miRNA in the human is miR-34c (Krawetz et al., 2011). It has also been identified in stallion and mouse (Peng et al., 2012; Das et al., 2013), and has been shown to be essential for the first cleavage division in mouse zygotes (Liu et al., 2012). Except for miR-34c-5p, where we have a glimpse, their mechanism of action and functional role in spermiogenesis and/or fertility remain to be fully delineated (Curry et al., 2011; Krawetz et al., 2011; Govindaraju et al., 2012; Das et al., 2013). For example, in mouse testes, miR-34c expression is p53 independent (Bouhallier et al., 2010), whereas miR-34c targets p53 in cancer cells (Corney et al., 2007). This is somewhat in line with their ability to influence growth status during periods of rapid growth like oncogenesis (reviewed in Shivdasani, 2006; Croce, 2009; Luningschror et al., 2013). Spermatozoa also contain several intact miRNA precursors (pri-miRNAs, 100–150 nt). Since the zygote has the capacity to process immature miRNAs (Liu et al., 2012), the potential role of the pri-miRNAs requires consideration. For example, pri-miRNA-181c is the most abundant immature miRNA in human spermatozoa. Predicted targets of this miRNA include those critical to early embryonic development and globally decrease at the 4–8 cell stage of human embryo development (Vassena et al., 2011; Sendler et al., 2013). One specific target of miR-181c is CARM1 (Coactivator-Associated aRginine Methyltransferase 1), an embryonic stem cell pluripotency factor. CARM1 directly catalyzes the methylation of H3 arginine in the promoters of POU5F1 (POU domain, class 5, transcription factor 1) and SOX2 (Transcription factor SOX-2). This forms an active chromatin mark coinciding with induction (Xu et al., 2013). At the 2-cell stage overexpression of CARM1 in one of the blastomeres predisposes its derivatives to contribute to the pluripotent cells of inner cell mass (Torres-Padilla et al., 2007). It is tempting to speculate that through the delivery by sperm miR-34c and pri-miR-181c, the division and partitioning of the targeted CARM1 are, respectively, ensured, thereby decreasing some pluripotency factors in one blastomere while pushing the other towards the trophoectoderm lineage.
piRNAs
Piwi-interacting RNA (piRNAs) are abundant in the mammalian male germline (reviewed in Girard et al., 2006) and their presence in spermatozoa has been confirmed in several species (Krawetz et al., 2011; Kawano et al., 2012; Peng et al., 2012). They are typically organized in the genome as clusters that range up to 100 kb in size. piRNAs precursors are processed to their 23–32 nt mature form by a PIWI protein-dependent mechanism (reviewed in Ishizu et al., 2012). Although not clearly articulated, several functions have been proposed for this class of transcripts. These include regulation of RNA stability and epigenetic states as well as protecting the germline genome from transposition (reviewed in Aravin and Hannon, 2008; Gangaraju and Lin, 2009). During spermatogenesis, the activation of mobile transposable elements is suppressed by piRNAs. The absence of these regulatory RNAs can induce spermatogenic arrest (Kuramochi-Miyagawa et al., 2004; Carmell et al., 2007). The piRNAs may act in a similar protective manner during early embryo development as the genome undergoes extensive demethylation and remethylation. They could protect genome integrity by binding to DNA and thus preventing the action of various classes of repetitive and transposable elements like SINE, LINE, MER and LTR at specific stages of embryogenesis (Krawetz et al., 2011).
Potentially novel classes of snc-RNAs, sperm RNAs (spR)-12 and -13, were recently identified in mouse spermatozoa. They are ∼20 nt in length and are likely derived from additional processing of mature piRNAs (Kawano et al., 2012). This has defined yet another snc-RNA biogenesis pathway. These abundant spRs are maintained post-fertilization until the blastocyst stage, suggestive of their potential role in early embryo development perhaps to ensure genome integrity at this critical stage.
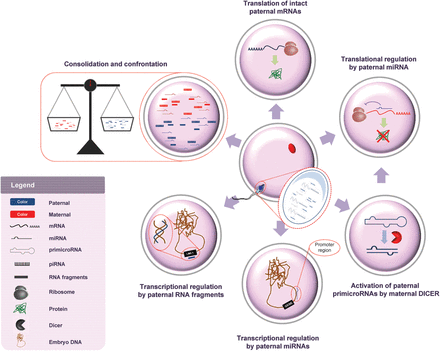
Confrontation and consolidation
The classes of spermatozoal RNAs described above may play an integral role in the confrontation and consolidation mechanism that has been described in plants and animals (Bourc'his and Voinnet, 2010; Goring and Indriolo, 2010; Krawetz et al., 2011; Miller and Iles, 2013). When the sperm and oocyte meet, it is necessary to ensure the compatibility of the genomic contributions of each parent to ensure that their combination will be conducive to embryonic development. During confrontation, the pairing of paternal RNAs, such as repeat associated spermatozoal RNAs, with complementary maternal repetitive elements, may activate or suppress their partner. Once compatibility between gametes is assured, the RNA-based information could then be transferred to a chromatized state, i.e. consolidation, likely by modifying the epigenome. Interestingly, snc-RNAs have been associated with heterochromatization, perhaps consolidation (reviewed in Lippman et al., 2004; Lippman and Martienssen, 2004).
Examples of this surveillance pathway and its consequence are apparent when one considers the various outcomes that can occur when two different species or breeds are crossed to produce hybrid offspring. The potential consequences include failure at fertilization, inappropriate embryo development or compromised fertility of the offspring. The latter outcome is best characterized by the mule (mare and donkey hybrid) which is infertile/sterile (Short, 1975). Similarly, blastomere formation is halted at the 8-cell stage when hybrid embryos are created by in vitro fertilization of a water buffalo (Bubalus bubalis) oocyte with bovine (Bos taurus) spermatozoa. This parallels a failure to undergo zygotic genome activation leading to developmental arrest (Patil and Totey, 2003). Perhaps this reflects an incompatible paternal contribution in which the specific mechanism necessary to activate or suppress elements necessary for embryo development is absent.
Transposable elements
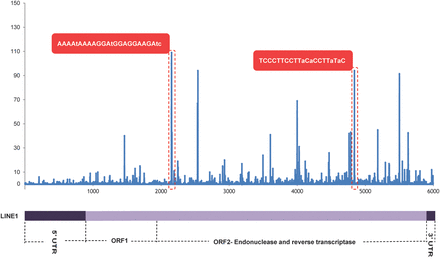
A large proportion of human spermatozoal snc-RNAs map to repetitive elements. The most abundant repeat classes represented in mature spermatozoa are the various members of the, LTR, SINE/ALU and LINE families of transposable elements (Krawetz et al., 2011). The role that transposable elements may play in the germline and early embryo remains controversial (Beraldi et al., 2006; Georgiou et al., 2009; van der Heijden and Bortvin, 2009). For example, LINE1 has a dynamic activity during early embryo development. Interrupting this activity results in embryonic arrest at the 2- or 4-cell stage (Beraldi et al., 2006). This may reflect the disruption of LINE1-associated reverse transcriptase (Pittoggi et al., 2003; reviewed in Spadafora, 2008). At this stage, LINE1 transcription is thought to be mediated by polypurine enriched LINE1 RNA fragments. These fragments form a triple helix within several regions of LINE1 potentially serving as a scaffolding that alters the association of chromatin modifiers and the transcriptional machinery (Fadloun et al., 2013) thereby promoting their own expression. Perhaps the large number of LINE1 fragments observed in and delivered by sperm activates this feedback loop. The distribution of short reads derived from RNA-seq of human spermatozoal snc-RNAs over the length of LINE1 exhibits very high enrichment of specific fragments, with some being homopurine polymers or near polypurine in sequence (Fig. 4). A similar distribution of highly enriched fragments is observed for other transposable elements in sperm such as ERVL-MaLR, for which early embryo function is also noted (Kigami et al., 2003; Inoue et al., 2012) and for which sperm-delivered fragments may play a similar activating role. It remains to be determined whether the transcribed transposable elements found in sperm such as SINE/ALU, which are complementary to coding and/or regulatory regions, may modulate host gene expression in early embryogenesis, since such behavior has been observed in other developmental processes (Polak and Domany, 2006). It appears though, that far from being ‘junk’ or simply an unintended consequence of global genomic demethylation, perhaps the abundant repeat associated sperm RNAs modulate other regulatory elements in the early embryonic stages of development.
Alignment of short RNAs (18–24 nt) from human sperm sample AS062 to the LINE1 repeat. Some LINE1 elements in the genome act as active transposable elements encoding both an ORF2 endonuclease and reverse transcriptase as well as the RNA-binding protein p40 encoded by ORF1. Specific LINE1 fragments are abundant in the small RNA fraction (blue peaks). Some of these fragments are purine rich sequences (red boxes), which may, through the formation of triplex structures, promote expression of complete LINE1 elements in the fertilized oocyte.
‘Other’ sperm RNAs
The distribution of poly(A+) selected sperm and testes sequencing reads demonstrates that while ∼10% of testes reads correspond to intergenic or intronic regions, more than two-thirds of sperm reads align to regions of unknown annotation or function (Sendler et al., 2013). Many of these unidentified yet prominent transcripts appear in all sperm samples from normal individuals and show little or no expression in other cell types. Although the majority of these RNAs have no known function, their abundance suggests that they may play a significant functional role. Whether this occurs during the final stages of sperm maturation, at delivery to the oocyte or during early preimplantation development remains to be determined. The observation that these transcripts correspond to genomic regions that retain histones, specifically H3K4me3, a histone modification correlated with transcriptional activity, suggests that sperm chromatin is uniquely structured to facilitate the transcription of these RNAs and that they are not artifacts (Hammoud et al., 2009; Sendler et al., 2013). Some classes of these elements with specific characteristics are described below.
Intronic retained elements
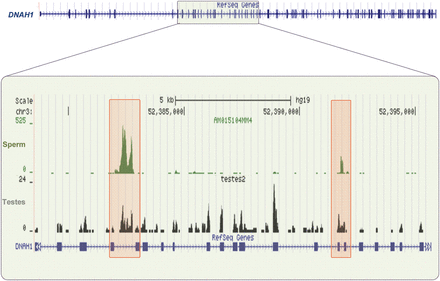
Non-coding RNAs contained within introns of coding mRNAs have been described in other systems (Hill et al., 2006). Both precursor miRNAs and snoRNAs often originate from these regions (Kiss et al., 1996; Lin et al., 2006; Li et al., 2007b). At least 200 distinctive non-coding sperm transcripts appear to be full-length introns specifically retained in sperm (Sendler et al., 2013). The mechanism by which they escape degradation after splicing remains to be defined. Interestingly, the corresponding mRNAs are often abundant in testes, while in sperm these coding segments show a marked reduced presence. Genes from which these intronic elements are derived do not classify into a distinct ontological category nor do they correlate with a specific pattern of early embryonic expression (Vassena et al., 2011). No evidence has been found to show that the intronic elements observed in sperm comply with the computationally predicted models for precursor elements of either sno- or mi-RNAs. Figure 5 shows an example of the sperm transcript DNAH1 (Dynein heavy chain 1, axonemal) in which several introns are retained. Several abundant intron spanning RNAs are apparent. Comparison of relative abundance of these intronic elements in sperm (A+) and (A−) fractions shows that the vast majority are not polyadenylated. Examination by RT–PCR of three such elements found within transcripts, TRIM66 (Tripartite motif-containing protein 66), KAT8 (Histone acetyltransferase KAT8) and QRICH1 (Glutamine-rich protein 1), confirmed that they were transcribed in the same orientation as the transcript in which they are embedded. Interestingly, they are present in much higher levels in sperm than in testes (unpublished data). These observations suggest that they are retained in mature sperm perhaps as part of a separate regulatory mechanism. The recent suggestion that some intronic ncRNAs are specifically regulated by a drop in temperature (Heo and Sung, 2011) to target their host transcripts for rapid degradation is intriguing. Spermatogenesis is temperature-sensitive and this may act as a physiological monitor.
Sperm intronic retained elements. The structure of DNAH1 is shown in the upper panel. The sequence reads obtained from sperm (green) and testes (black) RNA-seq within the highlighted region are shown (lower panel). The levels of specific intronic sperm RNAs are enhanced, while the coding regions of this transcript are absent in sperm. In equivalently sequenced testes samples, these intronic regions are underrepresented and resemble levels observed across the complete transcript (note y-axis). (See Supplementary data, Fig. S2 for more details.)
Long non-coding RNAs
Long non-coding RNAs (lnc-RNAs) range in size from ∼200 to 10,000 nt and are scattered throughout the genome. They are generally classified as a function of their relative position to protein coding genes (reviewed in Ponting et al., 2009). This includes intronic or intergenic regions where strand orientation cannot be directly determined, exonic regions primarily derived from the reverse strand, or from pseudogenes and retrotransposons. Spermatogenesis is in part regulated through the action of lnc-RNAs (Nolasco et al., 2012) some of which are certain to be antisense. Specifically, the abundance of antisense transcripts in testes may add to the mechanisms strictly regulating expression and function during spermatogenesis (Lee et al., 2009a). Lnc-RNA mechanisms have been described to operate in somatic cells at both the transcriptional or posttranscriptional levels (reviewed in Mercer et al., 2009; Lee, 2012; Rinn and Chang, 2012). At the transcriptional level this is accomplished by promoting specific histone modifications. For example, HOTAIR (HOX transcript antisense RNA) can modulate transcription through chromatin structure by recruiting PRC2 (Polychrome Recruiting Complex) to the HoxD locus thereby repressively marking histone H3 (Tsai et al., 2010). Transcription can also be modulated through the interaction of an lnc-RNA with an associated promoter region as exemplified by DHFR (Dihydrofolate reductase). Transcription of this gene by an alternative promoter results in a regulatory transcript that targets the usual promoter via triplex formation repressing the expression of DHFR (Martianov et al., 2007). Lnc-RNAs can also function post-transcriptionally during splicing (reviewed in Yoon et al., 2012a). For example, MALAT1 (Metastasis-Associated Lung Adenocarcinoma Transcript 1) regulates the alternative splicing of a subset of transcripts through its interaction with splicing factors (Tripathi et al., 2010). Translation of RNAs can also be modulated through the interaction of lnc-RNAs with specific repressors (Yoon et al., 2012b) or by general interference with the translation initiation complex (reviewed in Kindler et al., 2005). Finally, a similar regulatory effect can be achieved by modifying RNA stability. This can be affected through sense–antisense pairing that protects the target from miRNA-mediated degradation (Faghihi et al., 2008).
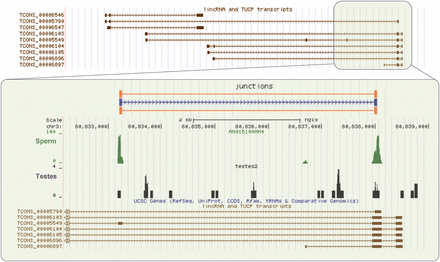
A number of predicted lnc-RNAs (Trapnell et al., 2010; Cabili et al., 2011) correspond to both abundant poly (A+) and poly (A−) sperm transcripts. In some cases, the sperm lnc-RNAs appear to represent different isoforms than the predicted forms. This is not unexpected given that the list of lnc-RNAs was primarily derived from expression in somatic cells. In many cases, the sperm lnc-RNAs appear more abundant in sperm than in testes (Fig. 6). The function of such lnc-RNAs in sperm maturation, fertilization and early embryo development remains to be explored.
Unique sperm lnc-RNAs isoforms. A 30 kb region of chromosome 3 containing a series of putative lnc-RNAs as identified by the Human Body Map lincRNA UCSC track (Trapnell et al., 2010; Cabili et al., 2011) is shown in upper panel. Although low- level expression of a number of identified lnc-RNAs is evident across this region in testes, a single highly expressed two exon RNA is observed in sperm (lower panel). Many junction reads, as measured by RUM (Grant et al., 2011) (box), confirm that these two exons are part of a single spliced transcript, which was not previously identified as a unique lnc-RNA isoform. (See Supplementary data, Fig. S3 for more details.)
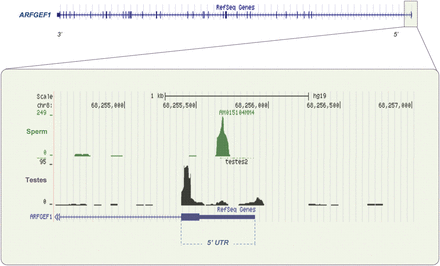
A specific class of lnc-RNAs overlaps coding transcripts and they are derived from the reverse strand (NAT: natural antisense transcripts). Several NAT have been described in mature spermatozoa (Ostermeier et al., 2005; Sendler et al., 2013). Roles in gene silencing, selective transcript editing, promoter inactivation and epigenetic modifications of the genome have been revealed for such RNAs (Lavorgna et al., 2004; Lapidot and Pilpel, 2006; Faghihi and Wahlestedt, 2009; Werner et al., 2009; Werner and Swan, 2010). For example, independent regulation of sense–antisense pairs of some nc-RNAs at specific stages of cellular development (Cawley et al., 2004; Katayama et al., 2005; Werner et al., 2007) results in the rapid processing of the longer transcripts into short ∼23 nt fragments (Borsani et al., 2005; Carlile et al., 2008). There are several examples of abundant of 100–300 nt sperm RNAs that overlap either the coding or UTR portion of an otherwise low-expressed or absent transcript. A striking example is the antisense transcript that overlaps ARFGEF1 (Brefeldin A-inhibited guanine nucleotide-exchange protein 1). This transcript is an ADP-ribosylation factor, required for maintenance of Golgi structure and function (Manolea et al., 2008). The corresponding fragment is abundant in sperm and corresponds to the middle of the 5′UTR. The full-length transcript is present in testes but virtually absent in sperm (Fig. 7). It would appear likely that this may be one example of a processing mechanism in sperm perhaps targeting specific transcripts for rapid degradation and which may be achieved throughout spermatogenesis through a variety of means. In addition to the possible action of NATs in the physiology of spermatozoa, some NATs present in mature spermatozoa overlap genes involved in early embryo development (Ostermeier et al., 2005) suggesting that such antisense RNAs may also have a role during fertilization and in the first steps of embryogenesis (Li et al., 2002).
Exonic sperm element. An overview of the structure of ARFGEF1 is provided in the upper panel. In testes, significant coverage of the complete transcript is observed (Supplementary data, Fig. S4). In contrast, sperm show virtually no transcript within the length of the coding region. A ∼100 nt sperm-specific element is observed however within the 5′UTR (highlighted region), but is virtually absent in equivalently prepared testes samples. This unique sperm element may serve to accelerate degradation of its containing transcript. (See Supplementary data, Fig. S4 for more details.)
Specific classes of lnc-RNAs
A number of elements from previously identified nc-RNA classes are abundant in the longer fraction of sperm RNA. These include Chromatin-associated-(CAR) and some small-nuclear ILF3/NF30 associated-(snaR) RNAs. CARs are found associated with chromatin and may act in cis or in trans to influence genomic architecture or regulate gene expression (Rodriguez-Campos and Azorin, 2007; Mondal et al., 2010). Three intronic and three intergenic regions in sperm, which show substantial sequencing coverage, overlapped with CARs recently identified in human fibroblast (HF) cells (Mondal et al., 2010). Perhaps a significant number of the unidentified lone elements in sperm are CARs and serve to aid the unique packaging requirements of the paternal genome. Several of the small NF90-associated RNAs (snaR) including snaR-G1 are also abundant. While their role remains uncertain, it is significant that snaR-G1 resides within the promoter region of the embryonic developmentally important human chorionic gonadotrophin (hCG1) (Parrott and Mathews, 2007). The level of this sperm snaR is elevated relative to that observed in testes, which is already at a level that is ∼100 times that of somatic tissues. This is certainly suggestive of a prominent functional role for these transcripts in mature spermatozoa.
tRNA-derived snc-RNAs and YRNAs
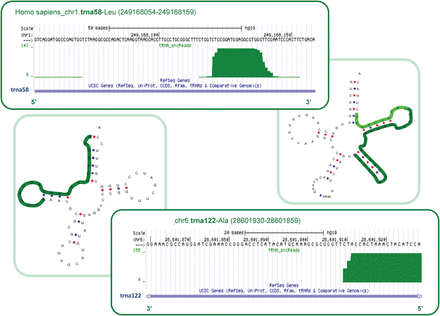
The majority of mouse and human mse-tsRNAs (mature-sperm-enriched tRNA-derived small RNAs) correspond to specific cleavage products (Krawetz et al., 2011; Peng et al., 2012). They typically represent 5′ end fragments between the D-loop and anticodon loop (Peng et al., 2012). However, as illustrated in Fig. 8, unlike mouse, human-specific mse-tsRNAs are also derived from the 3′ region (Krawetz et al., 2011; Peng et al., 2012). While initial inspection suggested that the fragmentation of rRNAs and tRNAs is to ensure translational silence (Johnson et al., 2011b), perhaps some of the mse-tsRNAs are functional. For example, their action as a stress responder appears conserved as far back as bacteria. In response to stress, an abundant 5′ end fragment of Val-tRNA in Haloferaz volcanii specifically targets and inhibits the translational machinery (Gebetsberger et al., 2012). This effect can be mimicked by the transfection of natural and synthetic tRNAs fragments (Ivanov et al., 2011). Knock-down of trF-1001, (a 3′ end tRNA-derived fragment from the Ser-TGA tRNA precursor) inhibits cancer cell proliferation (Lee et al., 2009b), suggesting a role in maintaining proliferation. Perhaps mse-tRNAs acts in a similar manner upon delivery to the oocyte (Peng et al., 2012).
Enrichment of mse-tRNA fragments in human sperm samples. Short read alignment to specific tRNAs is highlighted in the dark green box. Significant enrichment of fragments corresponding to particular regions of each tRNA is observed. The corresponding region within each folded structure is marked in green. The upper panel highlights the enrichment of the 3′ fragment of tRNA58Leu. Lower panel, enrichment of the 5′ fragment of tRNA122-Ala.
A minor portion of the snc-RNAs corresponds to YRNAs. YRNAs are a small cytoplasmic RNAs (85–115 nt) associated with Ro protein forming a RNP complex. In humans, specific YRNAs fragments that bind the Ro RNP complex have been observed (Krawetz et al., 2011). It has been proposed that this RNP complex participates in a quality control pathway for misfolded small RNAs (Stein et al., 2005). After ultraviolet irradiation, bacterial YRNAs and Ro protein increase suggesting that Ro RNP complex could have a role in the recognition or repair of DNA damage (Chen et al., 2003). Similarly, this RNP complex could act in the first steps of embryogenesis to initiate repair.
Spermatozoal RNA as epigenetic modifiers
Sperm-specific RNAs can influence fertilization and early embryo development but may also epigenetically modify the phenotype of the offspring (reviewed in Cuzin et al., 2008; Lalancette et al., 2008a; Johnson et al., 2011a; Hamatani, 2012; Rando, 2012). Following somatic cell nuclear transfer, some pathological changes in the placenta and congenital defects in the fetus as well as in the offspring are observed (reviewed in Shiels et al., 1999; Lanza et al., 2000; Xu and Yang, 2003). These changes reflect inappropriate epigenetic reprogramming of the donor and recipient cells leading to aberrant inner cell mass and trophectoderm formation (Niemann et al., 2008). It is possible that this mechanistic perturbation reflects the absence of the early effects of paternal elements (Krawetz, 2005; Krawetz et al., 2011) as somatic cells lack spermatozoal specific RNAs (Krawetz et al., 2011) that are likely to be integral to this pathway. This could involve targeting by epigenetic RNAs (Krawetz et al., 2011) that modify chromatin structure (Taft et al., 2011), e.g. through DNA methylation (Khraiwesh et al., 2010).
While the role of RNAs as modifiers of the epigenome altering gene expression is generally accepted, their transmission through the mammalian germline has been debated (Rassoulzadegan et al., 2006). The transgenerational epigenetic effect of paramutation is well established in the plant kingdom. Paramutation is the transfer of an epigenetic state to an unlinked homologous wild-type allele (paramutated allele) yielding a heritable phenotype in absence of an altered gene. Paramutation was first observed in maize (Brink, 1956) of the r1 gene that affects plant color. The most well-characterized paramutated gene in maize is b1, which employs the siRNA silencing pathway to modify methylation. At least three-repeat sequences upstream of the b1 gene are required to exceed a threshold to observe the effect conveyed by the siRNAs (reviewed in Arteaga-Vazquez and Chandler, 2010) for full penetrance and stability across generations. Paramutation in mammals seems to be reflected in complex processes like color, growth and disease, e.g. cardiac hypertrophy (Rassoulzadegan et al., 2006; Wagner et al., 2008; Grandjean et al., 2009). For example, while complete disruption of the mouse Kit (Mast/stem cell growth factor receptor Kit) gene was lethal, the heterozygote and paramutated animal presented a white tail and feet. Spermatozoa from heterozygote and paramutated progeny were enriched with truncated kit RNA. Interestingly, microinjection of heterozygote RNAs or miRNAs that target Kit (miR-221, miR-222) into fertilized oocyte induced the heterozygote phenotype (Rassoulzadegan et al., 2006). Other miRNAs, with a paramutation function that display a transgenerational effect have been described. These include miR-1 and miR-124 that paramutate Cdk9 (Cyclin-dependent kinase 9) and Sox9 (Transcription factor SOX-9), respectively. Although, the mechanism remains unknown, transactivation through methytransferases is being aggressively pursued.
Paramutation of human genes may reconcile familiar predisposition of some non-Mendelian genetic diseases. Transgenerational epigenetic effects could be a mechanism to confer increased competitiveness that allows the progeny to adapt to new environments to which the parents have been exposed. Alternatively, exposure to a toxic environment could hijack this response compromising the offspring. Transgenerational genetic effects occur when genetic factors in one generation affect the phenotype in the following generations without inheritance of the parental genetic factor. For example, daughters from genetically equal fathers but with a different Y chromosome differ in behavioral phenotype. This is remarkable, considering the low number of genes present on the Y chromosome (Nelson et al., 2010). On the one hand, it may simply reflect the high level of recombination during spermatogenesis yielding diverse gamete genomes (Lu et al., 2012; Wang et al., 2012). On the other hand, the variability may reflect epigenetic and/or transcript sharing among the maturing sperm cells through the cytoplasmic bridges (Caldwell and Handel, 1991). Evidence for the latter is provided by the apparent unequal distribution of transcripts among each sperm (Wykes et al., 2000).
Several reviews are available that provide examples of transgenerational epigenetic effects in mammals (reviewed in Jirtle and Skinner, 2007; Curley et al., 2011; Rando, 2012). For example, the progeny of mice receiving a high-fat diet during pregnancy are at increased risk of obesity and metabolic disease with subsequent passage through the paternal lineage (Dunn and Bale, 2011). Exposure to endocrine disruptors in female rats during gonadal sex determination increases the incidence of F1 male infertility. Some consequences of transgenerational inheritance are reflected by changes in the pattern of male germ cell methylation (Anway et al., 2005). Perhaps spermatozoal non-coding RNAs known to regulate DNA methylation and chromatin structure are components of transgenerational epigenetic mechanisms (reviewed in Lee, 2012; Rinn and Chang, 2012).
Models in agriculture
Animal models including cattle (Adams and Pierson, 1995; Burns et al., 2005) and equine (Carnevale, 2008) have proved essential to developing various assisted reproductive techniques (Bavister, 2002) and for providing a framework to study human reproductive disease (reviewed in Matsunari and Nagashima, 2009). While many critical sperm transcripts are conserved among different mammals (mouse, sheep, cattle, horse, pig and human) some appear species-specific (Card et al., 2013; Das et al., 2013; Sendler et al., 2013). Several orthologous spermatozoal transcripts observed among human, bovine and stallion are presented in Table I. Among the roles identified for these critical genes, are sperm DNA condensation (PRM1: Protamine 1), sperm motility (AKAP4: A-kinase anchor protein 4), sperm capacitation and sperm–oocyte interaction (CRISP2: Cysteine-rich secretory protein 2) and nucleocytoplasmic exchange during spermatogenesis (KIF5C: Kinesin heavy chain isoform 5C). Orthologous transcripts which appear at high levels in all species, but are of unknown function, like FAM71D (Family with sequence similarity 71, member D) warrant further study. As summarized in Supplementary data, Table SI and SII, many of the abundant human spermatozoal transcripts are orthologous to those present in either stallion or bull spermatozoa. Although some of these orthologous genes like GPX4 and DDX4 are involved in spermatogenesis (Phospholipid hydroperoxide glutathione peroxidase and Probable ATP-dependent RNA helicase DDX4, respectively), sperm function (CA2, Carbonic anhydrase 2) or embryo development (PAFAH1B1, Platelet-activating factor acetylhydrolase IB subunit alpha), the functional role for many others remains to be delineated. Understanding their role should prove valuable in elucidating factors regulating spermatogenesis and the underlying cause(s) of male infertility.
Orthologous spermatozoa transcripts identified among human, bovine and stallion.
| Transcript name (percentile ranking in human) . | Transcript symbol . | Reported functiona . | References . |
|---|---|---|---|
| Protamine 1 (0.99) | PRM1 | Sperm DNA condensation | Cho et al. (2001) (reviewed in Miller et al., 2005; Oliva, 2006) |
| Cysteine-rich secretory protein 2 (0.99) | CRISP2 | Sperm capacitation and sperm–egg interaction | Busso et al. (2007), Wang et al. (2004) |
| A-kinase anchor protein 4 (0.99) | AKAP4 | Sperm motility | Miki et al. (2002) (reviewed in Turner, 2006) |
| Kinesin heavy chain isoform 5C (0.98) | KIF5C | Nucleocytoplasmic exchange activities during spermatogenesis | Mannowetz et al. (2010) |
| Family with sequence similarity 71, member D (0.95) | FAM71D | Functional role in sperm not reported | Platts et al. (2007) |
| Involved in sperm morphogenesis with likely role in genome stability, cell division, survival and/or proliferation | Kittler et al. (2007), Paulsen et al. (2009), Chia et al. (2010) |
| Transcript name (percentile ranking in human) . | Transcript symbol . | Reported functiona . | References . |
|---|---|---|---|
| Protamine 1 (0.99) | PRM1 | Sperm DNA condensation | Cho et al. (2001) (reviewed in Miller et al., 2005; Oliva, 2006) |
| Cysteine-rich secretory protein 2 (0.99) | CRISP2 | Sperm capacitation and sperm–egg interaction | Busso et al. (2007), Wang et al. (2004) |
| A-kinase anchor protein 4 (0.99) | AKAP4 | Sperm motility | Miki et al. (2002) (reviewed in Turner, 2006) |
| Kinesin heavy chain isoform 5C (0.98) | KIF5C | Nucleocytoplasmic exchange activities during spermatogenesis | Mannowetz et al. (2010) |
| Family with sequence similarity 71, member D (0.95) | FAM71D | Functional role in sperm not reported | Platts et al. (2007) |
| Involved in sperm morphogenesis with likely role in genome stability, cell division, survival and/or proliferation | Kittler et al. (2007), Paulsen et al. (2009), Chia et al. (2010) |
The transcripts were selected and compared based on the RNA-seq data from human (Sendler et al., 2013), bovine (Card et al., 2013) and stallion (Das et al., 2013). The top 5% of the transcripts of the RNA-seq data from human were compared with bovine (FPKM > 100) and stallion (FPKM > 40).
Ortholgous genes were identified with Genomatix RegionMiner: Search for orthologous GeneIDs module.
Only stallion, bovine were compared for abundance, as these are the only species for which equivalent RNA-seq data are available.
aThe functions were verified in www.gopubmed.com, www.iHop-net.com and www.uniprot.org.
Orthologous spermatozoa transcripts identified among human, bovine and stallion.
| Transcript name (percentile ranking in human) . | Transcript symbol . | Reported functiona . | References . |
|---|---|---|---|
| Protamine 1 (0.99) | PRM1 | Sperm DNA condensation | Cho et al. (2001) (reviewed in Miller et al., 2005; Oliva, 2006) |
| Cysteine-rich secretory protein 2 (0.99) | CRISP2 | Sperm capacitation and sperm–egg interaction | Busso et al. (2007), Wang et al. (2004) |
| A-kinase anchor protein 4 (0.99) | AKAP4 | Sperm motility | Miki et al. (2002) (reviewed in Turner, 2006) |
| Kinesin heavy chain isoform 5C (0.98) | KIF5C | Nucleocytoplasmic exchange activities during spermatogenesis | Mannowetz et al. (2010) |
| Family with sequence similarity 71, member D (0.95) | FAM71D | Functional role in sperm not reported | Platts et al. (2007) |
| Involved in sperm morphogenesis with likely role in genome stability, cell division, survival and/or proliferation | Kittler et al. (2007), Paulsen et al. (2009), Chia et al. (2010) |
| Transcript name (percentile ranking in human) . | Transcript symbol . | Reported functiona . | References . |
|---|---|---|---|
| Protamine 1 (0.99) | PRM1 | Sperm DNA condensation | Cho et al. (2001) (reviewed in Miller et al., 2005; Oliva, 2006) |
| Cysteine-rich secretory protein 2 (0.99) | CRISP2 | Sperm capacitation and sperm–egg interaction | Busso et al. (2007), Wang et al. (2004) |
| A-kinase anchor protein 4 (0.99) | AKAP4 | Sperm motility | Miki et al. (2002) (reviewed in Turner, 2006) |
| Kinesin heavy chain isoform 5C (0.98) | KIF5C | Nucleocytoplasmic exchange activities during spermatogenesis | Mannowetz et al. (2010) |
| Family with sequence similarity 71, member D (0.95) | FAM71D | Functional role in sperm not reported | Platts et al. (2007) |
| Involved in sperm morphogenesis with likely role in genome stability, cell division, survival and/or proliferation | Kittler et al. (2007), Paulsen et al. (2009), Chia et al. (2010) |
The transcripts were selected and compared based on the RNA-seq data from human (Sendler et al., 2013), bovine (Card et al., 2013) and stallion (Das et al., 2013). The top 5% of the transcripts of the RNA-seq data from human were compared with bovine (FPKM > 100) and stallion (FPKM > 40).
Ortholgous genes were identified with Genomatix RegionMiner: Search for orthologous GeneIDs module.
Only stallion, bovine were compared for abundance, as these are the only species for which equivalent RNA-seq data are available.
aThe functions were verified in www.gopubmed.com, www.iHop-net.com and www.uniprot.org.
Differences between mice, ovine, bovine, equine, porcine and human RNA profiles may reflect basic interspecies differences in fertilization and early embryo development. In mouse, zygotic genome activation occurs just prior or at the 2-cell stage (reviewed in Schultz, 1993) whereas in bovine, ovine, porcine and human, genome activation appears to occur at the 4–8-cell stage (reviewed in Telford et al., 1990; Memili and First, 2000). The centrosome of the developing embryo is maternally derived in mouse (Schatten et al., 1986), whereas in other mammals, including human, it is paternally derived (Sathananthan et al., 1991, 1997; Manandhar et al., 2005). In mouse, pig and human, the male pronucleus is rapidly demethylated following fertilization whereas in bovine, sheep and rabbit, demethylation is comparatively delayed (Fulka et al., 2004). In part, this may reflect the degree of sperm chromatin condensation (Beaujean et al., 2004). In mammals, early developmental failure or altered phenotype has been associated with the perturbation of demethylation following in vitro fertilization (Yoshizawa et al., 2010), ovulation induction (Shi and Haaf, 2002), embryo culture (Zaitseva et al., 2007) or somatic cell nuclear transfer (reviewed in Morgan et al., 2005; Ma et al., 2012).
Artificial insemination (AI) can be viewed as one of the most important techniques devised for the genetic improvement of animals. In the dairy sector, improving reproductive efficiency is 5–10 times more economically important than any of the other production parameters including milk production and carcass quality (Wiltbank, 1994). Unexpectedly, the genetic selection of animals for higher milk production has lowered fertility (Veerkamp et al., 2003). One of the factors influencing fertility in the herd is the quality of the semen. However, sires with equivalent measurable semen parameters may produce vastly different pregnancy rates (reviewed in Kastelic and Thundathil, 2008). Since a single bull is used to inseminate hundreds of females, the use of semen from subfertile or infertile animals can have devastating consequences for the dairy industry. Selection of bulls or semen samples-based primarily on progressive forward motility invariably does not equally yield bulls of high or equal fertility (Selvaraju et al., 2008). Efficient semen evaluation methods including cellular and molecular approaches are required to predict fertility potential of a bull with high reproductive efficiency. In this regard, the potential of sperm transcripts to provide a marker of sperm quality and embryonic development in farm animals is of considerable interest. The levels of specific sperm RNAs associated with sperm functional parameters (Bissonnette et al., 2009; Curry et al., 2011) and conception (Lalancette et al., 2008b; Arangasamy et al., 2011; Kasimanickam et al., 2012) have been explored (Table II). These studies have now been extended to miRNAs in bovine (Govindaraju et al., 2012), porcine (Curry et al., 2009, 2011) and stallion (Das et al., 2013). As in the human (Krawetz et al., 2011), they may have critical roles both in mature sperm or after delivery to the oocyte where they may regulate fertilization and/or early embryonic development.
Mature spermatozoa RNAs associated with semen parameters and fertility in animals determined by RT–PCR.
| Study . | Technique . | Phenotype . | Altered specific RNAsa . |
|---|---|---|---|
| Bissonnette et al. (2009) | RTPCR | High motility bovine sperm fraction | ↑TSSK6 and ADAM5P |
| Arangasamy et al. (2011) | RTPCR | High sire conception rate in bovine | ↑CRISP2 |
| ↓CCT8 | |||
| Curry et al. (2011) | RTPCR | Low motility in porcine | ↑miR let 7d and 7e |
| Altered morphology in porcine | ↑miR let 7a, 7d,7e and miR22 | ||
| Hwang et al. (2012) | RTPCR | Low porcine embryo cleavage after IVF | ↑MYC, CYP19, ADAM2, PRM1 and PRM2 |
| High capacitated porcine spermatozoa | ↓MYC | ||
| Kasimanickam et al. (2013) | RTPCR | Fertile males in bovine | ↑Adiponectine and receptors ADR1 and ADR2 |
| Ganguly et al. (2013) | RTPCR | Motility impaired in bovine | ↓PRM1 |
| Study . | Technique . | Phenotype . | Altered specific RNAsa . |
|---|---|---|---|
| Bissonnette et al. (2009) | RTPCR | High motility bovine sperm fraction | ↑TSSK6 and ADAM5P |
| Arangasamy et al. (2011) | RTPCR | High sire conception rate in bovine | ↑CRISP2 |
| ↓CCT8 | |||
| Curry et al. (2011) | RTPCR | Low motility in porcine | ↑miR let 7d and 7e |
| Altered morphology in porcine | ↑miR let 7a, 7d,7e and miR22 | ||
| Hwang et al. (2012) | RTPCR | Low porcine embryo cleavage after IVF | ↑MYC, CYP19, ADAM2, PRM1 and PRM2 |
| High capacitated porcine spermatozoa | ↓MYC | ||
| Kasimanickam et al. (2013) | RTPCR | Fertile males in bovine | ↑Adiponectine and receptors ADR1 and ADR2 |
| Ganguly et al. (2013) | RTPCR | Motility impaired in bovine | ↓PRM1 |
aTranscript abundance: ↑ increased and ↓ decreased.
Mature spermatozoa RNAs associated with semen parameters and fertility in animals determined by RT–PCR.
| Study . | Technique . | Phenotype . | Altered specific RNAsa . |
|---|---|---|---|
| Bissonnette et al. (2009) | RTPCR | High motility bovine sperm fraction | ↑TSSK6 and ADAM5P |
| Arangasamy et al. (2011) | RTPCR | High sire conception rate in bovine | ↑CRISP2 |
| ↓CCT8 | |||
| Curry et al. (2011) | RTPCR | Low motility in porcine | ↑miR let 7d and 7e |
| Altered morphology in porcine | ↑miR let 7a, 7d,7e and miR22 | ||
| Hwang et al. (2012) | RTPCR | Low porcine embryo cleavage after IVF | ↑MYC, CYP19, ADAM2, PRM1 and PRM2 |
| High capacitated porcine spermatozoa | ↓MYC | ||
| Kasimanickam et al. (2013) | RTPCR | Fertile males in bovine | ↑Adiponectine and receptors ADR1 and ADR2 |
| Ganguly et al. (2013) | RTPCR | Motility impaired in bovine | ↓PRM1 |
| Study . | Technique . | Phenotype . | Altered specific RNAsa . |
|---|---|---|---|
| Bissonnette et al. (2009) | RTPCR | High motility bovine sperm fraction | ↑TSSK6 and ADAM5P |
| Arangasamy et al. (2011) | RTPCR | High sire conception rate in bovine | ↑CRISP2 |
| ↓CCT8 | |||
| Curry et al. (2011) | RTPCR | Low motility in porcine | ↑miR let 7d and 7e |
| Altered morphology in porcine | ↑miR let 7a, 7d,7e and miR22 | ||
| Hwang et al. (2012) | RTPCR | Low porcine embryo cleavage after IVF | ↑MYC, CYP19, ADAM2, PRM1 and PRM2 |
| High capacitated porcine spermatozoa | ↓MYC | ||
| Kasimanickam et al. (2013) | RTPCR | Fertile males in bovine | ↑Adiponectine and receptors ADR1 and ADR2 |
| Ganguly et al. (2013) | RTPCR | Motility impaired in bovine | ↓PRM1 |
aTranscript abundance: ↑ increased and ↓ decreased.
Biomarkers of human fertility
Infertility is a growing problem in contemporary society, affecting ∼10–15% of reproductive aged couples (reviewed in Evers, 2002). The evaluation of observable semen parameters is well suited to diagnosing some obvious forms of male infertility. However, even when the sample is deemed suitable based on external characteristics, fertilization potential is still in question. Hence, there is significant need for additional markers of sperm fertility status. Differences in the levels of individual or transcript groups between infertile patients and fertile controls may provide a means to assess the fidelity of past spermatogenic events and/or potential post-fertilization success (reviewed in Anton and Krawetz, 2012). Microarray analysis has identified altered mRNA profiles in infertile patients presenting suboptimal seminal parameters (Platts et al., 2007; Jodar et al., 2012; Montjean et al., 2012). These results identified some altered pathways allowing further insight into the pathogenic mechanisms involved in male infertility. For example, the ubiquitin–proteosome pathway is severely disrupted in teratozoospermic patients (Platts et al., 2007) and in oligozoospermic patients a decrease in the transcripts involved in DNA repair and oxidative stress regulation has been observed (Montjean et al., 2012). Because of the relatively high cost of microarrays, the use of real-time PCR has been explored.
Protamine transcripts are among those most strongly associated with the different seminal parameters such as sperm concentration and motility (Lambard et al., 2004; Kempisty et al., 2007) as well as with sperm fertilization ability and embryo quality (Depa-Martynow et al., 2007, 2012; Steger et al., 2008; Jodar et al., 2012; Rogenhofer et al., 2013). This is likely reflective of the relative abundance of the protamines and their requirement for chromatin packaging. Although the spermatozoa contain a heterogenous population of transcripts, some transcript pairs are proposed to have a stable correlation of expression among different fertile individuals (Lima-Souza et al., 2012). All of these reported RNA factors could provide a useful suite of fertility biomarkers, and are summarized in Table III.
Altered spermatozoa transcripts and pathways associated with human male infertility determined by microarray or RT–PCR.
| Study . | Technique . | Phenotype . | Altered specific RNAsa . | Altered pathways . |
|---|---|---|---|---|
| Lambard et al. (2004) | RTPCR | Low motility sperm fraction | ↑PRM1, eNOS and nNOS | |
| High capacitated spermatozoa | ↓MYC | |||
| Wang et al. (2004) | RTPCR | Asthenozoospermic patients | ↓TPX1 and LDHC | |
| Depa-Martynow et al. (2007) | RTPCR | IVF failure | ↓Fetilin beta, PRM1 and PRM2 | |
| Guo et al. (2007) | RTPCR | Oligozoospermic patients | ↓VASA | |
| Jedrzejczak et al. (2007) | RTPCR | Athenozoospermic patients | ↓HILS1, TNP1, and TNP2 | |
| Kempisty et al. (2007) | RTPCR | Athenozoospermic patients | ↓PRM1 and PRM2 | |
| Li et al. (2007a, b) | RTPCR | Low motility sperm fraction | ↓CatSper2 and CatSper3 | |
| Platts et al. (2007) | Array | Teratozoospermic patients | Ubiquitin-proteosome pathway, apoptotic pathway and MAP kinase signaling | |
| Steger et al. (2008) | RTPCR | Infertile patients | Aberrant PRM1/PRM2 | |
| ↑Bcl2 | ||||
| Avendano et al. (2009) | RTPCR | Infertile patients | ↓PSG1 and HLA-E | |
| Garrido et al. (2009) | Array | Infertile normozoospermic patients | Spermatozoa differentiation | |
| RTPCR | ↓TRY1, GGF1 and CAB39L | |||
| Nguyen et al. (2009) | Array | Cryptorchid male | Germ cell maturation and sperm tail formation | |
| RTPCR | ↓TPX1 | |||
| Ferlin et al. (2010) | RTPCR | Varicocele, oligozoospermia | ↑ HSPA4, HSF1 and HSF2 | |
| Varicocele, normozoospermia | ↑HSFY | |||
| Oligozoospermic patients | ↑HSP90 | |||
| Garcia-Herrero et al. (2011) | Array | ICSI failure | Testicular function, spermatogenesis and sperm physiology | |
| Zheng et al. (2011) | RTPCR | Oligoasthenozoospermic patients | ↓BDNF | |
| Depa-Martynow et al. (2012) | RTPCR | Low concentration, motility, morphology, fertilization ability, embryo quality | ↓PRM1 and PRM2 | |
| Jodar et al. (2012) | Array | Asthenozoospermic patients | Spermatid development and the ubiquinone biosynthesis pathway | |
| RTPCR | Asthenozoospermic patients | ↓ ANXA2, BRD2 and OAZ3 | ||
| Infertile patients | ↓PRM1 and PRM2 | |||
| Montjean et al. (2012) | Array | Oligozoospermic patients | Spermatogenesis, sperm motility, DNA repair and oxidative stress regulation | |
| RTPCR | ↓ PRM2, TPD52L3, JMJD1A and NIPBL | |||
| Rogenhofer et al. (2013) | RTPCR | Low fertilization capacity (IVF and ICSI) | Aberrant PRM1/PRM2 |
| Study . | Technique . | Phenotype . | Altered specific RNAsa . | Altered pathways . |
|---|---|---|---|---|
| Lambard et al. (2004) | RTPCR | Low motility sperm fraction | ↑PRM1, eNOS and nNOS | |
| High capacitated spermatozoa | ↓MYC | |||
| Wang et al. (2004) | RTPCR | Asthenozoospermic patients | ↓TPX1 and LDHC | |
| Depa-Martynow et al. (2007) | RTPCR | IVF failure | ↓Fetilin beta, PRM1 and PRM2 | |
| Guo et al. (2007) | RTPCR | Oligozoospermic patients | ↓VASA | |
| Jedrzejczak et al. (2007) | RTPCR | Athenozoospermic patients | ↓HILS1, TNP1, and TNP2 | |
| Kempisty et al. (2007) | RTPCR | Athenozoospermic patients | ↓PRM1 and PRM2 | |
| Li et al. (2007a, b) | RTPCR | Low motility sperm fraction | ↓CatSper2 and CatSper3 | |
| Platts et al. (2007) | Array | Teratozoospermic patients | Ubiquitin-proteosome pathway, apoptotic pathway and MAP kinase signaling | |
| Steger et al. (2008) | RTPCR | Infertile patients | Aberrant PRM1/PRM2 | |
| ↑Bcl2 | ||||
| Avendano et al. (2009) | RTPCR | Infertile patients | ↓PSG1 and HLA-E | |
| Garrido et al. (2009) | Array | Infertile normozoospermic patients | Spermatozoa differentiation | |
| RTPCR | ↓TRY1, GGF1 and CAB39L | |||
| Nguyen et al. (2009) | Array | Cryptorchid male | Germ cell maturation and sperm tail formation | |
| RTPCR | ↓TPX1 | |||
| Ferlin et al. (2010) | RTPCR | Varicocele, oligozoospermia | ↑ HSPA4, HSF1 and HSF2 | |
| Varicocele, normozoospermia | ↑HSFY | |||
| Oligozoospermic patients | ↑HSP90 | |||
| Garcia-Herrero et al. (2011) | Array | ICSI failure | Testicular function, spermatogenesis and sperm physiology | |
| Zheng et al. (2011) | RTPCR | Oligoasthenozoospermic patients | ↓BDNF | |
| Depa-Martynow et al. (2012) | RTPCR | Low concentration, motility, morphology, fertilization ability, embryo quality | ↓PRM1 and PRM2 | |
| Jodar et al. (2012) | Array | Asthenozoospermic patients | Spermatid development and the ubiquinone biosynthesis pathway | |
| RTPCR | Asthenozoospermic patients | ↓ ANXA2, BRD2 and OAZ3 | ||
| Infertile patients | ↓PRM1 and PRM2 | |||
| Montjean et al. (2012) | Array | Oligozoospermic patients | Spermatogenesis, sperm motility, DNA repair and oxidative stress regulation | |
| RTPCR | ↓ PRM2, TPD52L3, JMJD1A and NIPBL | |||
| Rogenhofer et al. (2013) | RTPCR | Low fertilization capacity (IVF and ICSI) | Aberrant PRM1/PRM2 |
aTranscript abundance: ↑ increased and ↓ decreased.
Altered spermatozoa transcripts and pathways associated with human male infertility determined by microarray or RT–PCR.
| Study . | Technique . | Phenotype . | Altered specific RNAsa . | Altered pathways . |
|---|---|---|---|---|
| Lambard et al. (2004) | RTPCR | Low motility sperm fraction | ↑PRM1, eNOS and nNOS | |
| High capacitated spermatozoa | ↓MYC | |||
| Wang et al. (2004) | RTPCR | Asthenozoospermic patients | ↓TPX1 and LDHC | |
| Depa-Martynow et al. (2007) | RTPCR | IVF failure | ↓Fetilin beta, PRM1 and PRM2 | |
| Guo et al. (2007) | RTPCR | Oligozoospermic patients | ↓VASA | |
| Jedrzejczak et al. (2007) | RTPCR | Athenozoospermic patients | ↓HILS1, TNP1, and TNP2 | |
| Kempisty et al. (2007) | RTPCR | Athenozoospermic patients | ↓PRM1 and PRM2 | |
| Li et al. (2007a, b) | RTPCR | Low motility sperm fraction | ↓CatSper2 and CatSper3 | |
| Platts et al. (2007) | Array | Teratozoospermic patients | Ubiquitin-proteosome pathway, apoptotic pathway and MAP kinase signaling | |
| Steger et al. (2008) | RTPCR | Infertile patients | Aberrant PRM1/PRM2 | |
| ↑Bcl2 | ||||
| Avendano et al. (2009) | RTPCR | Infertile patients | ↓PSG1 and HLA-E | |
| Garrido et al. (2009) | Array | Infertile normozoospermic patients | Spermatozoa differentiation | |
| RTPCR | ↓TRY1, GGF1 and CAB39L | |||
| Nguyen et al. (2009) | Array | Cryptorchid male | Germ cell maturation and sperm tail formation | |
| RTPCR | ↓TPX1 | |||
| Ferlin et al. (2010) | RTPCR | Varicocele, oligozoospermia | ↑ HSPA4, HSF1 and HSF2 | |
| Varicocele, normozoospermia | ↑HSFY | |||
| Oligozoospermic patients | ↑HSP90 | |||
| Garcia-Herrero et al. (2011) | Array | ICSI failure | Testicular function, spermatogenesis and sperm physiology | |
| Zheng et al. (2011) | RTPCR | Oligoasthenozoospermic patients | ↓BDNF | |
| Depa-Martynow et al. (2012) | RTPCR | Low concentration, motility, morphology, fertilization ability, embryo quality | ↓PRM1 and PRM2 | |
| Jodar et al. (2012) | Array | Asthenozoospermic patients | Spermatid development and the ubiquinone biosynthesis pathway | |
| RTPCR | Asthenozoospermic patients | ↓ ANXA2, BRD2 and OAZ3 | ||
| Infertile patients | ↓PRM1 and PRM2 | |||
| Montjean et al. (2012) | Array | Oligozoospermic patients | Spermatogenesis, sperm motility, DNA repair and oxidative stress regulation | |
| RTPCR | ↓ PRM2, TPD52L3, JMJD1A and NIPBL | |||
| Rogenhofer et al. (2013) | RTPCR | Low fertilization capacity (IVF and ICSI) | Aberrant PRM1/PRM2 |
| Study . | Technique . | Phenotype . | Altered specific RNAsa . | Altered pathways . |
|---|---|---|---|---|
| Lambard et al. (2004) | RTPCR | Low motility sperm fraction | ↑PRM1, eNOS and nNOS | |
| High capacitated spermatozoa | ↓MYC | |||
| Wang et al. (2004) | RTPCR | Asthenozoospermic patients | ↓TPX1 and LDHC | |
| Depa-Martynow et al. (2007) | RTPCR | IVF failure | ↓Fetilin beta, PRM1 and PRM2 | |
| Guo et al. (2007) | RTPCR | Oligozoospermic patients | ↓VASA | |
| Jedrzejczak et al. (2007) | RTPCR | Athenozoospermic patients | ↓HILS1, TNP1, and TNP2 | |
| Kempisty et al. (2007) | RTPCR | Athenozoospermic patients | ↓PRM1 and PRM2 | |
| Li et al. (2007a, b) | RTPCR | Low motility sperm fraction | ↓CatSper2 and CatSper3 | |
| Platts et al. (2007) | Array | Teratozoospermic patients | Ubiquitin-proteosome pathway, apoptotic pathway and MAP kinase signaling | |
| Steger et al. (2008) | RTPCR | Infertile patients | Aberrant PRM1/PRM2 | |
| ↑Bcl2 | ||||
| Avendano et al. (2009) | RTPCR | Infertile patients | ↓PSG1 and HLA-E | |
| Garrido et al. (2009) | Array | Infertile normozoospermic patients | Spermatozoa differentiation | |
| RTPCR | ↓TRY1, GGF1 and CAB39L | |||
| Nguyen et al. (2009) | Array | Cryptorchid male | Germ cell maturation and sperm tail formation | |
| RTPCR | ↓TPX1 | |||
| Ferlin et al. (2010) | RTPCR | Varicocele, oligozoospermia | ↑ HSPA4, HSF1 and HSF2 | |
| Varicocele, normozoospermia | ↑HSFY | |||
| Oligozoospermic patients | ↑HSP90 | |||
| Garcia-Herrero et al. (2011) | Array | ICSI failure | Testicular function, spermatogenesis and sperm physiology | |
| Zheng et al. (2011) | RTPCR | Oligoasthenozoospermic patients | ↓BDNF | |
| Depa-Martynow et al. (2012) | RTPCR | Low concentration, motility, morphology, fertilization ability, embryo quality | ↓PRM1 and PRM2 | |
| Jodar et al. (2012) | Array | Asthenozoospermic patients | Spermatid development and the ubiquinone biosynthesis pathway | |
| RTPCR | Asthenozoospermic patients | ↓ ANXA2, BRD2 and OAZ3 | ||
| Infertile patients | ↓PRM1 and PRM2 | |||
| Montjean et al. (2012) | Array | Oligozoospermic patients | Spermatogenesis, sperm motility, DNA repair and oxidative stress regulation | |
| RTPCR | ↓ PRM2, TPD52L3, JMJD1A and NIPBL | |||
| Rogenhofer et al. (2013) | RTPCR | Low fertilization capacity (IVF and ICSI) | Aberrant PRM1/PRM2 |
aTranscript abundance: ↑ increased and ↓ decreased.
Most of the high abundant transcripts in human sperm have a relationship with testicular function and spermatogenesis (Sendler et al., 2013). Microarray analysis of serially sectioned testes has produced transcript profiles from different stages of spermatogenesis (Chalmel et al., 2012). These data, in conjunction with the profiles obtained from mature spermatozoa, will be instrumental in correlating perturbations during spermattogenesis and specific forms of male infertility. For example, using sperm transcript profiling, the initial effect of teratozoospermia was traced to the pachytene spermatocyte (Platts et al., 2007). Identification of these disruptions by profiling sperm transcripts rather than invasive testicular biopsy offers obvious benefits to the patient (Yatsenko et al., 2006). These novel techniques are expected to be useful in identifying the origins, prognosis and treatment of various forms of what was previously considered to be male idiopathic infertility.
In recent years, there has been speculation about the phenotype and health of the offspring born out of assisted reproductive technologies (ART) especially after IVF and ICSI (reviewed in Batcheller et al., 2011; Savage et al., 2011). The children born with the use of ART have been reported, by some, to have a higher risks to health (e.g. fertility disorders) when compared with those naturally conceived. However, considerable controversy remains as to the composition of the appropriate ‘control’ group that would permit such comparisons. The infertility associated with a genetic defect (reviewed in Matzuk and Lamb, 2008), if any, would also be carried to the next generation along with the increased risk of epigenetic disorders (Kobayashi et al., 2009) that could be mirrored by changes in the pattern of DNA methylation (reviewed in Savage et al., 2011; Feuer et al., 2013; Hart and Norman, 2013).
Maternally, it is well established that advanced age increases the risk of cytogenetic abnormalities that can manifest as Down syndrome (Hassold et al., 1984). With the ability of ART to ‘bypass’ some of the boundaries that have limited conception, studies are now beginning to suggest an association between increasing paternal age at conception and neurological disorders like autism, with a noticeable effect between 30 and 39 years and a substantial effect at ≥50 years (Grether et al., 2009; Hultman et al., 2011; van Balkom et al., 2012). Recent data show that the autism spectrum of disorders are strongly associated with de novo mutations (Sanders et al., 2012) present in spermatozoa. This likely reflects the continuity of sperm production during the life of an adult male that arises from the ∼840 divisions from each stem cell that give rise to the mature spermatozoon. The resulting cumulative effect of mutations at each division (Kong et al., 2012) along with effects on chromatin integrity (Wyrobek et al., 2006) and methylation errors (Flanagan et al., 2006) may contribute to the growing prevalence of age-related effects as observed, e.g. autism.
Paternal factors are thought to underlie the etiology of infertility/subfertility in approximately half of the couples undergoing ART (Jarow et al., 2002). This may, to some extent, be reflective of the sperm transcript profile that often varies between infertile and fertile males (Platts et al., 2007; Garrido et al., 2009; Garcia-Herrero et al., 2010a; Jodar et al., 2012; Montjean et al., 2012). Thus, the use of the paternal transcriptome as a biomarker deserves consideration. Moreover, different transcript profiles have been suggested that may coincide with successful pregnancy in different fertility treatments (Garcia-Herrero et al., 2010b, 2011). This has further supported the notion (Ostermeier et al., 2002; Platts et al., 2007; Lima-Souza et al., 2012) of the potential use of the microarray strategy as a clinical diagnostic tool (Garrido et al., 2013). Perhaps, sperm transcript profiling of patients undergoing ART (Garcia-Herrero et al., 2011) will aid in identifying both specific paternal factors and pathways which are negatively affecting fertility outcomes (reviewed in Carrell, 2008). As costs continue to decline, it is very likely that sperm transcript sequencing will reach the clinic shortly. These data and their analysis provide several advantages compared with the microarray assays. These include a quantitative description of abundance, immediate assessment of the fidelity of the information content and allele-specific expression reflective of Expressed Quantitative Trait Loci and hence genotype. This truly heralds the beginning of Male Personalized Reproductive Medicine.
Conclusion
Rapid development of RNA assay technologies including RNA-seq has detailed the specific presence of a wide variety of spermatozoal transcripts. Such transcripts appear to reflect both the past course of spermatogenesis, yet also include factors critical to fertilization and successful embryo development (Fig. 9). This likely includes a significant epigenetic component that is mediated by the action of sperm-borne RNAs on both genotype and phenotype of the offspring. An increasing number of comparative animal models will be of use in identifying key transcripts, many of which may be unique to sperm, and in elucidating their functional role. With this understanding of the differentiative history of the pool of spermatozoal transcripts, the outcome of ART, and perhaps in the future the ability to both treat underlying causes of infertility and ensure the birth of a healthy child, will be optimized.
The potential actions of spermatozoal RNAs during early embryo development. Spermatozoal RNAs are delivered to the oocyte acting during the first steps of embryogenesis. Some intact paternal mRNAs like INST1 could be translated by maternal machinery. On one hand, paternal mature miRNAs like mouse miR-34c are essential for the first cell division. On the other hand, primicroRNAs like 181c, can be processed and thus activated by maternal DICER to their mature miRNAs regulating transcript stability, whereas others may target promoters. Interestingly, some non-coding RNAs act through triplex structures and perhaps are transcriptional regulators. For example, homopurine fragments of LINE1 provided by spermatozoa induce LINE1 transcription during the first divisions of the zygote. It has also been proposed that piRNAs, miRNAs and other potential RNAs may be the pathway to confrontation and consolidation.
Authors' roles
M.J., S.S. and E.S. analyzed the data, performed the literature searches and wrote the manuscript with M.P.D. and S.A.K. S.A.K. directed the data analysis, writing and editing of the manuscript. The authors alone are responsible for the content and writing of the paper.
Funding
This work was supported in part by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD039005. S.S. is supported by a Cutting-edge Research Enhancement and Scientific Training Award, Department of Biotechnology, Government of India. M.P.D. and S.A.K. are recipients of an EMD Serono grant to Wayne State University. Otherwise the authors report no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or NIH.
Acknowledgements
The authors would like to thank Mr G. Johnson for his critical review of this manuscript and Mr R. Sanchez Giones and Mr Yitzchok Sendler for their assistance in the preparation of the illustrations. We apologize to others that we were not able to include their work in this review. The authors would like to thank the members of RMN for their invaluable assistance and for providing some of the samples used to illustrate the properties of spermatozoal RNAs. Prerelease access to the SEQR Whole Transcription Amplification system from Sigma Chemical Corporation is gratefully acknowledged.
References
Author notes
Reproductive Medicine Network.