-
PDF
- Split View
-
Views
-
Cite
Cite
Nahid G. Robertson, Cor W.R.J. Cremers, Patrick L.M. Huygen, Tetsuo Ikezono, Bryan Krastins, Hannie Kremer, Sharon F. Kuo, M. Charles Liberman, Saumil N. Merchant, Constance E. Miller, Joseph B. Nadol, David A. Sarracino, Wim I.M. Verhagen, Cynthia C. Morton, Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction, Human Molecular Genetics, Volume 15, Issue 7, 1 April 2006, Pages 1071–1085, https://doi.org/10.1093/hmg/ddl022
Close - Share Icon Share
Abstract
Seven missense mutations and one in-frame deletion mutation have been reported in the coagulation factor C homology (COCH) gene, causing the adult-onset, progressive sensorineural hearing loss and vestibular disorder at the DFNA9 locus. Prevalence of COCH mutations worldwide is unknown, as there is no systematic screening effort for late-onset hearing disorders; however, to date, COCH mutations have been found on four continents and the possibility of COCH playing an important role in presbycusis and disorders of imbalance has been considered. Cochlin (encoded by COCH) has also been shown as a major target antigen for autoimmune sensorineural hearing loss. In this report, we present histopathology, immunohistochemistry and proteomic analyses of inner ear tissues from post-mortem DFNA9 temporal bone samples of an individual from a large Dutch kindred segregating the P51S mutation and adult human unaffected controls, and wild-type (+/+) and Coch null (−/−) knock-out mice. DFNA9 is an inner ear disorder with a unique histopathology showing loss of cellularity and aggregation of abundant homogeneous acellular eosinophilic deposits in the cochlear and vestibular labyrinths, similar to protein aggregation in well-known neurodegenerative disorders. By immunohistochemistry on the DFNA9 temporal bone sections, we have shown cochlin staining of the characteristic cochlear and vestibular deposits, indicating aggregation of cochlin in the same structures in which it is normally expressed. Proteomic analysis identified cochlin as the most abundant protein in mouse and human cochleae. The high-level expression and stability of cochlin in the inner ear, even in the absence and severe atrophy of the fibrocytes that normally express COCH, are shown through these studies and further elucidate the pathobiologic events occurring in DFNA9 leading to hearing loss and vestibular dysfunction.
INTRODUCTION
A large number of loci have been mapped for syndromic and non-syndromic hereditary hearing loss, and the gene mutations responsible for these disorders are being continually discovered and characterized (1). Elucidation of the functions of these genes and their roles in the inner ear and in pathogenesis of hearing and balance disorders are important ongoing endeavors.
The autosomal-dominant deafness disorder at the DFNA9 locus has been described and the clinical aspects extensively characterized, showing adult-onset, progressive sensorineural hearing loss and vestibular dysfunction (2–9). Different missense mutations in the COCH (coagulation factor C homology) gene were found initially in three families in the United States, and subsequently in families in the Netherlands, Belgium and Australia (Table 1 and Fig. 1) (10–14). Two simplex cases of another missense mutation and an in-frame deletion in the same domain of COCH [factor C-homology (FCH)/limulus factor C, cochlin, lung gestational protein (LCCL) domain] have been reported in Japan and Hungary, respectively (15,16). A recent report describes the first finding of a COCH mutation outside of the FCH/LCCL domain in the von Willebrand factor A-like (vWFA) domain in a large DFNA9 kindred in the United States (9). The prevalence of COCH mutations worldwide is not known, as systematic genetic testing of adult-onset hearing loss is not currently performed.
COCH was isolated initially by organ-specific subtractive approaches from a human fetal cochlear cDNA library and found to be expressed at high levels in the inner ear by northern blot, tissue in situ hybridization and immunohistochemistry (17–20). The secreted protein, cochlin, was detected by proteomic analysis as the most abundant protein in the bovine inner ear (21). Histopathological analyses of DFNA9-affected temporal bones in the three original US families have revealed very valuable information about the endpoint changes in these inner ears (3,22). A striking and unique finding in these temporal bones, which actually allowed initial identification of several of these families as DFNA9 kindreds, is the presence of homogeneous extracellular eosinophilic deposits in the same areas as fibrocyte atrophy. Other well-characterized neurodegenerative disorders with aberrant protein accumulation include Alzheimer disease (β-amyloid precursor protein) (23), Huntington disease (huntingtin) (24–26) and Parkinson disease (α-synuclein) (27). However, DFNA9 is the only known inner ear disorder showing this type of aggregate as the signature pathological finding, whereas findings in other disorders of the inner ear, such as endolymphatic hydrops, and degeneration of structures, such as the sensory epithelium, ganglion cells, spiral ligament and the stria vascularis, are observed in a variety of different conditions showing hearing loss and vestibular dysfunction.
The cochlear and vestibular fibrocytes, which are severely atrophied in DFNA9, are cells expressing COCH, and the homogenous acellular deposits are found in the same areas as cochlin immunostaining in the normal inner ear (19). However, previous to this report, it has not been shown whether this eosinophilic substance in DFNA9 is the abnormal cochlin which has precipitated and aggregated or whether it is another component of the inner ear, a cochlin-interacting protein, or some other downstream effect of the COCH mutations.
A recent post-mortem donation of a temporal bone from an individual from the Netherlands with DFNA9 (COCH P51S mutation) provided the opportunity to obtain additional histopathological data for DFNA9. By using an antibody to the vWFA domain of cochlin, which detects all known size isoforms of cochlin (Fig. 1), we have undertaken a thorough characterization of cochlin immunostaining in the cochlear and vestibular labyrinths. We present proteomic analysis of DFNA9-affected and unaffected adult human temporal bone sections, as well as of wild-type (+/+) and Coch null (−/−) knock-out mouse inner ears. These studies have enabled examination of the content of the abnormal deposits seen in DFNA9 and provided insight into the role of cochlin in the inner ear and the mechanism of pathobiology underlying DFNA9 by COCH mutations.
RESULTS AND DISCUSSION
Histopathology of DFNA9 temporal bones with the P51S cochlin mutation
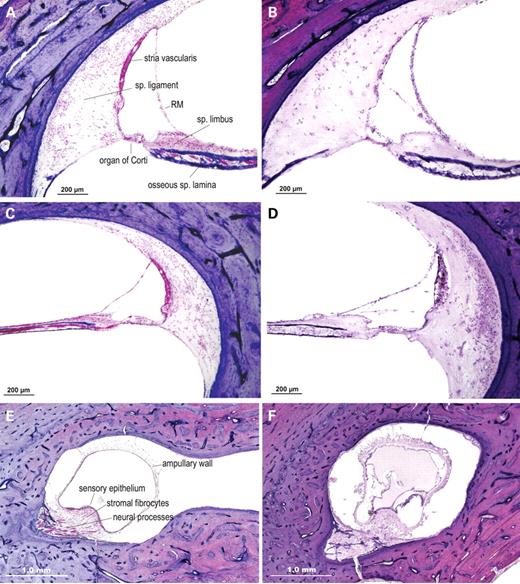
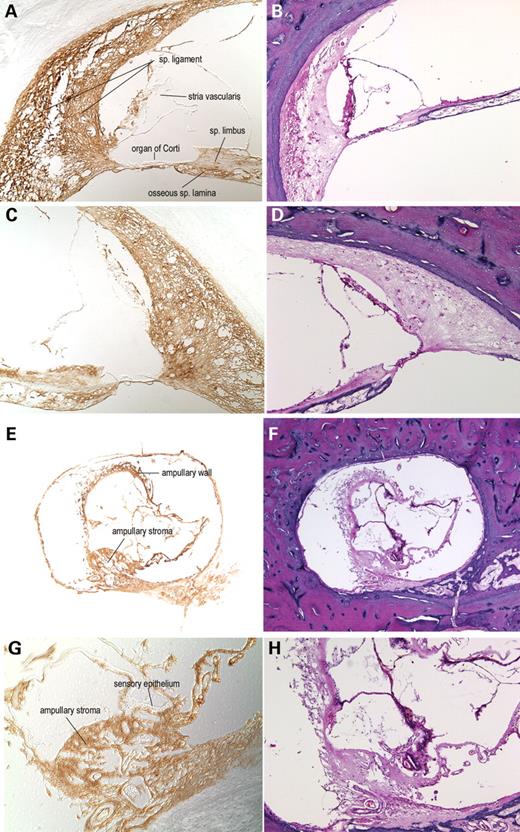
Histopathologic findings in the P51S DFNA9 temporal bone (Fig. 2) are consistent with those previously reported for other DFNA9 families designated 1W (V66G mutation), 1Su (G88E mutation) and 1St (W117R) (3,22,28), suggesting that the P51S mutation causes the same pathologic changes and acts via the same mechanism as the other mutations in the FCH/LCCL domain of COCH. A marked reduction in the number of fibrocytes is observed throughout the spiral ligament and limbus of the cochlear duct and in the vestibular organs. In the same areas of fibrocyte loss, and atrophy is the DFNA9-characteristic eosinophilic-staining extracellular ground substance. The deposits are particularly prominent in the more medial parts of the ligament underlying the stria vascularis and in the area of the insertion of the ligament into the basilar membrane. Eosinophilic material is also present in the osseous spiral lamina, along with loss of dendrites in these channels and in the modiolus.
Cochlin immunostaining in normal inner ear
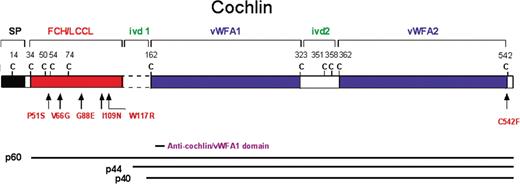
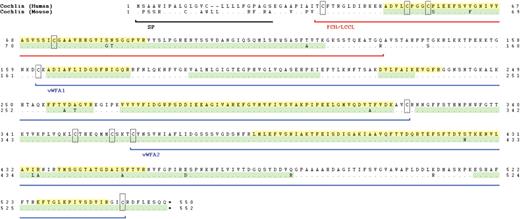
Prior to performing immunohistochemistry on DFNA9-affected temporal bones, we optimized anti-cochlin antibody staining on normal mouse and human adult tissues, as well as on a Coch (−/−) mouse (29,30). In our previous studies, we used a polyclonal antibody to the entire FCH/LCCL and ivd1 domain of cochlin (19). However, given different-sized isoforms of cochlin detected by proteomic analysis and N-terminal sequencing (21) (Fig. 1), four antibodies to small peptides in different regions of cochlin were developed and shown by western blot analysis to be specific for the isoforms that each was expected to recognize (31). The anti-cochlin antibody to the vWFA1 domain reacts with all three known cochlin isoforms: p60 (full-length) and p44 and p40 (both lacking the FCH/LCCL domain) (Fig. 1). We chose this antibody (anti-cochlin/vWFA1 domain) for our studies because it would provide a more complete representation of cochlin localization in unaffected and affected tissues.
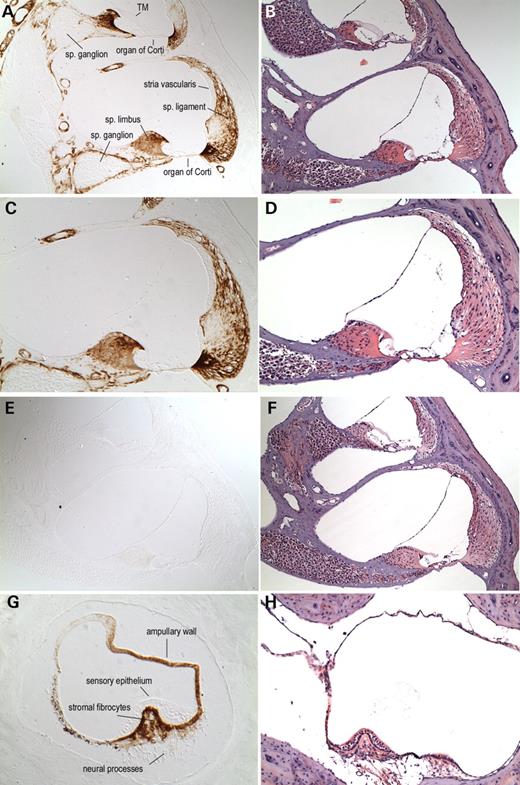
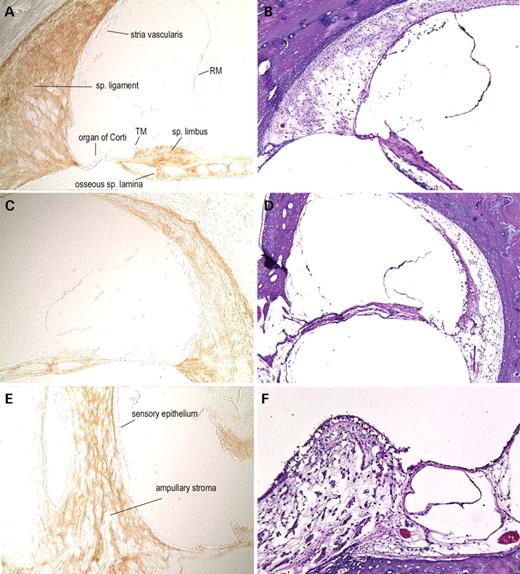
In the normal adult mouse cochlea (Fig. 3), cochlin immunostaining is strong in the spiral ligament and spiral limbus. In the ligament, the staining is darkest in the basilar crest, near the basilar membrane and weakest in the spiral prominence. Cells lining Rosenthal's canal and the channels of the osseous spiral lamina are cochlin-positive, whereas the cochlear ganglion cell bodies and the neural processes are cochlin-negative. Distinct cochlin staining of pericytes surrounding blood vessels in the modiolus and throughout the cochlear duct is observed. In contrast, adjacent areas of surrounding bony tissues clearly lack cochlin staining. Cochlin-negative structures in the cochlear duct are the organ of Corti, including the sensory epithelium and tectorial membrane, stria vascularis, Reissner's membrane, cochlear ganglion cells and their neuronal processes. In the vestibular labyrinth, the cristae (Fig. 3G) show intense cochlin staining in the fibrocytes and stroma underlying the sensory epithelium as well as in the ampullary wall. The sensory epithelium, the neuronal processes within the ampullary stroma as well as the surrounding bone and connective tissues are all cochlin-negative.
To confirm antibody specificity, we immunostained sections from a Coch (−/−) mouse (29,30) and no staining was detected (Fig. 3E). Negative controls with secondary antibody alone also show no background staining (data not shown). The intense staining for cochlin in the (+/+) mouse inner ear corroborates the finding of cochlin by proteomic analysis as a very abundant and stable protein in cochlea and vestibular organs.
In the unaffected control human adult inner ear (Fig. 4), a similar pattern of cochlin staining is detected. Cochlin immunoreactivity was prominent throughout the spiral ligament, spiral limbus and within the osseous spiral lamina. Immunostaining in the modiolus was also observed around the blood vessels (data not shown). As in the mouse, the organ of Corti, the neuronal cell bodies and central and peripheral axons lack cochlin expression. The surrounding outer bony and mesenchymal tissues are also unstained. In the human adult vestibular labyrinth, the cristae also show immunostaining in the area of the stromal fibrocytes and a lack of staining in the adjacent overlying sensory epithelium. The ampullary wall also contains cochlin, as observed in the mouse sections.
In both mouse and human cochlea and vestibular organs, cochlin immunostaining is restricted to tissues that are mesodermal in origin; neuroectodermally derived structures clearly lack cochlin expression. Within the mesodermal structures, there is widespread and high-level expression of cochlin in areas such as the spiral ligament, which comprises a large percentage of the total mass of the membranous cochlea, in agreement with findings of high levels of cochlin mRNA by EST, northern blot and tissue in situ hybridization analyses (17,19,32), and with abundance and stability of cochlin protein as observed by western blot and proteomic analyses (19,21).
Cochlin immunostaining in DFNA9-affected inner ear
The cochlin staining pattern in the DFNA9 temporal bone sections (Fig. 5) is similar to that in the unaffected control sections. Immunostaining is strong throughout the spiral ligament, spiral limbus, stroma of the crista ampullaris and the ampullary wall. As a negative control, no staining was detected using the secondary antibody alone (data not shown). There is no detectable background staining in the tissues immediately adjacent to the spiral ligament lateral wall and tissues surrounding the ampulla. The regions of the osseous spiral lamina normally occupied by cochlear peripheral axons are immunopositive for cochlin, as are perivascular areas in the modiolus. Other structures such as the organ of Corti, vestibular sensory epithelium and stria vascularis, which are cochlin-negative in normal tissues, also lack staining.
The large amounts of eosinophilic acellular deposits contained throughout the spiral ligament, limbus and osseous spiral lamina are darkly and evenly immunostained with anti-cochlin, but completely lack non-specific staining with secondary antibody alone. The ampullary stroma and wall, which show distortion, collapse and thickening, and contain the acellular material, also show prominent cochlin staining. These results are consistent with the view that cochlin is intimately associated with the eosinophilic deposits characteristic of the temporal bone histopathology in DFNA9.
Proteomic analysis in mouse inner ear
Proteomic analysis of the cochlear and vestibular labyrinths of (+/+) and Coch (−/−) mice were performed and an abridged list of the representative peptide matches is presented (Table 2). The number of tryptic peptides identified from mass spectrometry analysis reflects the relative abundance of proteins detected within each tissue by this method. A striking finding is the presence of cochlin peptides as the most abundantly detected protein in the cochlea of (+/+) mice. In the vestibular organs, cochlin is the second most frequently detected protein, with albumin being primary. In both tissues, cochlin is more abundant than β-hemoglobin. Findings for two other proteins, α-tectorin and keratin 9, are representative of structural proteins expressed in these tissues. Our proteomic analysis combines results of four gel fractions where digestion and mass spectrometry were performed separately. Identification of cochlin as the most abundant protein in mouse inner ear lysates corroborates previous proteomic analysis by the alternative method of 2D gel electrophoresis, also revealing cochlin as the most prevalent protein in bovine inner ear (21). As a negative control, we studied the Coch (−/−) mouse. No cochlin peptides were detected in either cochlear or vestibular tissues, confirming the lack of cochlin protein as shown also by our immunohistochemistry and by previous western blot analysis (30).
In (+/+) mouse cochlea, a total of 123 cochlin tryptic peptides representing 38 unique peptides are detected, ranging from 7 to 35 amino acid residues each. Extensive peptide coverage for cochlin was observed (Fig. 6) throughout all domains of the full-length protein (excluding the signal peptide). In conjunction with detection of cochlin as a highly abundant and stable protein in the cochlear and vestibular organs, and its fairly restricted expression at a high level in the inner ear, it is interesting to note that several studies have implicated cochlin as a target antigen for autoimmune sensorineural hearing loss via both immunoglobulin and T-cell-mediated mechanisms. Elevated serum levels of anti-cochlin immunoglobulins have been detected in a number of patients with autoimmune hearing loss (33,34). In addition, cochlin has been shown to co-immunoprecipitate with choline transporter-like protein 2 as targets of antibody-induced hearing loss (35,36). Studies have also demonstrated experimentally induced CD4+ T-cell-mediated autoimmune hearing loss with cochlin as the target antigen (37,38). Furthermore, recent investigations have revealed significantly higher frequencies of cochlin-specific circulating T-cells as well as elevated cochlin-specific serum antibody titers in individuals with autoimmune sensorineural hearing loss, as compared with unaffected age-matched controls (39). These reports implicate cochlin as a prominent inner ear target antigen in both antibody and T-cell-mediated autoimmune hearing loss.
Proteomic analysis in human adult unaffected and DFNA9-affected and temporal bones
Because the only material available from the DFNA9-affected and unaffected human samples is formalin-fixed, paraffin-embedded temporal bones, we had to employ a different approach than that used in the mouse. Thus, proteomic analysis was performed on proteins extracted from 8-µm paraffin-embedded sections, whereas fresh whole tissue lysates or frozen tissues are typically used.
In the human adult unaffected temporal bone sample, cochlin is also the most abundantly detected protein by mass spectrometry, as was the case in the mouse using fresh cochlear lysates. A total of 66 cochlin peptides, representing 17 unique peptides, are detected (Table 3), ranging from 10 to 35 amino acid residues each. Cochlin peptides identified in the human sample are also representative throughout the protein in all domains of cochlin, although peptide coverage is not as complete as that found in the mouse sample (Fig. 6). This is not surprising given that peptide extraction from archival formalin-fixed, paraffin-embedded sections (40) is much more difficult than from fresh total tissue lysates. Nonetheless, these results indicate that cochlin is also a prevalent and stable component of the human adult inner ear.
A complete alphabetical listing of all proteins detected from the proteomic analysis of the human adult temporal bone sections is presented in Table 3. The major classes of proteins found are extracellular and structural components of the cochlea, cochlin being the primary. Many of the proteins are known to be expressed in the spiral ligament, which comprises a major amount of the total cochlear mass. Collagen types I, II, IX and XI, which are also abundantly detected in our analysis, are very representative of the known and stable components of cochlear tissue and involved in both non-syndromic and syndromic types of hearing loss (1). Other proteins frequently represented in our analysis are those comprising cytoskeletal elements such as keratins, β-actin and tubulin, which are also known to be expressed in the cochlea. A total of 13 unique peptides are identified, representing five distinct keratins. The intermediate filament, vimentin, is the second most prevalent protein detected in our analysis (29 peptide matches, reflecting 17 unique peptides), followed by collagen type II, alpha 1 (11 peptide matches, representing two unique peptides). Immunohistochemical localization of vimentin in the gerbil inner ear shows high-level expression of this protein in most connective tissue cells and in several different types of epithelial cells, including some types of organ of Corti supporting cells (41).
Our proteomic analysis of formalin-fixed, paraffin-embedded adult human tissue sections has some limitations in the number and complexity of cochlear proteins identified, likely as a result of the inaccessibility and possible degradation of proteins during the extraction process from very small amounts of tissue in 8-µm paraffin sections. However, even with these limitations, many representative proteins were detected. Cochlin is the most prevalent, showing over twice as many detected peptides as vimentin and reflects its high expression and stability even after the processing and extraction protocol from fixed and embedded tissues.
In the proteomic analysis of the DFNA9-affected temporal bone sections (Table 3), there are substantially fewer proteins identified, as well as fewer peptide matches for each protein. However, there is significant overlap in the proteins that were identified in the DFNA9-affected and unaffected samples. Aside from blood components, albumin and α- and β-globins, the major proteins found in the DFNA9-affected temporal bone (and also present in the unaffected sample) are cochlin, collagen types I alpha 1 and alpha 2, and keratins 1 and 2a. A quantitative comparison of proteins across the DFNA9-affected and unaffected samples cannot be made because the two samples varied greatly in complexity and number of total peptide matches for each protein, likely as a result of the greater difficulty of extraction and solubility of proteins in the DFNA9 sections which was apparent during the processing of samples (also discussed below). For every protein identified in the DFNA9 sample, the total number of peptide matches ranges from 1 to 3, whereas in the unaffected sample 1 to 66 matches were found. For instance, in the unaffected sample, the total number of peptide matches are 66 for cochlin, five for keratin 1 and two for collagen type I alpha 2; corresponding peptide matches in the DFNA9-affected sample are three, one and two, respectively. The number of cochlin peptides found in the DFNA9 sample is greater than or equivalent to that for any other protein. Cochlin peptides identified in the DFNA9 sample are from both the N- and C-terminal portions of cochlin spanning amino acid residues 81 to 91, in the FCH/LCCL domain and amino acid residues 526 to 539 in the vWFA2 domain.
One major factor in identification of proteins was that the tissue composition of DFNA9-affected temporal bone samples presented a challenge in total protein extraction due to relative insolubility of these samples. Much of this sample remained as insoluble aggregates during the extraction protocol. Therefore, a large number of proteins were likely not solubilized or extracted, making them inaccessible for mass spectrometry analysis and resulting in lower complexity and number of total proteins as well as a smaller overall number of peptides. The difficulty in extraction and solubility of proteins from DFNA9-affected sections is perhaps not surprising given the observation of the large amounts of DFNA9 eosinophilic aggregates in the spiral ligament and limbus by light microscopy. Nevertheless, cochlin is detected in the DFNA9-affected tissue at least as often as other major proteins such as collagens, keratins and the blood components albumin and globins. Detection of cochlin in the DFNA9 temporal bone as one of the primary proteins identified by proteomic analysis suggests the stability of cochlin aggregates even in the absence and severe degeneration of the fibrocytes that normally express COCH, and corroborates our immunohistochemical finding of intense cochlin staining of the abundant deposits found in the DFNA9-affected inner ears.
RT–PCR analysis
To evaluate whether the mutant COCH allele in individuals with the P51S mutation (nucleotide C207T) displays stable expression of the mutant COCH transcript, reverse transcription–polymerase chain reaction (RT–PCR) was performed on RNA from Epstein-Barr virus (EBV)-transformed cell lines. One distinct band of the expected size was obtained (data not shown) using primers flanking the mutation. Sequence chromatographs show approximately equal peak heights for both the normal and mutated base pairs in the heterozygote DFNA9-affected sample and one single peak of higher amplitude for the normal base pair in the unaffected sample.
These results suggest that the mutant COCH transcript shows stable expression in P51S DFNA9 patients and is not subjected to degradation. Given that this missense mutation and all other known COCH mutations (seven missense and one in-frame deletion) do not cause any premature termination or truncation of the predicted protein sequence, it may be expected that the mutant allele would show stable expression. However, some missense mutations result in unstable transcripts or proteins, which are subjected to degradation by cellular mechanisms and effectively result in lack of a functional protein. In the case of DFNA9, stable detection of the mutant COCH transcript is consistent with the hypothesis of a dominant-negative effect of the mutation in DFNA9 pathology rather than haploinsufficiency of cochlin.
Cochlin deposition in DFNA9
Our previous studies have shown cochlin to be secreted and glycosylated in cultured mammalian cells transfected with full-length COCH cDNA (42). As an extracellular glycoprotein, with the FCH/LCCL and vWFA domains, cochlin is likely to bind to, or interact with, other cellular components such as extracellular proteins, glycoproteins and proteoglycans. Such interactions have been shown for other proteins containing these types of domains (43–47). Our light microscopy studies of DFNA9 sections have identified the prominent eosinophilic material as an acellular extracellular homogeneous material. Movats pentachrome staining suggests that this material may contain a mucopolysaccharide-like substance (3). Electron microscopic examination of DNFA9 inner ear sections shows these extracellular deposits to be a highly branched, disarrayed, microfibrillar substance, along with scattered glycosaminoglycan-like granules (48). These observations are consistent with our finding of cochlin immunostaining of the eosinophilic ground substance, suggesting that these aggregates contain extracellularly deposited cochlin. It is also possible that other proteins associated with cochlin may be present and that the nature of these interactions is altered as a result of the presence of mutated cochlin.
In terms of the actual mechanism of the missense mutations leading to misfolding and aggregation of cochlin, several in vitro studies have been performed to investigate this possibility. Initial studies of the FCH/LCCL domain of cochlin in bacterial cells showed misfolding of this domain due to several of the known disease-causing mutations and precipitation of the mutant FCH/LCCL peptide during the folding process (49). Our transient transfections of full-length COCH with several of the inherited mutations did not reveal any differences in secretion or apparent steady-state levels of cochlin (9,42). Another study corroborated these findings and reported differences in cochlin deposition in the extracellular matrix (ECM) of cultured cells, suggesting altered integration of mutated cochlin into the matrix (50). However, a caveat of in vitro studies is the lack of the appropriate extracellular environment of the inner ear. Furthermore, the late-onset and progressive nature of DFNA9 suggests that the effects of mutant cochlin such as aggregation or altered interaction with other ECM components may be a cumulative process. A DFNA9 mouse model would more clearly address long-term and progressive changes in the inner ear as a result of Coch mutation and such a model is currently being evaluated in our laboratory.
Possible etiology and pathogenesis of DFNA9
Cochlin immunostaining of eosinophilic aggregates in DFNA9 and the severe atrophy of fibrocytes in the same areas point toward the primary sites where pathological changes are likely to have initiated as a result of COCH mutations. Other observations in DFNA9 post-mortem sections are neuroepithelial and neural degeneration in the inner ear. Because cochlin is not expressed in these neuroectodermal structures, it is likely that these changes are secondary to those in mesodermal tissues. The striking reduction and degeneration of fibrocytes and replacement by aggregates throughout the spiral ligament and spiral limbus are in the very same sites as the pathways of K+ recycling from epithelial cells of the organ of Corti back into the endolymphatic scala media compartment (51), indicating a disruption of the integrity of the network of gap junctions that normally exists between these cells and plays a critical role in the ion homeostasis necessary for proper hair cell function. Therefore, a disruption of inner ear ionic balance likely occurs in DFNA9 as a result of the lack of fibrocytes and presence of deposits throughout the areas critical for K+ recycling.
Another observation in both unaffected and DFNA9 inner ears is cochlin immunostaining throughout the osseous spiral lamina and in modiolar areas surrounding neurons and their processes, but not in the neural cell bodies or processes. The detection of cochlin deposits within these neural channels suggests that obstruction of these channels and neuronal damage may also be occurring as a result of mutant cochlin aggregates.
An intriguing finding is immunostaining of perivascular areas in the modiolus and throughout the cochlear duct. Such a finding suggests the possibility that cochlin deposition in perivascular aggregates in organs outside the inner ear is implicated in the high prevalence of vascular disorders that has been found in some P51S DFNA9 kindreds (4), including the one to which the present 67-year-old female individual belonged (52,53). These observations warrant further studies of cochlin expression in extralabyrinthine blood vessels.
Another interesting observation is the lack of any similar histopathological findings between DFNA9-affected temporal bones and those of Coch (−/−) mice (at ∼5 months of age) (30) (Figs 3 and 5). In fact, these mice, which do not express cochlin, do not show any apparent inner ear abnormalities and any significant hearing loss although later time-points have yet to be evaluated. Our studies showing cochlin-staining eosinophilic deposits in DFNA9, in addition to the absence of overt pathology in Coch (−/−) mice at 5 months of age, are further support that this disorder is not likely due to COCH haploinsufficiency, but rather a result of deleterious effects by a ‘gain-of-function’ molecular mechanism of COCH missense mutations.
Cochlin deposits found in glaucoma
Recent proteomic studies have revealed cochlin as the most frequently detected protein by mass spectrometry in the trabecular meshwork (TM) of glaucomatous human eyes, but absent in normal age-matched control donor eyes (54). Immunohistochemistry revealed cochlin-staining deposits co-localizing with mucopolysaccharide substance in the TM around Schlemm's canal in glaucomatous eyes in the human and in the DBA/2J mouse model for glaucoma (54,55). Western blot analysis showed an increase in cochlin levels with increasing age in the human and mouse, along with progression of disease, as well as a parallel decrease in type II collagen, an important component of normal TM. It is hypothesized that the altered architecture of this tissue may cause obstruction of the aqueous flow in the eye (54,55). These studies suggest dysregulation of cochlin expression in a subset of human and mouse glaucomas. The finding of cochlin deposits in these eyes is concomitant with an increase in intraocular pressure and precedes optic nerve damage and ganglion cell degeneration (54,55). Interesting similarities such as cochlin deposition and neuronal damage exist between the findings in glaucoma and in DFNA9; parallel studies will provide insight into cochlin function and its role in disease processes in these two sensory systems.
MATERIALS AND METHODS
Tissues
Mouse tissues were obtained according to the guidelines and protocols approved by the Harvard Medical School Standing Committee on Animals (Boston, MA, USA). Human temporal bones were obtained in accordance with the guidelines established by the Human Research Committees at the Massachusetts Eye and Ear Infirmary (Boston, MA, USA) and the Canisius Wilhelmina Hospital (Nijmegen, the Netherlands).
Postnatal mice (3–6 months of age) were used in this study. Wild-type (+/+) mice were used for assessment of normal histology, cochlin localization and proteomic analysis. Coch (−/−) mice, used as negative controls, were a gift of Drs Colin Stewart and Clara Rodriguez (29). Mice were perfused intracardially and tissues fixed in 4% paraformaldehyde. After removal of the stapes from the oval window and piercing of the round window, 4% paraformaldehyde fixative was perfused gently through the cochlea. Inner ears were immersed in fixative for 2–4 h, followed by decalcification in 120 mm ethylenediaminetetraacetic acid (EDTA) for 1 week at room temperature, and embedded in paraffin by standard histologic procedures. Serial sections were obtained at 5–8-µm thickness and used for staining with hematoxylin and eosin (H&E) and for immunohistochemistry. For proteomic analysis, cochlear and vestibular tissues were dissected separately and processed as described in ‘Proteomic analysis’.
For human tissues, temporal bones from a 67-year-old female who was a member of a large DFNA9 kindred in the Netherlands segregating the P51S cochlin mutation (5,11) were donated and processed at the Otopathology Laboratory of the Massachusetts Eye and Ear Infirmary, Boston, MA. Post-mortem time for obtaining tissues was 6 h. In this DFNA9 individual, the onset of bilateral sensorineural hearing loss and disequilibrium occurred around age 40 years with progression to profound deafness by age 63, which was documented by audiological evaluation. Vestibular symptoms consisted of gait imbalance, instability in the dark and oscillopsia. Vestibular testing revealed bilateral peripheral vestibular hypofunction. For unaffected controls (with no history of hearing loss), temporal bones obtained from two donors, a 63-year-old female and a 75-year-old male were used. Post-mortem times for obtaining control tissues were 8 and 15 h, respectively. Temporal bones were fixed in 10% formalin, decalcified in EDTA and reduced in size using razor blades to contain only the otic capsule and inner ear. To obtain optimal morphology of sections, one of the DFNA9 temporal bones (left side) was embedded in celloidin, as is standard with temporal bones processed for light microscopic study. Immunostaining is challenging in celloidin-embedded sections, even though there is much better tissue integrity in this medium (Fig. 2); therefore, to facilitate immunohistochemistry, the other temporal bone (right side) was embedded in paraffin. This is the first and only temporal bone from a DFNA9 family member that has been embedded in paraffin to date, thereby facilitating our analyses. For the unaffected controls, the temporal bones from the 63-year-old female were embedded in celloidin, and those from the 75-year-old male in paraffin. Specimens were serially sectioned at a thickness of 20 µm for celloidin and 8 µm for paraffin, and selected sections stained with H&E. Paraffin sections were processed for immunohistochemistry and proteomic analysis as described below.
Immunohistochemistry
Immunostaining was performed using an anti-cochlin antibody generated against a peptide in the vWFA1 domain of cochlin (Fig. 1), corresponding to amino acid residues 163–181 of human cochlin, identical to the residues in murine and bovine cochlin (31). Anti-serum was purified through a protein A sepharose column, followed by peptide-affinity chromatography. This antibody (anti-cochlin/vWFA1 domain) recognizes all three different-sized isoforms of cochlin (Fig. 1).
Immunohistochemistry was performed as previously described (19), except for modifications as described below, including lack of the antigen-retrieval step. Paraffin-embedded sections from postnatal (+/+) and Coch (−/−) mice, unaffected control and DFNA9-affected human adult temporal bones were incubated with anti-cochlin/vWFA1 domain antibody overnight at room temperature, washed and incubated with a secondary biotinylated anti-rabbit IgG (Vector Labs, Burlingame, CA, USA). Immunostaining was visualized by incubation with the Vectastain ABC reagent (Vector Labs) followed by 3,3′-diaminobenzidine (DAB). Sections were not counterstained.
Proteomic analysis
For proteomic analysis in the mouse, membranous cochlear and vestibular labyrinths were dissected separately from approximately 3-month-old (+/+) and Coch (−/−) mice. Protein lysates were prepared by incubation of tissues in lysis buffer [2% sodium dodecyl sulfate (SDS), 100 mm ammonium bicarbonate, 10 mm dithiothreitol (DTT), pH 8.5] at 90°C for 10 min, 37°C for 60 min, with subsequent sonication in 0.2% SDS, and incubation at 90°C for 10 min. Three rounds of sonication and boiling were performed, followed by alkylation with 30 mm iodoacetamide. The reaction was quenched with 10 mm DTT and samples were separated by SDS gel electrophoresis in 8–16% polyacrylamide gels. Gels were size-fractionated into four sections, destained with two washes of 50% methanol and 5% acetic acid, followed by three alternating washes of ammonium bicarbonate and acetonitrile. Gel slices were dried and subsequently suspended individually in trypsin (5.5 µg/ml in 50 mm ammonium bicarbonate) prior to incubation at 37°C for 18 h for digestion of proteins. Peptides were extracted with two rinses of 50 mm ammonium bicarbonate and two rinses of 50% acetonitrile and 0.1% formic acid. Samples were prepared for mass spectrometry by lyophilization and rehydration in 5% acetonitrile and 0.1% formic acid.
For proteomic analysis of the adult human inner ear, the only materials available were formalin-fixed, paraffin-embedded tissues. Sections of 8 µm in thickness were used from the same DFNA9-affected temporal bone as used for immunohistochemistry. For a human adult temporal bone control, formalin-fixed, paraffin-embedded, 8 µm thick sections were used from an 85-year-old female with otosclerosis, but showing no inner ear histopathology and with normal cochlear duct structures including the spiral ligament and spiral limbus.
Extraction of proteins from human paraffin-embedded sections was performed by adding heptane and incubating at room temperature for 60 min, followed by the addition of methanol to pellet-extracted proteins. Protein extracts were resolubilized and sonicated in 2% SDS, 100 mm ammonium bicarbonate, 10 mm DTT, pH 8.5. Reduction of proteins was achieved by boiling samples at 90°C for 20 min, followed by incubation at 37°C for 60 min and alkylation in 30 mm iodoacetamide for 60 min at room temperature. Trypsin digestion of proteins and preparation for mass spectrometry were performed as described for the mouse samples.
For mass spectrometry, samples were run on an LCQ DECA XP plus Proteome X workstation (Thermo Electron Corporation, San Jose, CA, USA). Peptide identifications were made using Sequest through the Bioworks Browser 3.1 (Thermo Electron Corporation). Database searches were made using the NCBI RefSeqHuman and RefSeqMurine databases using static carbamidomethyl-modified cysteines and differential oxidized methionines, followed by further searches using differential modifications.
Reverse transcription–polymerase chain reaction
RT–PCR was performed with total RNA isolated from EBV-transformed cell lines from two P51S DFNA9 individuals, using the RNAeasy midi-kit (Qiagen, Leusden, the Netherlands). cDNA synthesis was performed as described (56) and PCR was done for 35 cycles under standard conditions using the following primers flanking the mutation: forward 5′-ACCAGAGGCTTGGACATCAG-3′ in exon 4 and reverse 5′-TTTGAGACTGGATGCCATTG-3′ in exon 5. Amplified products were gel-isolated and sequenced using an ABI PRISM Big Dye Terminator Cycle Sequencing V2 Ready reaction kit and the ABI 3730 DNA sequencing apparatus (Applied Biosystems, Foster City, CA, USA).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online. The supplementary figure shows immunohistochemistry performed on human adult temporal bone sections with secondary antibody alone, as a negative control; no staining is detected.
ACKNOWLEDGEMENTS
We are especially grateful to the individuals and their families for donation of temporal bones, and a more detailed description of the P51S DFNA9 histopathology is in preparation for publication. We would like to thank Drs Roderick Bronson and Li Zhang at the Dana Farber/Harvard Cancer Center Rodent Histopathology Core. We also thank Drs Colin Stewart and Clara Rodriguez for their gift of the Coch (−/−) mice. This work was supported by NIH/NIDCD grants R01-DC03402 (to C.C.M.), R01-DC0188 and P30-05209 (to M.C.L.), the NIDCD National Temporal Bone, Hearing and Balance Pathology Resource Registry and by Mr Axel Eliasen, and the Health and Labor Sciences Research Grants in Japan (Research on Measures for Intractable Diseases, and Sensory and Communicative Disorders) (to T.I.).
This manuscript is dedicated by Cynthia Morton to the memory of Craig Philip Morton, who passed away on July 9, 2005 from pancreatic cancer, and who upon his death donated his temporal bones to the NIDCD National Temporal Bone, Hearing and Balance Pathology Resource Registry (http://www.tbregistry.org/) having struggled with Meniere's disease during his too short life.
Conflict of Interest statement. None declared.
Figure 1. Schematic representation of the deduced amino acid structure of human COCH, encoding the protein cochlin, shows a predicted signal peptide (SP), followed by a domain initially designated as FCH, also known as the LCCL domain, followed by an intervening domain (ivd1) and two von Willebrand factor A-like domains (vWFA1 and vWFA2) separated by an intervening domain (ivd2). Six familial missense mutations (five in the FCH/LCCL domain and one in the vWFA2 domain) causing DFNA9 deafness and vestibular disorder are indicated by arrows. The positions of all cysteine residues are shown as ‘C’. The three known isoforms of cochlin are represented by horizontal lines corresponding to their sequence and designated as p60 (full-length, excluding the SP) and two shorter isoforms (p44 and p40, both lacking the FCH/LCCL domain). The cochlin antibody used in this study was made against a small peptide in the N-terminus of the vWFA1 domain (amino acid residues 163–181), shown in the figure. This antibody recognizes all three isoforms of cochlin.
Figure 2. H&E-stained celloidin-embedded temporal bone sections from an individual with DFNA9 from a Dutch kindred segregating the P51S mutation (67-year-old female) (B, D, F) and from an age-matched unaffected control (63-year-old female) (A, C, E). In the cochlear duct of the DFNA9-affected individual (B, D, ×100), as compared with the unaffected control (A, C, ×100), the most striking findings are the presence of abundant extracellular eosinophilic aggregates throughout the spiral ligament, spiral limbus and the osseous spiral lamina, and significant loss and degeneration of fibrocytes in the ligament and limbus. In particular, the more medial parts of the spiral ligament, underlying the stria vascularis and the area of the insertion of the ligament into the basilar membrane are more severely affected with presence of deposits and fibrocyte atrophy. Degeneration of the organ of Corti and of neural processes in the osseous spiral lamina is also observed. In the ampulla of the posterior semi-circular canal in the vestibular labyrinth (E, F, ×40) pathologic changes, similar to those in the cochlear duct, are present in the DFNA9-affected ampulla (F) as compared with the unaffected control (E). Abundant eosinophilic deposition is present within the DFNA9 ampullary stroma, with reduction and atrophy of the stromal fibrocytes, as well as degeneration of the sensory epithelium of the crista, and atrophy of the ampullary nerve. In addition, there is appreciable thickening and partial collapse of the ampullary wall, also showing the presence of eosinophilic aggregates.
Figure 3. Immunohistochemistry on postnatal (5-month-old) (+/+) (A, C, G) and Coch (−/−) (E) mouse inner ear sections with anti-cochlin. Immunostaining (on the left panels) appears as a reddish brown DAB reaction product; no counterstain was applied on these sections. Serial sections stained with H&E are shown in parallel on the right panels (B, D, F, H). In the (+/+) cochlear duct (A, ×100; C, ×150), prominent cochlin immunostaining is present in fibrocytes and in the ECM throughout the spiral ligament and spiral limbus. Cells lining Rosenthal's canal (surrounding the spiral ganglion) and the channels of the osseous spiral lamina also contain cochlin, whereas the cochlear ganglion cell bodies and the neural processes are negative for cochlin immunostaining. Distinct perivascular rings around blood vessels in the modiolus and throughout the cochlear duct are stained. In contrast, adjacent areas of surrounding bony tissues clearly lack cochlin staining. The structures of the cochlea that show the absence of cochlin expression are the organ of Corti, including the sensory epithelium and tectorial membrane (TM), stria vascularis, Reissner's membrane and spiral ganglion cells. The cochlear duct in the Coch (−/−) mouse (E, ×100) was used as a negative control and lacks any cochlin immunostaining, confirming the specificity of this antibody. In the (+/+) crista of the posterior ampulla in the vestibular labyrinth (G, ×200), intense cochlin staining is observed in the fibrocytes and stroma underlying the sensory epithelium, as well as in the ampullary wall. Neuronal processes within the ampullary stroma as well as the surrounding bone and connective tissues lack any immunostaining. The sensory epithelium is also completely devoid of any cochlin staining, as observed in the cochlear duct.
Figure 4. Immunohistochemistry on unaffected human adult (75-year-old male) temporal bone sections with anti-cochlin (A, C, E). No counterstain was used on these sections; serial H&E sections are shown (B, D, F). In the cochlear duct (A, C, ×100), cochlin immunostaining is prominent throughout the spiral ligament, spiral limbus and the channels of the osseous spiral lamina. Adjacent areas of surrounding bony tissues are not stained with the anti-cochlin antibody. Structures of the cochlea shown in this figure, which lack cochlin expression, are the organ of Corti, including the sensory epithelium and tectorial membrane (TM), stria vascularis and Reissner's membrane (RM). Some of these structures show artifactual disruption as a result of paraffin embedding of adult temporal bones. In the posterior crista ampullaris of the vestibular labyrinth (E, ×200), intense cochlin staining is observed in the fibrocytes and stroma underlying the sensory epithelium. The sensory epithelium is completely devoid of any cochlin expression, as was observed in the cochlear duct.
Figure 5. Immunohistochemistry on DFNA9-affected human adult (67-year-old female) temporal bone sections with the anti-cochlin antibody (A, C, E, G). No counterstain was used on these sections; serial H&E sections are shown (B, D, F, H). In the cochlear duct (A, C, ×100), cochlin immunostaining is observed throughout the spiral ligament, spiral limbus and the channels of the osseous spiral lamina. The homogeneous eosinophilic deposits seen on the H&E sections are stained darkly and evenly. The cochlin immunostaining of this acellular material is prominent in the spiral ligament, particularly in the area of insertion into the basilar membrane, the spiral limbus and within the channels of the osseous spiral lamina. The organ of Corti and the stria vascularis are negative for cochlin staining. Some stained tissue underlying the stria vascularis appears to be a part of the spiral ligament that was detached along with the stria. Adjacent areas of surrounding bony tissues do not show any immunostaining. In the posterior ampulla of the vestibular labyrinth (E, ×4; G, ×100), cochlin staining of the ampullary stroma containing the eosinophilic deposits is observed. The collapsed ampullary wall showing prominent thickening and acellular deposition (F) also contains cochlin (E). The sensory epithelium does not show cochlin expression, as seen in the cochlear duct in both DFNA9 and unaffected control inner ears.
Figure 6. Amino acid sequence of human and mouse cochlin, showing distribution of tryptic digest fragments identified by mass spectrometry from cochlear tissue. The domains of cochlin are underlined and labeled (FCH/LCCL in red, vWFA1 and vWFA2 in blue), as well as the SP, cleaved in mature cochlin and therefore not detected among tryptic fragments. Amino acid residues identical between human and mouse are indicated by a dot. Cochlin peptide coverage, as found in our proteomic analysis from human unaffected formalin-fixed, paraffin-embedded temporal bone sections is highlighted in yellow and from mouse fresh whole cochlear tissue is highlighted in green.
COCH mutations in DFNA9
| Origin . | Exon with mutation . | Nucleotide changea . | Amino acid changeb . | Protein domain . | References . |
|---|---|---|---|---|---|
| The Netherlandsc; Belgiumc | 4 | C207T | P51S | FCH/LCCL | (11,12) |
| United Statesc | 4 | T253G | V66G | FCH/LCCL | (10) |
| United Statesc; The Netherlandsc | 5 | G319A | G88E | FCH/LCCL | (10,14) |
| Hungaryd | 5 | 366_368delGTA | V104del | FCH/LCCL | (16) |
| Australiac | 5 | T382A | I109N | FCH/LCCL | (13) |
| United Statesc | 5 | T405C | W117R | FCH/LCCL | (10) |
| Japand | 5 | G411A | A119T | FCH/LCCL | (15) |
| United Statesc | 12 | G1681T | C542F | vWFA2 | (9) |
| Origin . | Exon with mutation . | Nucleotide changea . | Amino acid changeb . | Protein domain . | References . |
|---|---|---|---|---|---|
| The Netherlandsc; Belgiumc | 4 | C207T | P51S | FCH/LCCL | (11,12) |
| United Statesc | 4 | T253G | V66G | FCH/LCCL | (10) |
| United Statesc; The Netherlandsc | 5 | G319A | G88E | FCH/LCCL | (10,14) |
| Hungaryd | 5 | 366_368delGTA | V104del | FCH/LCCL | (16) |
| Australiac | 5 | T382A | I109N | FCH/LCCL | (13) |
| United Statesc | 5 | T405C | W117R | FCH/LCCL | (10) |
| Japand | 5 | G411A | A119T | FCH/LCCL | (15) |
| United Statesc | 12 | G1681T | C542F | vWFA2 | (9) |
aNumbering of nucleotides is according to the human COCH cDNA sequence (GenBank Accession No. AF006740), which starts in the 5′ untranslated region, 56 bp upstream of the start ATG.
bNumbering of amino acids begins at the start methionine.
cFamilial (autosomal-dominant) cases.
dSimplex cases.
COCH mutations in DFNA9
| Origin . | Exon with mutation . | Nucleotide changea . | Amino acid changeb . | Protein domain . | References . |
|---|---|---|---|---|---|
| The Netherlandsc; Belgiumc | 4 | C207T | P51S | FCH/LCCL | (11,12) |
| United Statesc | 4 | T253G | V66G | FCH/LCCL | (10) |
| United Statesc; The Netherlandsc | 5 | G319A | G88E | FCH/LCCL | (10,14) |
| Hungaryd | 5 | 366_368delGTA | V104del | FCH/LCCL | (16) |
| Australiac | 5 | T382A | I109N | FCH/LCCL | (13) |
| United Statesc | 5 | T405C | W117R | FCH/LCCL | (10) |
| Japand | 5 | G411A | A119T | FCH/LCCL | (15) |
| United Statesc | 12 | G1681T | C542F | vWFA2 | (9) |
| Origin . | Exon with mutation . | Nucleotide changea . | Amino acid changeb . | Protein domain . | References . |
|---|---|---|---|---|---|
| The Netherlandsc; Belgiumc | 4 | C207T | P51S | FCH/LCCL | (11,12) |
| United Statesc | 4 | T253G | V66G | FCH/LCCL | (10) |
| United Statesc; The Netherlandsc | 5 | G319A | G88E | FCH/LCCL | (10,14) |
| Hungaryd | 5 | 366_368delGTA | V104del | FCH/LCCL | (16) |
| Australiac | 5 | T382A | I109N | FCH/LCCL | (13) |
| United Statesc | 5 | T405C | W117R | FCH/LCCL | (10) |
| Japand | 5 | G411A | A119T | FCH/LCCL | (15) |
| United Statesc | 12 | G1681T | C542F | vWFA2 | (9) |
aNumbering of nucleotides is according to the human COCH cDNA sequence (GenBank Accession No. AF006740), which starts in the 5′ untranslated region, 56 bp upstream of the start ATG.
bNumbering of amino acids begins at the start methionine.
cFamilial (autosomal-dominant) cases.
dSimplex cases.
Relative abundance of inner ear peptides in wild-type +/+ and Coch −/− mice
| . | Cochlea . | Vestibular . | ||
|---|---|---|---|---|
| . | Wild-type +/+ . | Coch −/− . | Wild-type +/+ . | Coch −/− . |
| Cochlin | 123 | 0 | 50 | 0 |
| Albumin | 68 | 109 | 55 | 38 |
| β-Hemoglobin | 76 | 81 | 47 | 28 |
| α-Tectorin | 12 | 19 | 1 | 1 |
| Keratin 9 | 18 | 20 | 31 | 36 |
| . | Cochlea . | Vestibular . | ||
|---|---|---|---|---|
| . | Wild-type +/+ . | Coch −/− . | Wild-type +/+ . | Coch −/− . |
| Cochlin | 123 | 0 | 50 | 0 |
| Albumin | 68 | 109 | 55 | 38 |
| β-Hemoglobin | 76 | 81 | 47 | 28 |
| α-Tectorin | 12 | 19 | 1 | 1 |
| Keratin 9 | 18 | 20 | 31 | 36 |
Relative abundance of inner ear peptides in wild-type +/+ and Coch −/− mice
| . | Cochlea . | Vestibular . | ||
|---|---|---|---|---|
| . | Wild-type +/+ . | Coch −/− . | Wild-type +/+ . | Coch −/− . |
| Cochlin | 123 | 0 | 50 | 0 |
| Albumin | 68 | 109 | 55 | 38 |
| β-Hemoglobin | 76 | 81 | 47 | 28 |
| α-Tectorin | 12 | 19 | 1 | 1 |
| Keratin 9 | 18 | 20 | 31 | 36 |
| . | Cochlea . | Vestibular . | ||
|---|---|---|---|---|
| . | Wild-type +/+ . | Coch −/− . | Wild-type +/+ . | Coch −/− . |
| Cochlin | 123 | 0 | 50 | 0 |
| Albumin | 68 | 109 | 55 | 38 |
| β-Hemoglobin | 76 | 81 | 47 | 28 |
| α-Tectorin | 12 | 19 | 1 | 1 |
| Keratin 9 | 18 | 20 | 31 | 36 |
Proteins identified from DFNA9-affected and unaffected adult human temporal bones
| Protein name . | Accession No. . | Unaffected control . | DFNA9-affected . | ||
|---|---|---|---|---|---|
| . | . | No. of total peptide matches . | No. of unique peptide matches . | No. of total peptide matches . | No. of unique peptide matches . |
| β-Actin | gi|4501885| | 3 | 2 | — | — |
| Albumin precursor | gi|4502027| | 4 | 4 | 3 | 3 |
| Annexin A2; annexin II | gi|4757756| | 3 | 3 | — | — |
| Annexin V; endonexin II | gi|4502107| | 5 | 5 | — | — |
| ATP synthase, mitochondrial F1 complex | gi|4757810| | 1 | 1 | — | — |
| ATPase, alpha 1 polypeptide | gi|21361181| | 1 | 1 | — | — |
| Chondromodulin I precursor | gi|5901932| | 1 | 1 | — | — |
| Clusterin | gi|4502905| | 7 | 4 | — | — |
| Cochlin (coagulation factor C homology) | gi|4758022| | 66 | 17 | 3 | 2 |
| Collagen type I, alpha 1 | gi|4502945| | 2 | 1 | 3 | 3 |
| Collagen type I, alpha 2 | gi|4502947| | 2 | 2 | 2 | 2 |
| Collagen type II, alpha 1, isoform 1 | gi|13435125| | 11 | 2 | — | — |
| Collagen type IX, alpha 2 | gi|11386161| | 3 | 2 | — | — |
| Collagen type IX, alpha 3 | gi|17921995| | 1 | 1 | — | — |
| Collagen type XI, alpha isoform A | gi|18375518| | 2 | 1 | — | — |
| Dermatan sulfate proteoglycan 3 | gi|4826704| | 1 | 1 | — | — |
| Desmin | gi|18105050| | 3 | 2 | — | — |
| Eukaryotic translation initiation factor 4A1 | gi|30153769| | 1 | 1 | — | — |
| Globin, alpha 2 | gi|4504345| | 1 | 1 | 2 | 2 |
| β-Globin | gi|4504349| | 3 | 2 | 3 | 3 |
| Growth factor receptor-bound protein 10 | gi|19923303| | 1 | 1 | — | — |
| Histone H2a | gi|15617199| | 1 | 1 | — | — |
| Histone, H1, member 2 | gi|4885375| | 2 | 2 | — | — |
| Histone, H2A, member Q | gi|24638446| | 5 | 4 | — | — |
| Histone, H2A, member Z | gi|4504255| | 1 | 1 | — | — |
| Histone, H2B, member Q | gi|4504277| | 2 | 2 | — | — |
| Histone, H3, family 3A | gi|4504279| | 1 | 1 | — | — |
| Histone, H4, family 2, member N | gi|4504323| | 9 | 6 | — | — |
| Hypothetical protein FLJ10618 | gi|8922551| | 1 | 1 | — | — |
| Hypothetical protein LOC221302 | gi|21450836| | 2 | 1 | — | — |
| Hypothetical protein XP_298917 | gi|29735940| | 1 | 1 | — | — |
| IMP (inosine monophosphate) dehydrogenase 2 | gi|30150307| | — | — | 1 | 1 |
| Keratin 1 | gi|17318569| | 5 | 5 | 1 | 1 |
| Keratin 10 | gi|4557697| | 3 | 3 | — | — |
| Keratin 2a | gi|4557703| | 2 | 2 | 1 | 1 |
| Keratin 8 | gi|30154839| | 1 | 1 | — | — |
| Keratin 9 | gi|4557705| | 2 | 2 | — | — |
| Lamin A/C isoform 2 | gi|5031875| | 2 | 2 | — | — |
| Lanthionine synthetase C-like protein 1 | gi|5174445| | 1 | 1 | — | — |
| Norrin (Norrie disease protein) | gi|4557789| | 1 | 1 | — | — |
| Nuclear ribonucleoprotein A2/B1 isoform A2 | gi|4504447| | 1 | 1 | — | — |
| Peroxiredoxin 1 | gi|4505591| | 3 | 3 | — | — |
| Peroxiredoxin 2 | gi|5902726| | 1 | 1 | — | — |
| Phosphatidylethanolamine-binding protein | gi|18543899| | 1 | 1 | — | — |
| Phosphodiesterase 8A isoform 1 | gi|27734721| | 1 | 1 | — | — |
| Plectin 1 | gi|4505877| | 4 | 4 | — | — |
| Protease, serine, 1 preproprotein | gi|4506145| | 2 | 1 | — | — |
| Pyruvate kinase, liver and RBC | gi|10835121| | 1 | 1 | — | — |
| Pyruvate kinase, M2 isozyme | gi|29727204| | 1 | 1 | — | — |
| Pyruvate kinase-3 | gi|4505839| | 2 | 2 | — | — |
| Ring finger protein 14 | gi|4757762| | 1 | 1 | — | — |
| S100 calcium-binding protein, beta | gi|5454034| | 1 | 1 | — | — |
| Similar to natural killer cell transcript 4 | gi|30158950| | 1 | 1 | — | — |
| Tolloid-like 1 | gi|22547221| | 1 | 1 | — | — |
| Tropomyosin 2, beta | gi|4507649| | 1 | 1 | — | — |
| Tropomyosin 3 | gi|24119203| | 1 | 1 | — | — |
| α-Tubulin | gi|30149013| | 1 | 1 | — | — |
| Tumor necrosis factor type 1 receptor | gi|7706485| | 1 | 1 | — | — |
| Ubiquitin and ribosomal protein S27 | gi|4506713| | 1 | 1 | — | — |
| Vimentin | gi|4507895| | 29 | 17 | — | — |
| Vinculin isoform VCL | gi|4507877| | 1 | 1 | — | — |
| Vitronectin precursor | gi|18201911| | 2 | 2 | — | — |
| Protein name . | Accession No. . | Unaffected control . | DFNA9-affected . | ||
|---|---|---|---|---|---|
| . | . | No. of total peptide matches . | No. of unique peptide matches . | No. of total peptide matches . | No. of unique peptide matches . |
| β-Actin | gi|4501885| | 3 | 2 | — | — |
| Albumin precursor | gi|4502027| | 4 | 4 | 3 | 3 |
| Annexin A2; annexin II | gi|4757756| | 3 | 3 | — | — |
| Annexin V; endonexin II | gi|4502107| | 5 | 5 | — | — |
| ATP synthase, mitochondrial F1 complex | gi|4757810| | 1 | 1 | — | — |
| ATPase, alpha 1 polypeptide | gi|21361181| | 1 | 1 | — | — |
| Chondromodulin I precursor | gi|5901932| | 1 | 1 | — | — |
| Clusterin | gi|4502905| | 7 | 4 | — | — |
| Cochlin (coagulation factor C homology) | gi|4758022| | 66 | 17 | 3 | 2 |
| Collagen type I, alpha 1 | gi|4502945| | 2 | 1 | 3 | 3 |
| Collagen type I, alpha 2 | gi|4502947| | 2 | 2 | 2 | 2 |
| Collagen type II, alpha 1, isoform 1 | gi|13435125| | 11 | 2 | — | — |
| Collagen type IX, alpha 2 | gi|11386161| | 3 | 2 | — | — |
| Collagen type IX, alpha 3 | gi|17921995| | 1 | 1 | — | — |
| Collagen type XI, alpha isoform A | gi|18375518| | 2 | 1 | — | — |
| Dermatan sulfate proteoglycan 3 | gi|4826704| | 1 | 1 | — | — |
| Desmin | gi|18105050| | 3 | 2 | — | — |
| Eukaryotic translation initiation factor 4A1 | gi|30153769| | 1 | 1 | — | — |
| Globin, alpha 2 | gi|4504345| | 1 | 1 | 2 | 2 |
| β-Globin | gi|4504349| | 3 | 2 | 3 | 3 |
| Growth factor receptor-bound protein 10 | gi|19923303| | 1 | 1 | — | — |
| Histone H2a | gi|15617199| | 1 | 1 | — | — |
| Histone, H1, member 2 | gi|4885375| | 2 | 2 | — | — |
| Histone, H2A, member Q | gi|24638446| | 5 | 4 | — | — |
| Histone, H2A, member Z | gi|4504255| | 1 | 1 | — | — |
| Histone, H2B, member Q | gi|4504277| | 2 | 2 | — | — |
| Histone, H3, family 3A | gi|4504279| | 1 | 1 | — | — |
| Histone, H4, family 2, member N | gi|4504323| | 9 | 6 | — | — |
| Hypothetical protein FLJ10618 | gi|8922551| | 1 | 1 | — | — |
| Hypothetical protein LOC221302 | gi|21450836| | 2 | 1 | — | — |
| Hypothetical protein XP_298917 | gi|29735940| | 1 | 1 | — | — |
| IMP (inosine monophosphate) dehydrogenase 2 | gi|30150307| | — | — | 1 | 1 |
| Keratin 1 | gi|17318569| | 5 | 5 | 1 | 1 |
| Keratin 10 | gi|4557697| | 3 | 3 | — | — |
| Keratin 2a | gi|4557703| | 2 | 2 | 1 | 1 |
| Keratin 8 | gi|30154839| | 1 | 1 | — | — |
| Keratin 9 | gi|4557705| | 2 | 2 | — | — |
| Lamin A/C isoform 2 | gi|5031875| | 2 | 2 | — | — |
| Lanthionine synthetase C-like protein 1 | gi|5174445| | 1 | 1 | — | — |
| Norrin (Norrie disease protein) | gi|4557789| | 1 | 1 | — | — |
| Nuclear ribonucleoprotein A2/B1 isoform A2 | gi|4504447| | 1 | 1 | — | — |
| Peroxiredoxin 1 | gi|4505591| | 3 | 3 | — | — |
| Peroxiredoxin 2 | gi|5902726| | 1 | 1 | — | — |
| Phosphatidylethanolamine-binding protein | gi|18543899| | 1 | 1 | — | — |
| Phosphodiesterase 8A isoform 1 | gi|27734721| | 1 | 1 | — | — |
| Plectin 1 | gi|4505877| | 4 | 4 | — | — |
| Protease, serine, 1 preproprotein | gi|4506145| | 2 | 1 | — | — |
| Pyruvate kinase, liver and RBC | gi|10835121| | 1 | 1 | — | — |
| Pyruvate kinase, M2 isozyme | gi|29727204| | 1 | 1 | — | — |
| Pyruvate kinase-3 | gi|4505839| | 2 | 2 | — | — |
| Ring finger protein 14 | gi|4757762| | 1 | 1 | — | — |
| S100 calcium-binding protein, beta | gi|5454034| | 1 | 1 | — | — |
| Similar to natural killer cell transcript 4 | gi|30158950| | 1 | 1 | — | — |
| Tolloid-like 1 | gi|22547221| | 1 | 1 | — | — |
| Tropomyosin 2, beta | gi|4507649| | 1 | 1 | — | — |
| Tropomyosin 3 | gi|24119203| | 1 | 1 | — | — |
| α-Tubulin | gi|30149013| | 1 | 1 | — | — |
| Tumor necrosis factor type 1 receptor | gi|7706485| | 1 | 1 | — | — |
| Ubiquitin and ribosomal protein S27 | gi|4506713| | 1 | 1 | — | — |
| Vimentin | gi|4507895| | 29 | 17 | — | — |
| Vinculin isoform VCL | gi|4507877| | 1 | 1 | — | — |
| Vitronectin precursor | gi|18201911| | 2 | 2 | — | — |
Proteins identified from DFNA9-affected and unaffected adult human temporal bones
| Protein name . | Accession No. . | Unaffected control . | DFNA9-affected . | ||
|---|---|---|---|---|---|
| . | . | No. of total peptide matches . | No. of unique peptide matches . | No. of total peptide matches . | No. of unique peptide matches . |
| β-Actin | gi|4501885| | 3 | 2 | — | — |
| Albumin precursor | gi|4502027| | 4 | 4 | 3 | 3 |
| Annexin A2; annexin II | gi|4757756| | 3 | 3 | — | — |
| Annexin V; endonexin II | gi|4502107| | 5 | 5 | — | — |
| ATP synthase, mitochondrial F1 complex | gi|4757810| | 1 | 1 | — | — |
| ATPase, alpha 1 polypeptide | gi|21361181| | 1 | 1 | — | — |
| Chondromodulin I precursor | gi|5901932| | 1 | 1 | — | — |
| Clusterin | gi|4502905| | 7 | 4 | — | — |
| Cochlin (coagulation factor C homology) | gi|4758022| | 66 | 17 | 3 | 2 |
| Collagen type I, alpha 1 | gi|4502945| | 2 | 1 | 3 | 3 |
| Collagen type I, alpha 2 | gi|4502947| | 2 | 2 | 2 | 2 |
| Collagen type II, alpha 1, isoform 1 | gi|13435125| | 11 | 2 | — | — |
| Collagen type IX, alpha 2 | gi|11386161| | 3 | 2 | — | — |
| Collagen type IX, alpha 3 | gi|17921995| | 1 | 1 | — | — |
| Collagen type XI, alpha isoform A | gi|18375518| | 2 | 1 | — | — |
| Dermatan sulfate proteoglycan 3 | gi|4826704| | 1 | 1 | — | — |
| Desmin | gi|18105050| | 3 | 2 | — | — |
| Eukaryotic translation initiation factor 4A1 | gi|30153769| | 1 | 1 | — | — |
| Globin, alpha 2 | gi|4504345| | 1 | 1 | 2 | 2 |
| β-Globin | gi|4504349| | 3 | 2 | 3 | 3 |
| Growth factor receptor-bound protein 10 | gi|19923303| | 1 | 1 | — | — |
| Histone H2a | gi|15617199| | 1 | 1 | — | — |
| Histone, H1, member 2 | gi|4885375| | 2 | 2 | — | — |
| Histone, H2A, member Q | gi|24638446| | 5 | 4 | — | — |
| Histone, H2A, member Z | gi|4504255| | 1 | 1 | — | — |
| Histone, H2B, member Q | gi|4504277| | 2 | 2 | — | — |
| Histone, H3, family 3A | gi|4504279| | 1 | 1 | — | — |
| Histone, H4, family 2, member N | gi|4504323| | 9 | 6 | — | — |
| Hypothetical protein FLJ10618 | gi|8922551| | 1 | 1 | — | — |
| Hypothetical protein LOC221302 | gi|21450836| | 2 | 1 | — | — |
| Hypothetical protein XP_298917 | gi|29735940| | 1 | 1 | — | — |
| IMP (inosine monophosphate) dehydrogenase 2 | gi|30150307| | — | — | 1 | 1 |
| Keratin 1 | gi|17318569| | 5 | 5 | 1 | 1 |
| Keratin 10 | gi|4557697| | 3 | 3 | — | — |
| Keratin 2a | gi|4557703| | 2 | 2 | 1 | 1 |
| Keratin 8 | gi|30154839| | 1 | 1 | — | — |
| Keratin 9 | gi|4557705| | 2 | 2 | — | — |
| Lamin A/C isoform 2 | gi|5031875| | 2 | 2 | — | — |
| Lanthionine synthetase C-like protein 1 | gi|5174445| | 1 | 1 | — | — |
| Norrin (Norrie disease protein) | gi|4557789| | 1 | 1 | — | — |
| Nuclear ribonucleoprotein A2/B1 isoform A2 | gi|4504447| | 1 | 1 | — | — |
| Peroxiredoxin 1 | gi|4505591| | 3 | 3 | — | — |
| Peroxiredoxin 2 | gi|5902726| | 1 | 1 | — | — |
| Phosphatidylethanolamine-binding protein | gi|18543899| | 1 | 1 | — | — |
| Phosphodiesterase 8A isoform 1 | gi|27734721| | 1 | 1 | — | — |
| Plectin 1 | gi|4505877| | 4 | 4 | — | — |
| Protease, serine, 1 preproprotein | gi|4506145| | 2 | 1 | — | — |
| Pyruvate kinase, liver and RBC | gi|10835121| | 1 | 1 | — | — |
| Pyruvate kinase, M2 isozyme | gi|29727204| | 1 | 1 | — | — |
| Pyruvate kinase-3 | gi|4505839| | 2 | 2 | — | — |
| Ring finger protein 14 | gi|4757762| | 1 | 1 | — | — |
| S100 calcium-binding protein, beta | gi|5454034| | 1 | 1 | — | — |
| Similar to natural killer cell transcript 4 | gi|30158950| | 1 | 1 | — | — |
| Tolloid-like 1 | gi|22547221| | 1 | 1 | — | — |
| Tropomyosin 2, beta | gi|4507649| | 1 | 1 | — | — |
| Tropomyosin 3 | gi|24119203| | 1 | 1 | — | — |
| α-Tubulin | gi|30149013| | 1 | 1 | — | — |
| Tumor necrosis factor type 1 receptor | gi|7706485| | 1 | 1 | — | — |
| Ubiquitin and ribosomal protein S27 | gi|4506713| | 1 | 1 | — | — |
| Vimentin | gi|4507895| | 29 | 17 | — | — |
| Vinculin isoform VCL | gi|4507877| | 1 | 1 | — | — |
| Vitronectin precursor | gi|18201911| | 2 | 2 | — | — |
| Protein name . | Accession No. . | Unaffected control . | DFNA9-affected . | ||
|---|---|---|---|---|---|
| . | . | No. of total peptide matches . | No. of unique peptide matches . | No. of total peptide matches . | No. of unique peptide matches . |
| β-Actin | gi|4501885| | 3 | 2 | — | — |
| Albumin precursor | gi|4502027| | 4 | 4 | 3 | 3 |
| Annexin A2; annexin II | gi|4757756| | 3 | 3 | — | — |
| Annexin V; endonexin II | gi|4502107| | 5 | 5 | — | — |
| ATP synthase, mitochondrial F1 complex | gi|4757810| | 1 | 1 | — | — |
| ATPase, alpha 1 polypeptide | gi|21361181| | 1 | 1 | — | — |
| Chondromodulin I precursor | gi|5901932| | 1 | 1 | — | — |
| Clusterin | gi|4502905| | 7 | 4 | — | — |
| Cochlin (coagulation factor C homology) | gi|4758022| | 66 | 17 | 3 | 2 |
| Collagen type I, alpha 1 | gi|4502945| | 2 | 1 | 3 | 3 |
| Collagen type I, alpha 2 | gi|4502947| | 2 | 2 | 2 | 2 |
| Collagen type II, alpha 1, isoform 1 | gi|13435125| | 11 | 2 | — | — |
| Collagen type IX, alpha 2 | gi|11386161| | 3 | 2 | — | — |
| Collagen type IX, alpha 3 | gi|17921995| | 1 | 1 | — | — |
| Collagen type XI, alpha isoform A | gi|18375518| | 2 | 1 | — | — |
| Dermatan sulfate proteoglycan 3 | gi|4826704| | 1 | 1 | — | — |
| Desmin | gi|18105050| | 3 | 2 | — | — |
| Eukaryotic translation initiation factor 4A1 | gi|30153769| | 1 | 1 | — | — |
| Globin, alpha 2 | gi|4504345| | 1 | 1 | 2 | 2 |
| β-Globin | gi|4504349| | 3 | 2 | 3 | 3 |
| Growth factor receptor-bound protein 10 | gi|19923303| | 1 | 1 | — | — |
| Histone H2a | gi|15617199| | 1 | 1 | — | — |
| Histone, H1, member 2 | gi|4885375| | 2 | 2 | — | — |
| Histone, H2A, member Q | gi|24638446| | 5 | 4 | — | — |
| Histone, H2A, member Z | gi|4504255| | 1 | 1 | — | — |
| Histone, H2B, member Q | gi|4504277| | 2 | 2 | — | — |
| Histone, H3, family 3A | gi|4504279| | 1 | 1 | — | — |
| Histone, H4, family 2, member N | gi|4504323| | 9 | 6 | — | — |
| Hypothetical protein FLJ10618 | gi|8922551| | 1 | 1 | — | — |
| Hypothetical protein LOC221302 | gi|21450836| | 2 | 1 | — | — |
| Hypothetical protein XP_298917 | gi|29735940| | 1 | 1 | — | — |
| IMP (inosine monophosphate) dehydrogenase 2 | gi|30150307| | — | — | 1 | 1 |
| Keratin 1 | gi|17318569| | 5 | 5 | 1 | 1 |
| Keratin 10 | gi|4557697| | 3 | 3 | — | — |
| Keratin 2a | gi|4557703| | 2 | 2 | 1 | 1 |
| Keratin 8 | gi|30154839| | 1 | 1 | — | — |
| Keratin 9 | gi|4557705| | 2 | 2 | — | — |
| Lamin A/C isoform 2 | gi|5031875| | 2 | 2 | — | — |
| Lanthionine synthetase C-like protein 1 | gi|5174445| | 1 | 1 | — | — |
| Norrin (Norrie disease protein) | gi|4557789| | 1 | 1 | — | — |
| Nuclear ribonucleoprotein A2/B1 isoform A2 | gi|4504447| | 1 | 1 | — | — |
| Peroxiredoxin 1 | gi|4505591| | 3 | 3 | — | — |
| Peroxiredoxin 2 | gi|5902726| | 1 | 1 | — | — |
| Phosphatidylethanolamine-binding protein | gi|18543899| | 1 | 1 | — | — |
| Phosphodiesterase 8A isoform 1 | gi|27734721| | 1 | 1 | — | — |
| Plectin 1 | gi|4505877| | 4 | 4 | — | — |
| Protease, serine, 1 preproprotein | gi|4506145| | 2 | 1 | — | — |
| Pyruvate kinase, liver and RBC | gi|10835121| | 1 | 1 | — | — |
| Pyruvate kinase, M2 isozyme | gi|29727204| | 1 | 1 | — | — |
| Pyruvate kinase-3 | gi|4505839| | 2 | 2 | — | — |
| Ring finger protein 14 | gi|4757762| | 1 | 1 | — | — |
| S100 calcium-binding protein, beta | gi|5454034| | 1 | 1 | — | — |
| Similar to natural killer cell transcript 4 | gi|30158950| | 1 | 1 | — | — |
| Tolloid-like 1 | gi|22547221| | 1 | 1 | — | — |
| Tropomyosin 2, beta | gi|4507649| | 1 | 1 | — | — |
| Tropomyosin 3 | gi|24119203| | 1 | 1 | — | — |
| α-Tubulin | gi|30149013| | 1 | 1 | — | — |
| Tumor necrosis factor type 1 receptor | gi|7706485| | 1 | 1 | — | — |
| Ubiquitin and ribosomal protein S27 | gi|4506713| | 1 | 1 | — | — |
| Vimentin | gi|4507895| | 29 | 17 | — | — |
| Vinculin isoform VCL | gi|4507877| | 1 | 1 | — | — |
| Vitronectin precursor | gi|18201911| | 2 | 2 | — | — |
References
Van Camp, G. and Smith, R.J.H. (
Halpin, C., Khetarpal, U. and McKenna, M. (
Khetarpal, U., Schuknecht, H.F., Gacek, R.R. and Holmes, L.B. (
Bom, S.J.H., Kemperman, M.H., De Kok, Y.J.M., Huygen, P.L.M., Verhagen, W.I.M., Cremers, F.P.M. and Cremers, C.W.R.J. (
Verhagen, W.I.M., Bom, S.J.H., Huygen, P.L.M., Fransen, E., Van Camp, G. and Cremers, C.W.R.J. (
Verstreken, M., Declau, F., Wuyts, F.L., D'Haese, P., Van Camp, G., Fransen, E., Van den Hauwe, L., Buyle, S., Smets, R.E., Feenstra, L. et al. (
Kemperman, M.H., Bom, S.J.H., Lemaire, F.X., Verhagen, W.I.M., Huygen, P.L.M. and Cremers, C.W.R.J. (
Lemaire, F.X., Feenstra, L., Huygen, P.L.M., Fransen, E., Devriendt, K., Van Camp, G., Vantrappen, G. and Cremers, C.W.R.J. (
Street, V.A., Kallman, J.C., Robertson, N.G., Kuo, S.F., Morton, C.C. and Phillips, J.O. (
Robertson, N.G., Lu, L., Heller, S., Merchant, S.N., Eavey, R.D., McKenna, M., Nadol, J.B., Jr, Miyamoto, R.T., Linthicum, F.H., Jr, Lubianca Neto, J.F. et al. (
de Kok, Y.J.M., Bom, S.J.H., Brunt, T.M., Kemperman, M.H., van Beusekom, E., van der Velde-Visser, S.D., Robertson, N.G., Morton, C.C., Huygen, P.L.M., Verhagen, W.I.M. et al. (
Fransen, E., Verstreken, M., Verhagen, W.I.M., Wuyts, F.L., Huygen, P.L.M., D'Haese, P., Robertson, N.G., Morton, C.C., McGuirt, W.T., Smith, R.J.H. et al. (
Kamarinos, M., McGill, J., Lynch, M. and Dahl, H. (
Kemperman, M.H., De Leenheer, E.M.R., Huygen, P.L.M., van Duijnhoven, G., Morton, C.C., Robertson, N.G., Cremers, F.P.M., Kremer, H. and Cremers, C.W.R.J. (
Usami, S., Takahashi, K., Yuge, I., Ohtsuka, A., Namba, A., Abe, S., Fransen, E., Patthy, L., Otting, G. and Van Camp, G. (
Nagy, I., Horvath, M., Trexler, M., Repassy, G. and Patthy, L. (
Robertson, N.G., Khetarpal, U., Gutiérrez-Espeleta, G.A., Bieber, F.R. and Morton, C.C. (
Robertson, N.G., Skvorak, A.B., Yin, Y., Weremowicz, S., Johnson, K.R., Kovatch, K.A., Battey, J.F., Bieber, F.R. and Morton, C.C. (
Robertson, N.G., Resendes, B.L., Lin, J.S., Lee, C., Aster, J.C., Adams, J.C. and Morton, C.C. (
Ikezono, T., Shindo, S., Ishizaki, M., Li, L., Tomiyama, S., Takumida, M., Pawankar, R., Watanabe, A., Saito, A. and Yagi, T. (
Ikezono, T., Omori, A., Ichinose, S., Pawankar, R., Watanabe, A. and Yagi, T. (
Merchant, S.N., Linthicum, F.H. and Nadol, J.B., Jr (
Selkoe, D.J. (
Davies, S.W., Turmaine, M., Cozens, B.A., DiFiglia, M., Sharp, A.H., Ross, C.A., Scherzinger, E., Wanker, E.E., Mangiarini, L. and Bates, G.P. (
Zoghbi, H.Y. and Orr, H.T. (
Waelter, S., Boeddrich, A., Lurz, R., Scherzinger, E., Lueder, G., Lehrach, H. and Wanker, E.E. (
Masliah, E., Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A. and Mucke, L. (
Khetarpal, U. (
Rodriguez, C.I., Cheng, J.G., Liu, L. and Stewart, C.L. (
Makishima, T., Rodriguez, C.I., Robertson, N.G., Morton, C.C., Stewart, C.L. and Griffith, A.J. (
Ikezono, T., Shindo, S., Li, L., Omori, A., Ichinose, S., Watanabe, A., Kobayashi, T., Pawankar, R. and Yagi, T. (
Skvorak, A.B., Weng, Z., Yee, A.J., Robertson, N.G. and Morton, C.C. (
Boulassel, M.R., Deggouj, N., Tomasi, J.P. and Gersdorff, M. (
Boulassel, M.R., Tomasi, J.P., Deggouj, N. and Gersdorff, M. (
Kommareddi, P.K., Nair, T.S. and Carey, T.E. (
Nair, T.S., Kozma, K.E., Hoefling, N.L., Kommareddi, P.K., Ueda, Y., Gong, T.W., Lomax, M.I., Lansford, C.D., Telian, S.A., Satar, B. et al. (
Solares, C.A., Edling, A.E., Johnson, J.M., Baek, M.J., Hirose, K., Hughes, G.B. and Tuohy, V.K. (
Baek, M.J., Park, H.M., Johnson, J.M., Altuntas, C.Z., Jaini, R., Thomas, D.M., Ball, E.J., Robertson, N.G., Morton, C.C., Hughes, G.B. et al. (
Palmer-Toy, D.E., Krastins, B., Sarracino, D.A., Nadol, J.B., Jr and Merchant, S.N. (
Schulte, B.A. and Adams, J.C. (
Robertson, N.G., Hamaker, S.A., Patriub, V., Aster, J.C. and Morton, C.C. (
Colombatti, A. and Bonaldo, P. (
Colombatti, A., Bonaldo, P. and Doliana, R. (
Lee, J.O., Rieu, P., Arnaout, M.A. and Liddington, R. (
Delrieu, I., Waller, C.C., Mota, M.M., Grainger, M., Langhorne, J. and Holder, A.A. (
Whittaker, C.A. and Hynes, R.O. (
Khetarpal, U. (
Liepinsh, E., Trexler, M., Kaikkonen, A., Weigelt, J., Banyai, L., Patthy, L. and Otting, G. (
Grabski, R., Szul, T., Sasaki, T., Timpl, R., Mayne, R., Hicks, B. and Sztul, E. (
Verhagen, W.I.M., Huygen, P.L.M., Theunissen, E.J.J.M. and Joosten, E.M.G. (
Verhagen, W.I.M., Bom, S.J.H., Fransen, E., Van Camp, G., Huygen, P.L.M., Theunissen, E.J.J.M. and Cremers, C.W.R.J. (
Bhattacharya, S.K., Rockwood, E.J., Smith, S.D., Bonilha, V.L., Crabb, J.S., Kuchtey, R.W., Robertson, N.G., Peachey, N.S., Morton, C.C. and Crabb, J.W. (
Bhattacharya, S.K., Annangudi, S.P., Salomon, R.G., Kuchtey, R.W., Peachey, N.S. and Crabb, J.W. (