-
PDF
- Split View
-
Views
-
Cite
Cite
T. Simoncini, X-D. Fu, A. Caruso, S. Garibaldi, C. Baldacci, M.S. Giretti, P. Mannella, M.I. Flamini, A.M. Sanchez, A.R. Genazzani, Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors, Human Reproduction, Volume 22, Issue 8, August 2007, Pages 2325–2334, https://doi.org/10.1093/humrep/dem109
Close - Share Icon Share
Abstract
Progestins have actions on the cardiovascular system, which depend on the structure as well as on receptor binding characteristics. Drospirenone (DRSP) is a progestin that uniquely interferes with the signaling of the mineralocorticoid receptor (MR). Hormone therapy containing DRSP results in blood pressure reduction in hypertensive post-menopausal women.
We describe the effects of DRSP on endothelial nitric oxide (NO) synthesis and compare them with those of progesterone (P) and of medroxyprogesterone acetate (MPA). In addition, we herein tested the relevance of the anti-mineralocorticoid activity of DRSP for NO synthesis.
DRSP results in rapid activation of the endothelial NO synthase (eNOS) through mitogen-activated protein kinases and phosphatidylinositol 3-kinase as well as in enhanced eNOS expression. These actions depend on P receptor. When the cells are exposed to aldosterone, a reduction of eNOS expression is found that is antagonized by DRSP. This action is not shared by P or MPA. In addition, DRSP does not interfere with the induction or activation of eNOS induced by estradiol, as opposed to MPA.
DRSP acts on endothelial cells via a combined action through the P and MRs. These results help to interpret the anti-hypertensive effects of hormonal therapies containing DRSP.
Introduction
The influence of sex steroid hormones on the cardiovascular system is controversial. Endogenous estrogens seem to play a protective role on cardiovascular cells, including endothelial, smooth muscle cells and cardiomyocytes (Mendelsohn and Karas, 1999), explaining part of the gender-related differences in coronary heart disease (CHD) (Mendelsohn and Karas, 2005). However, recent trials have raised questions as to whether post-menopausal hormone replacement therapy (HRT) is associated with a reduction in cardiovascular disease risk.
Between the issues raised by the recent trials, a compelling one is the role of progestins on the cardiovascular system. According to the Women's Health Initiative (WHI) trial, post-menopausal women receiving combined HRT with conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA) (Manson et al., 2003) have no protection from coronary disease, as opposed to those treated with CEE only (Hsia et al., 2006) who show a trend toward a significant reduction of CHD. A similar discrepancy extends to the incidence of breast cancer (Stefanick et al., 2006). These findings raise interest on the role of progesterone receptors (PRs) in human cells as well as on the implications of using different progestins during hormone therapies.
The suggestion that synthetic progestins, differently from progesterone (P), may counteract some of the positive effects of estrogens in vascular cells has been around for years. In ovariectomized monkeys, MPA blunts the vasodilatation induced by estrogens (Miyagawa et al., 1997) and reduces the inhibition of arterial remodeling associated with estrogen replacement (Register and Adams, 1998). These effects are not found with P (Adams et al., 1990). Similar observations have been made in humans, where P has positive effects on exercise-induced myocardial ischemia that are not shared by MPA (Rosano et al., 2000). These differential actions depend in part on distinct effects on the endothelium. P, but not MPA, enhances the synthesis of endothelial nitric oxide (NO) and reduces the expression of leukocyte adhesion molecules (Simoncini et al., 2004). The reason for these discrepancies relies in the activation of partially distinct signaling pathways by PRs in the presence of P or MPA, in addition to the binding of MPA to the glucocorticoid receptor (Simoncini et al., 2004).
Overall, this supports the hypothesis that the various progestins may induce partially different effects in vascular cells, which might be clinically relevant.
Drospirenone (DRSP) is a synthetic progestin structurally related to 17α-spirolactone (Krattenmacher, 2000) used in contraception and in post-menopausal hormone therapy. From the cardiovascular point of view, this compound is interesting due to its ability to interfere with the MR and with the renin–angiotensin–aldosterone system (Sitruk-Ware, 2005), therefore, having meaningful effects on blood pressure in post-menopausal women with hypertension (Preston et al., 2005; White et al., 2005, 2006). However, little is known on the effects of this compound on vascular cells.
Deranged NO synthesis by endothelial cells is associated with decreased vascular dilatation, hypertension and enhanced atherosclerosis (Hansson, 2005). Estrogens and progestogens share the ability to regulate the endothelial NO synthase (eNOS) (Chambliss and Shaul, 2002; Simoncini and Genazzani, 2003).
Here, we assess the effects of DRSP on eNOS activity and expression in human endothelial cells and we compare with P and the synthetic progestogen MPA. Furthermore, we highlight the differences in the signal transduction induced by these compounds through P and MRs.
Materials and Methods
Cell cultures and treatments
Human umbilical vein endothelial cells (HUVECs) were cultured as described (Simoncini et al., 1999). Before treatments, HUVECs were kept 48 h in Dulbecco's modified Eagle's medium (DMEM) containing steroid-deprived FBS. Before experiments investigating non-transcriptional effects, HUVECs were kept in DMEM containing no FBS for 8 h. P, MPA and RU486 were from Sigma-Aldrich (Saint-Louis, MO, USA), DRSP was obtained from Dr K.H. Fritzemeier, Bayer Schering Pharma AG (Germany).
eNOS activity assay
eNOS activity was determined as conversion of [3H]arginine to [3H]citrulline in endothelial cell lysates. Briefly, HUVECs were harvested in ice-cold PBS containing 1 mM EDTA. The cell lysates were pelleted in a microfuge (2 min, 13000 rpm, 4°C) and subsequently homogenized in a buffer containing 25 mM Tris–HCl, pH 7.4, 1 mM EDTA and 1 mM EGTA. eNOS activity was detected by measuring the conversion of [3H]l-arginine to [3H]l-citrulline with the nitric oxide synthase assay kit (Calbiochem, La Jolla, CA, USA), according to the manufacturer's instructions. About 0.001 mCi (0.037 MBq) of [3H]l-arginine were added to each sample well for the reaction. Rat cerebellum extracts, containing elevated amounts of iNOS, were used as positive controls, whereas endothelial cell extracts incubated in the presence of the competitive NOS inhibitor L-NAME (1 mM) served were used to subtract the non-specific activity.
Nitrite assay
NO production was determined by a nitrite assay using 2, 3 diaminonaphtalene. Fluorescence of 1-(H)-naphtotriazole was measured with excitation and emission wavelengths of 365 and 450 nm. Standard curves were constructed with sodium nitrite. Non-specific fluorescence was determined in the presence of LNMA (3 mM).
Immunoblottingss
Cell lysates were separated by 10% SDS–PAGE. Antibodies used were: eNOS (Transduction Laboratories, Lexington, KY, USA), wild type or Tyr204-P-ERK 1/2 (Calbiochem, San Diego, CA, USA), wild type and Thr308-P-Akt (Upstate Biotechnology, Lake Placid, NY, USA). Primary and secondary antibodies were incubated with the membranes with standard technique.
Statistical analysis
All values are expressed as mean ± SD. Statistical differences between mean values were determined by ANOVA, followed by the Fisher's protected least significance difference.
Results
DRSP increases NO synthesis
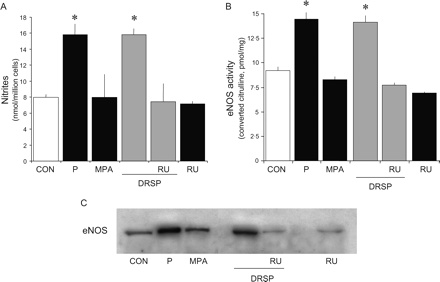
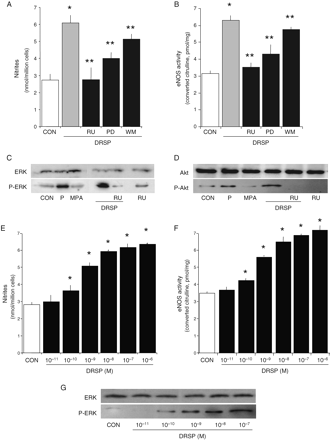
HUVECs were treated for 48 h with P (10−7 M), MPA (10−7 M) or DRSP (10−7 M), either alone or in the presence of the PR antagonist RU486 (10−5 M). DRSP and P increased NO synthesis (Fig. 1A) (measured as nitrites released in cell culture medium) and eNOS activity (Fig. 1B) (measured as conversion of [3H]arginine to [3H]citrulline). These effects were due to increased eNOS expression (Fig. 1C). As previously shown (Simoncini et al., 2004), MPA had no effect on NO synthesis or eNOS (Fig. 1A–C). The addition of RU486 to DRSP prevented the effects of this progestin (Fig. 1A–C), indicating that DRSP acts through PR.
DRSP increases NO synthesis and eNOS activity and expression via progesterone receptor Steroid-deprived HUVECs were treated for 48 h with P (10−7 M), MPA (10−7 M) or DRSP (10−7 M), either alone or in the presence of the PR antagonist RU486 (RU486, 10−5 M). (A) NO synthesis was assayed; (B) eNOS activity was tested and (C) shows eNOS protein amounts. *P < 0.01 versus control
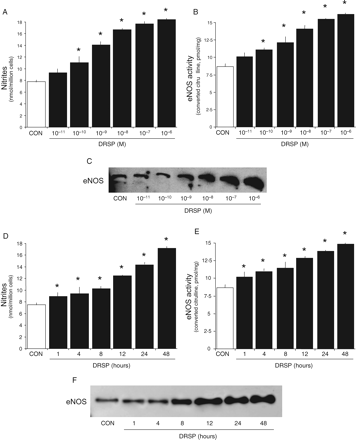
The modulation of NO synthesis by DRSP is concentration-related (Fig. 2A). Similar effects were found for eNOS activity and expression (Fig. 2B and C).
Dynamics of eNOS regulation by DRSP in endothelial cells Steroid-deprived HUVECs were treated for 48 h with increasing DRSP concentrations (A–C) or for different times (D–F). A and D show the amounts of NO released in the cell culture medium. B and E display the activity of eNOS. C and F present the cellular amount of eNOS. *P < 0.01 versus control
Based on the previous experiments we selected to use a concentration of 10−7 M for the single-dose treatments. This concentration appears to be effective and corresponds to luteal phase levels of P, so falling within a physiological range of concentrations.
DRSP (10−7 M) increases NO synthesis (Fig. 2D), eNOS activity and expression (Fig. 2E and F) in a time-dependent manner. However, a discrepancy can be seen between NO synthesis and eNOS activity (Fig. 2D and E), that were found to be significantly increased as early as after 1 h of exposure to DRSP, as opposed to eNOS expression (Fig. 2F) and that started to increase after 8 h. This suggests that DRSP might regulate eNOS through a non-genomic activation, as well as through the later enhancement of eNOS expression, as shown for other steroids (Chambliss and Shaul, 2002; Simoncini and Genazzani, 2003).
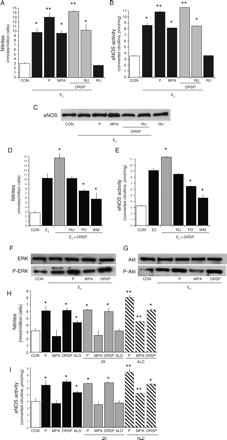
Effects on eNOS of DRSP in the presence of estradiol
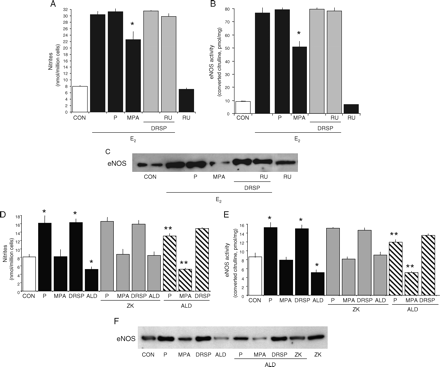
DRSP is used in association with estradiol (E2). We treated HUVEC with DRSP (10−7 M) in the presence or absence of E2 (10−8 M) and compared the effects of this progestin with P (10−7 M) and MPA (10−7 M). As shown (Simoncini et al., 2000; Simoncini et al., 2005), E2 was associated with enhanced synthesis of NO (Fig. 3A) and with increased eNOS activity and expression (Fig. 3B and C). Addition of DRSP or P to E2 did not change the effects of E2 (Fig. 3A–C), as opposed to MPA that interfered with E2 reducing the amount of NO synthesized due to reduced eNOS activation and expression (Fig. 3A–C). This indicates that DRSP does not alter the endothelial effects of E2 on NO.
Regulation of eNOS by DRSP in presence of E2 or aldosterone Steroid-deprived HUVECs were treated with 17β-E2 (10−8 M) for 48 h, either alone or in presence of DRSP (10−7 M), P (10−7 M), MPA (10−7 M) or RU486 (RU, 10−5 M). Alternatively, HUVECs were treated for 48 h with DRSP (10−7 M), P (10−7 M), MPA (10−7 M), aldosterone (ALD, 10−10 M) alone or in the presence of the MR antagonist ZK 91587 (ZK, 10−8 M). (A, D) NO synthesis by endothelial cells, (B, E) eNOS activity in whole cell extracts, (C, F) eNOS protein amounts in endothelial cells. (A, B) *P < 0.01 versus E2 alone. (D, E) *P < 0.01 versus control. **P < 0.01 respect to the corresponding compound in the absence of aldosterone
Anti-mineralocorticoid effects of DRSP on NO synthesis
To determine if DRSP exerts actions on endothelial cells through the MR, we treated HUVEC for 48 h with DRSP (10−7 M), P (10−7 M), MPA (10−7 M) or with a physiological concentration of aldosterone (10−10 M), either alone or in the presence of the MR antagonist ZK 91587 (10−8 M). Aldosterone was associated with a reduction of NO synthesis, eNOS activity and eNOS expression with respect to baseline (Fig. 3D–F). These effects of aldosterone are mediated through the MR, as they were prevented by ZK 91587 (Fig. 3D–F). However, ZK 91587 did not alter NO synthesis, eNOS activity and expression linked to P or DRSP (Fig. 3D–F), indicating that neither P nor DRSP regulate eNOS through the MR. The addition of P to aldosterone resulted in a synthesis of NO, eNOS activation and eNOS expression that were intermediate between the effects of the two compounds alone (Fig. 3D–F). The addition of MPA to aldosterone did not change the effects of aldosterone (Fig. 3D–F). In contrary, when DRSP was added to aldosterone the final effect was not different than in the presence of DRSP alone (Fig. 3D–F). These results suggest that the sustained exposure of endothelial cells to aldosterone is associated with a reduction of eNOS expression that turns into reduced NO synthesis. Although MPA does not alter these effects, P partially opposes this reduction. In parallel, DRSP completely blocks the negative effect of aldosterone.
Rapid regulation of eNOS by DRSP
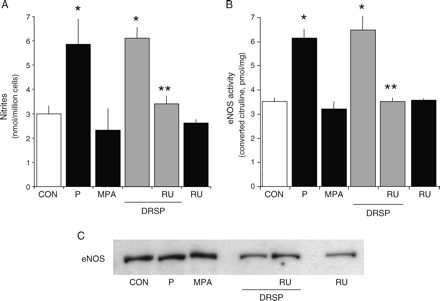
Since steroids exert rapid actions on eNOS through non-transcriptional mechanisms (Chambliss and Shaul, 2002; Simoncini and Genazzani, 2003), we tested whether DRSP regulates eNOS in this manner, as well. We tested the action of DRSP (10−7 M) in parallel to that of P (10−7 M) and MPA (10−7 M) in HUVEC throughout a 30 min treatment. DRSP and P resulted in a rapid NO synthesis (Fig. 4A) and eNOS activation (Fig. 4B). MPA had no effect (Fig. 4A and B). RU486 blocked the effect of DRSP, showing that this action depends on PR. The rapid actions of DRSP and P were exerted in the absence of modifications of eNOS expression (Fig. 4C).
Rapid activation of NO synthesis by DRSP in endothelial cells Steroid-deprived HUVECs were treated for 30 min with DRSP (10−7 M), P (10−7 M), MPA (10−7 M) either alone or in the presence of the PR antagonist RU486 (RU, 10−5 M). (A) NO release in the cell culture medium was assayed. (B) eNOS activity in whole cell lysates is shown. (C) eNOS protein amount in endothelial cells is presented. *P < 0.01 versus control. **P < 0.01 versus DRSP alone
Non-genomic signaling of DRSP to eNOSs
We exposed endothelial cells for 30 min to DRSP (10−7 M) in the presence of the MEK 1/2 inhibitor, PD98059 (PD, 5 × 10−6 M) or of the phosphatidylinositol 3-kinase (PI3K) inhibitor, wortmannin (WM, 3 × 10−8 M). The induction of NO synthesis and eNOS activity induced by DRSP was partially reduced by both the MEK 1/2 and the PI3K inhibitors (Fig. 5A and B), and completely by RU486 (Fig. 5A and B), implying that DRSP recruits the mitogen-activated protein kinases (MAPK) and PI3K cascades through PR.
Non-genomic signaling to eNOS by DRSP Steroid-deprived HUVECs were exposed for 30 (A, B, D) or 5 min (C) to DRSP (10−7 M), P (10−7 M), MPA (10−7 M) either alone or in the presence of RU486 (RU, 10−5 M), of the MEK inhibitor PD98056 (PD, 5 × 10−6 M) or of the PI3K inhibitor Wm (3 × 10−8 M). Alternatively, endothelial cells were exposed to increasing concentrations of DRSP for 30 min. A and E show NO production by endothelial cells; B and F present eNOS activity in whole endothelial cell lysates; C and G show cellular amounts of wild type or Tyr204-P-ERK 1/2. D wild type and Thr308-P-Akt endothelial cell amounts are shown. *P < 0.01 versus control. **P < 0.01 versus DRSP alone
In support of this, the administration of DRSP for 5 min resulted in a PR-dependent activation of ERK 1/2 (Fig. 5C). A similar activation was induced by P, but not by MPA (Fig. 5C). In addition, administration for 30 min of DRSP to HUVEC resulted in a PR-dependent activation of the PI3K effector, Akt (Fig. 5D). Again, although P induced a similar activation of Akt, MPA was ineffective (Fig. 5D). The non-genomic activation of NO synthesis and activity by DRSP is concentration-related (Fig. 5E and F), likewise the activation of ERK 1/2 (Fig. 5G). Overall, these results show that DRSP drives the signaling of PR to the activation of MAPK and PI3K, and that this does not happen in the presence of MPA.
Rapid signaling to eNOS of DRSP in the presence of E2
Since estrogen signals rapidly to eNOS via MAPK and PI3K (Simoncini and Genazzani, 2003), we tested the effect of rapidly co-administering DRSP with E2 to endothelial cells. We exposed HUVEC for 30 min to E2 (10−8 M), either alone or in the presence of DRSP (10−7 M), P (10−7 M) or MPA (10−7 M). DRSP and P potentiated the activation of NO synthesis and eNOS associated with E2 (Fig. 6A and B). DRSP acted through PR, as RU486 abrogated its effect (Fig. 6A and B). Co-administration of MPA did not change the E2-dependent effects (Fig. 6A and B). These actions were obtained without changes of cellular eNOS (Fig. 6C).
Rapid modulation of eNOS by DRSP in the presence of E2 or aldosterone Steroid-deprived HUVECs were treated for 30 min with 17β-E2 (10−8 M), either alone or with DRSP (10−7 M), P (10−7 M) or MPA (10−7 M), in the presence or absence of RU486 (RU, 10−5 M), of the MEK inhibitor PD98056 (PD, 5 × 10−6 M) or of the PI3K inhibitor WM (3 × 10−8 M). Alternatively, HUVECs were treated for 30 min with DRSP (10−7 M), P (10−7 M), MPA (10−7 M) or aldosterone (ALD, 10−10 M) either alone or in the presence of the MR antagonist ZK 91587 (ZK, 10−8 M). A, D and H show NO synthesis by endothelial cells. B, E and I present eNOS activity in endothelial cells. C shows cellular eNOS protein amounts. F presents the cellular amounts of wild type or Tyr204-P-ERK 1/2. Wild type and Thr308-P-Akt endothelial cell amounts are shown in E. (A, B, D and E) *P < 0.01 versus control, **P < 0.01 versus E2 alone. (H and I) *P < 0.01 versus control, **P < 0.01 versus compounds in the absence of aldosterone
Co-administration of PD98059 to E2 + DRSP reduced both NO synthesis and activity (Fig. 6D and E), and a similar effect was seen with WM (Fig. 6D and E). The addition of DRSP to E2 resulted in a potentiation of the recruitment of ERK 1/2 and Akt (Fig. 6F and G). A similar effect was seen with P but not with MPA (Fig. 6F and G). Cumulatively, these data suggest that PR recruited by DRSP or P, but not by MPA, signals to eNOS via an activation of MAPK and PI3K that is parallel (and additive) to that recruited by estrogen receptors
Relevance of the MR for the rapid signaling to eNOS of DRSP
Since the MR induces rapid effects in endothelial cells (Funder, 2005), we tested the effect of DRSP in the presence of aldosterone. A 30-min exposure to aldosterone (10−10 M) of endothelial cells resulted in increases of NO synthesis and eNOS activity (Fig. 6H and I) due to the recruitment of MR, as indicated by the blockade by ZK91587 (Fig. 6H and I). Furthermore, when P and aldosterone were co-administered, an additive effect on NO synthesis and eNOS activity was seen (Fig. 6H and I). In contrary, when DRSP was used with aldosterone, no additive effect was seen (Fig. 6H and I). Finally, MPA addition to aldosterone did not change the action of the mineralocorticoid (Fig. 6H and I).
Discussion
Synthetic progestins are a family of compounds characterized by significant clinical differences, as well as by different side effects. The recent data suggesting that post-menopausal women receiving a combination of CEE and MPA and CEE as hormone therapy do not present a reduction in coronary disease, as opposed to those who receive CEE only, have highlighted the relevance of the progestin with respect to cardiovascular health (The Writing Group for the Women's Health Initiative Investigators, 2002; The Women's Health Initiative Steering Committee, 2004).
Most synthetic progestins display promiscuous binding to steroid receptors, determining a multifaceted spectrum of effects (Sitruk-Ware, 2004). DRSP is characterized by a high affinity to PR as well as by the ability to bind and inhibit the androgen receptor (Sitruk-Ware, 2005). In addition, DRSP has the unique property of binding and antagonizing the MR (Sitruk-Ware, 2005), which translates into relevant actions at the cardiovascular level (Whitehead, 2006). Indeed, the administration of DRSP combined with E2 to hypertensive post-menopausal women results in a lowering of blood pressure (Preston et al., 2002, 2005; White et al., 2005, 2006), in addition to the expected benefits on menopausal symptoms (Archer et al., 2005). The administration of this hormonal combination results in the lowering of both systolic and diastolic blood pressure (White et al., 2005, 2006).
The main contribution of this manuscript is the demonstration that DRSP acts on endothelial cells inducing the synthesis of NO. This effect is mediated by PR. However, the interference with MR in endothelial cells explains part of the action of this progestin, as when DRSP is used in the presence of aldosterone, some of the detrimental effects of this hormone on NO synthesis are antagonized, which does not happen in the presence of other progestins. The induction of NO synthesis by DRSP may lead to enhanced vasodilatation and to the reduction of peripheral resistances, possibly explaining part of the blood pressure-lowering effects of this compound.
The binding of DRSP to PR leads to a combination of transcriptional and non-transcriptional signaling of this receptor, with a rapid activation of the MAPK and of the PI3K cascades, that are linked to rapid activation of the eNOS by different steroids (Chambliss and Shaul, 2002; Simoncini et al., 2004). Later on, the activation of PR by DRSP leads to the nuclear induction of eNOS expression, resulting in increased protein levels. These actions do not differ from those induced by natural P, supporting the concept that the signaling to eNOS in endothelial cells is mediated by PR. To this extent, significant differences exist between the effects of DRSP and P with respect to MPA, in agreement with our findings (Simoncini et al., 2004) as well as with previous reports of differential effects on NO synthesis by distinct progestins (Liao et al., 1996).
To explain such differences, it can be hypothesized that PR, depending on the ligand engaging the binding pocket, may be differently able to interfere with transcription factors regulating eNOS expression, such as Sp1 and GATA (Zhang et al., 1995). On the other hand, the ability of PR to activate MAPK or PI3K only in the presence of P or DRSP, but not of MPA, may depend on the different conformations of PR. Indeed, only selected PR conformations may allow the interaction of PR with Src and the following recruitment of the ERK 1/2 cascade. This may also be relevant for the behavior that endothelial cells show when exposed to E2 in the presence or absence of the progestins. In fact, PR-B and ERα have been shown to interact to activate c-Src in other cell types (Ballare et al., 2003), and the 3D conformation imposed to PR by P, DRSP or MPA may be different enough to explain the divergent activation of these cascades.
The interference with aldosterone signaling seems to be relevant in the generation of the vascular actions of DRSP. Although there is not much available on the effects of aldosterone on endothelial cells, suggestive evidence indicates that this mineralocorticoid may induce endothelial dysfunction and alter vascular dilatation (Funder, 2004). Indeed, exposure of endothelial cells to aldosterone decreases vascular endothelial growth factor-induced NO synthesis (Hashikabe et al., 2006) and increases endothelial stiffness (Oberleithner, 2005). These endothelial actions of aldosterone couple well with the evidence indicating that the blockade of aldosterone signaling improves endothelial-dependent vascular relaxation (Lahera et al., 2006) and that this depends in part on increased endothelial eNOS expression (Thai et al., 2006). Our data indicate that exposure of endothelial cells to aldosterone results in decreased expression of eNOS and NO synthesis. This ensues throughout several hours of exposure to the steroid, and follows a rapid increase in eNOS activity in the first minutes that is probably due to a non-transcriptional action of aldosterone (Funder, 2005). DRSP blocks the reduction of eNOS expression associated with aldosterone through its anti-mineralocorticoid activity. This action might result in beneficial cardiovascular actions in women, particularly in the presence of endothelial dysfunction, such as in hypertension.
When DRSP is provided together with E2, no changes of the NO-inducing actions of estrogen are found, differently than with MPA that decreases eNOS expression, supporting the idea that the effects of progestins on the vessels depend on the molecules used. Recent animal studies point out that MPA interferes with the protective actions of E2 on cardiac hypertrophy, perivascular fibrosis and NO-dependent relaxation of aortic rings, whereas DRSP has neutral or protective effects. In animals, receiving aldosterone infusion E2 reduces blood pressure levels. Combining DRSP with E2 results in a further reduction in blood pressure, whereas MPA abrogates the effect of E2 (Arias-Loza et al., 2006). These results are consistent with previous reports of anti-hypertensive effects involving NO regulation by DRSP in animal models (Elger et al., 2003).
In conclusion, we show that DRSP exerts a complex array of actions on human endothelial cells. Some of these effects are mediated by PRs but others depend on the interference with the MR. This unique mix of molecular actions on human endothelial cells sheds light on some of the actions of this compound on vascular function and on blood pressure.
Acknowledgements
We thank Christa Hegele-Hartung, from Bayer Schering Pharma AG, for the discussion on the results. This work has been supported by the PRIN grant 2004057090_007 by the Italian University and Scientific Research Ministry (MIUR) to T.S. and by a research grant from Bayer Schering AG.
References
- nitric oxide
- progestins
- hypertension
- signal transduction
- aldosterone
- endothelial cells
- estradiol
- 1-phosphatidylinositol 3-kinase
- cardiovascular system
- endothelium
- mitogen-activated protein kinases
- nitric oxide synthase
- postmenopause
- mineralocorticoid receptor
- mineralocorticoids
- progesterone
- drospirenone