-
PDF
- Split View
-
Views
-
Cite
Cite
Eric Bruckert, Impact of lipid treatment on cardiovascular risk reduction: new therapeutic targets, European Heart Journal Supplements, Volume 7, Issue suppl_L, November 2005, Pages L16–L20, https://doi.org/10.1093/eurheartj/sui081
Close - Share Icon Share
Abstract
High levels of LDL cholesterol (LDL-c) are causally related to the development of atherosclerosis. A large body of epidemiological evidence supports a direct relationship between the level of serum LDL-c and the risk of cardiovascular disease, and clinical trials have shown that lowering LDL-c with statins leads to a 30% decrease in the risk of cardiovascular events. However, many patients taking statins continue to suffer cardiovascular disease, highlighting the need both to improve the number of patients reaching LDL-c therapeutic goals and to develop new therapeutic targets. The apoB/apoA-I ratio has been shown to be a major contributor to the risk of myocardial infarction and hence increasing HDL-cholesterol (HDL-c) offers a promising approach to further decrease cardiovascular risk. Clinical trial data with fibrates and nicotinic acid, together with preliminary data on potent new treatments, support HDL-c as a therapeutic target. The cardioprotective effect of HDL-c may not solely be related to its role in reverse cholesterol transport.
Treatments to reduce LDL-cholesterol
A large body of epidemiological evidence supports a direct relationship between the level of serum LDL-cholesterol (LDL-c) and the risk of cardiovascular disease. Major trials comparing the effect of statins with placebo in a variety of populations have shown that lowering LDL-c reduces the risk of experiencing a cardiovascular event by 30%. Over 70 000 patients have completed placebo-controlled primary and secondary prevention trials with statins.
A trial of particular interest when considering obese patients or diabetic patients is the Collaborative Atorvastatin Diabetes Study (CARDS).1 This placebo-controlled study involved 2838 patients with Type 2 diabetes and at least one other cardiovascular risk factor who received atorvastatin 10 mg daily, or placebo, for a median duration of 3.9 years. Patients did not have high baseline concentrations of LDL-c. Treatment with atorvastatin decreased the risk of the primary endpoint (first occurrence of acute coronary events, coronary revascularization, or stroke) by 37% (P=0.001). These major cardiovascular events occurred in 9.0% of patients taking placebo and in 5.8% of those on atorvastatin.
However, CARDS showed that even with use of a potent statin, the risk of an event remains high in this population. It is of note that the mean level of LDL-c at baseline (3.02 mmol/L in the placebo group and 3.04 mmol/L in the atorvastatin group) was similar to that typically seen in obese patients and patients with the metabolic syndrome, as well as in diabetic patients.
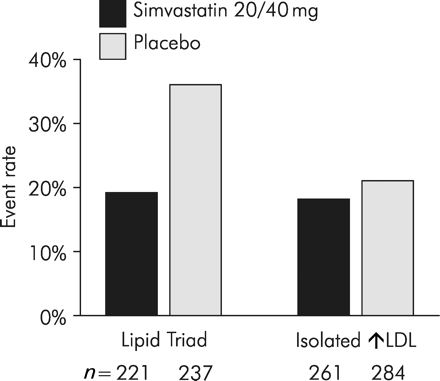
Post hoc analysis of the Scandinavian Simvastatin Survival Study (4S) showed that the subgroup of patients who had the combined abnormalities of elevated triglycerides and low HDL cholesterol (HDL-c), together with high LDL-c—the lipid triad—had the highest risk of major coronary events on placebo, but also achieved the greatest benefit from simvastatin therapy: there was a significantly greater reduction in major coronary events in the lipid triad subgroup compared with the subgroup of patients with isolated LDL-c elevation (P=0.03 for treatment by subgroup interaction)2 (Figure 1).
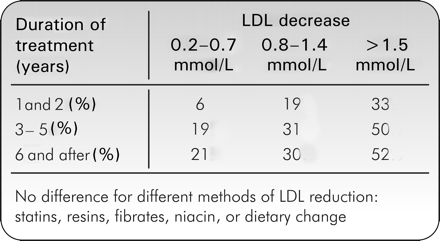
A meta-analysis of trials involving 148 321 patients has assessed the magnitude of cardiovascular risk reduction that can be achieved by reduction in LDL-c.3 It showed a clear relationship between LDL-c reduction, duration of treatment, and reduction in ischaemic heart disease events. With an LDL-c decrease of more than 1.5 mmol/L, there was a 52% reduction in the risk of ischaemic heart disease events after 5 years' treatment (Figure 2). These results are in agreement with the epidemiological observations in the INTERHEART4 study and the Apolipoprotein-related MOrtality RISk (AMORIS) study.5
The meta-analysis also indicated that the benefit derived by LDL decrease was independent of the therapy. This means that lowering LDL-c levels by 10%, for example, leads to the same decrease in cardiovascular events with any drug (statin, fibrate, nicotinic acid, and resins) and even with a dietary approach.
Treatments to increase HDL-c
The fact that a high proportion of patients taking statins continue to suffer cardiovascular events indicates that, in addition to improving the number of patients reaching LDL-c goals, there is also a need to consider new therapeutic targets to help reduce cardiovascular disease.
HDL-c is inversely related to the risk of cardiovascular disease and there are several lines of research supporting HDL-c as a promising therapeutic target. The prospective AMORIS study,5 which involved 175 553 individuals, indicated that apolipoprotein (apo) B, the apoB/apoA-I ratio, and apoA-I are highly predictive in evaluation of cardiovascular risk. More recently, the INTERHEART case–control study,4 involving 15 152 cases and 14 820 controls, showed the ratio of apo B/apoA-1 to be a major predictor of myocardial infarction at the population level. Interventions associated with raising HDL-c increase apoA-1 levels and, therefore, offer a promising approach to further decreasing cardiovascular risk.
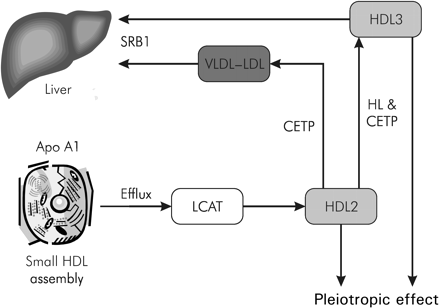
Increased understanding of the metabolism of HDL particles has identified potential targets. This metabolism is a complex process. The first step is cholesterol efflux by nascent, lipid-free apoA-I particles. These particles are progressively enriched with cholesterol and, under the influence of lecithin–cholesterol acyl transferase (LCAT) enzyme, which catalyses the esterification of free cholesterol, form the large cholesterol-enriched HDL2 particles. HDL2 particles can deliver cholesterol to the liver by two different pathways: the exchange of cholesterol through VLDL and LDL particles and the transformation into HDL3 for direct delivery of cholesterol to the liver (Figure 3).
Although the cardioprotective effect of HDL has been attributed to its role in this process of reverse cholesterol transport, other properties may also confer an important protective mechanism. HDL-c has pleiotropic effects, which are particularly relevant in relation to the treatment of obese patients. It has recently been shown that patients with metabolic syndrome have high oxidative stress and it is also known that obese patients have high C-reactive protein levels. The pleiotropic effects of HDL-c include a potent suppression of vascular inflammation as well as antioxidative properties.
One group of drugs that can influence HDL-c levels is the fibrates. A recent meta-analysis of 53 randomized, placebo controlled trials, involving 14 448 patients, showed that fibrate compounds (except clofibrate) can produce a 10% increase in HDL-c and an associated 36% decrease in triglycerides.6
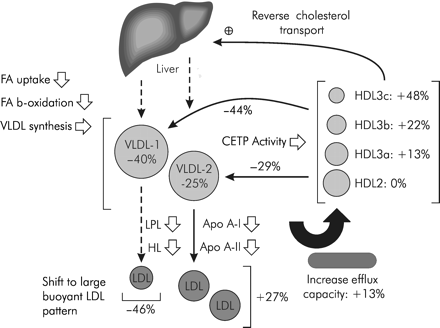
Guerin et al.7 studied the effect of a fibrate (ciprofibrate) on the lipoprotein subspecies in 10 patients with Type IIB combined hyperlipidaemia (Figure 4). This type of hyperlipidaemia is characterized by elevated levels of triglyceride-rich lipoproteins (including VLDL), a predominance of small dense LDL particles, and low concentrations of HDL. The main effect of the drug was to decrease plasma concentrations of large VLDL-1 (reduced by 40%) and of small VLDL-2 (reduced by 25%). The effects of fibrates are all driven by this action on triglycerides. The predominant impact of the drugs on the large VLDL particles explains the shift towards a buoyant-like LDL pattern: there was a dramatic decrease of dense LDL particles and an increase in light LDL particles. This observation could explain why globally there is no effect on LDL particles or even a slight increase in LDL-c.
The study also investigated the effect of the fibrate on HDL levels. In addition to an increase in HDL-c, there was found to be a profound modification in the distribution of HDL subspecies. HDL2 particles remained stable, whereas there was a significant increase in HDL3 particles. This can be explained by the dual effect of the action of fibrates on lipase and on cholesteryl ester transfer protein (CETP) activity. There was also shown to be a 13% increase in cholesterol efflux capacity.
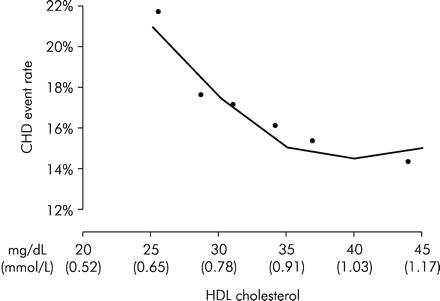
Patients in the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) had a lipid pattern similar to that seen in patients with the metabolic syndrome, i.e. normal LDL-c, slightly elevated triglycerides and low HDL-c. The trial compared gemfibrozil with placebo in 2531 men with coronary heart disease.8 After median follow-up of 5.1 years, in the active treatment group there was a 22% reduction in the relative risk of non-fatal myocardial infarction or death from coronary causes (P=0.006) and a 24% reduction in the combined outcome of death from coronary heart disease, non-fatal myocardial infarction, and stroke (P<0.001). There was a 6% increase in mean HDL-c level and a 31% decrease in triglyceride level in the gemfibrozil group; LDL-c levels did not differ significantly between the groups.8 The small increase in HDL-c explained almost all of the beneficial effects observed. The Expert Group on HDL Cholesterol9 notes that multivariate regression analysis shows that HDL-c concentration during gemfibrozil treatment was highly inversely predictive of coronary events, whereas LDL-c and triglycerides were not (Figure 5).
Nicotinic acid (niacin) is another drug that increases HLD-c level. The National Cholesterol Education Program ATP III report10 comments that nicotinic acid favourably affects all lipids and lipoproteins when given in pharmacological doses: it is effective at raising HDL-c and transforms small dense LDL particles into normal sized LDL particles. The report states that, among lipid lowering agents, nicotinic acid is the most effective HDL-raising drug. Nicotinic acid also causes a moderate reduction in LDL-c levels and it is the most effective drug for decreasing lipoprotein (a) [Lp(a)] levels.
The original immediate-release formulation of nicotinic acid is not well tolerated but a modified-release formulation with improved tolerability is now available. Clinical trials with this modified release formulation have shown dose-related effects on lipids comparable to those observed with the immediate release formulation.11 In meta-analyses of all trials with nicotinic acid, the increase in HDL-c was 21%. This level of HDL-c increase, which is twice that observed with fibrates, is the same as that which was observed in the STORM study with a combination of sibutramine and lifestyle modification.12
The Coronary Drug Project trial compared immediate release nicotinic acid and placebo in men with coronary heart disease. In the active treatment group, despite a high rate of drop out because of poor tolerability, over an observation period of 5 years there was a significant reduction in non-fatal myocardial infarction and stroke (P<0.05).13 The incidence of non-fatal MI was 8.9% for the 1119 patients randomized to nicotinic acid compared with 12.2% for the 2789 patients who received placebo (a reduction of 27% with nicotinic acid treatment). Total mortality was not significantly different in the two groups at 5 years. Stroke and transient ischaemic attack was reduced by 26% with nicotinic acid and cardiovascular surgery was reduced by 47%.
Combination therapy
A further approach to lipid lowering therapy is to combine agents that increase HDL-c with agents that reduce LDL-c. The combination of nicotinic acid and statin leads to a profound modification of both LDL-c (decreased by up to 50%), mainly due to the action of statin, and HDL-c (increased by up to 30%), due to the effect of both drugs.14
There are as yet no large clinical trials assessing efficacy of this combination therapy in terms of reduction in coronary risk. However, one double-blind study in 160 patients with coronary heart disease, low HDL-c levels, and normal LDL-c levels reported that, over 3 years, LDL-c decreased by 42% and HDL-c increased by 26% with a combination of simvastatin and nicotinic acid. In addition, the combination decreased the atherosclerosis burden in these high-risk patients: mean stenosis progressed by 3.9% in the placebo group and regressed by 0.4% in the simvastatin/nicotinic acid group (P<0.001 compared with placebo). The clinical endpoint was occurrence of a first cardiovascular event (death, myocardial infarction, stroke, or revascularization). The frequency of this endpoint was 24% with placebo and 3% with simvastatin/nicotinic acid.15 Interpretation of this trial requires caution, because the number of patients and the number of events were low but the data are promising and show the potential benefits of this type of combination therapy.16
New therapeutic approaches
Inhibitors of CETP are the most potent drugs for raising HDL-c level currently under development. These drugs can increase HDL-c by 50–60% (depending on whether or not they are given in combination with a statin).
The CETP inhibitor torcetrapib was investigated in a single-blind, placebo controlled trial in 19 patients with low levels of HDL-c [<1.0 mmol/L (40 mg/dL)], nine of whom were also treated with atorvastatin (20 mg daily).17 Treatment with torcetrapib increased plasma levels of HDL-c by 61% (P<0.001) and 46% (P=0.001) in the atorvastatin and non-atorvastatin cohorts, respectively. In a further phase of the trial, in which patients who had not received atorvastatin were given a higher dose of the CETP inhibitor, HDL-c was increased by 106% (P<0.001). LDL-c levels were also decreased with torcetrapib, both when administered on its own or with statin.
The potential clinical benefit of treatment with HDL-c or an HDL-c mimetic is also being investigated, with promising preliminary results. A double-blind randomized trial was carried out to assess the effect of treatment with recombinant apoA-I Milano on coronary atherosclerosis. ApoA-I Milano is a mutation of apoA-I that is associated with very low plasma levels of HDL-c (0.25–0.78 mmol/L; 10–30 mg/dL) but low levels of cardiovascular disease. It has been shown that these particles are catabolized rapidly and can deliver cholesterol to the liver. The study involved a group of 57 high-risk patients with acute coronary syndromes, 47 of whom completed the protocol. Patients received placebo or weekly infusions of apoA-I Milano phospholipid complexes (ETC-216). The primary endpoint of the study was change in per cent atheroma volume (follow-up minus baseline), as measured by intravascular ultrasound. After only 5 weeks, patients who received apoA-I had reduced atherosclerosis burden: in the combined treatment group (patients who received either high- or low-dose ETC-216) the mean per cent atheroma volume decreased by 1.06% (median value −0.81%; 95% confidence intervals −1.53 to −0.34; P=0.02 compared with baseline). For the placebo group, the mean per cent atheroma volume increased by 0.14% (median 0.03%; 95% CI −1.11 to 1.43%; P=0.97 compared with baseline).18
These data, which remain to be confirmed, indicate that the magnitude by which cardiovascular disease can be reduced by increasing HDL-c is potentially even greater than that produced by decreasing LDL-c.
Conclusion
Statin therapy is associated with at least a 30% decrease in the risk of cardiovascular events. Treatment is associated with reduced progression of atherosclerosis. Higher statin doses are associated with greater benefit, but with increased risk of dose-related side effects. Increasing HDL-c offers a promising approach to further decrease cardiovascular risk and reduce atherosclerotic burden. Fibrates and nicotinic acid are both associated with significant increases in HDL-c, which may explain the beneficial effect that has been observed in large clinical trials.
Key points
Lowering LDL-cholesterol with statins leads to a 30% decrease in risk of cardiovascular events.
Additional therapeutic targets are needed and there is particular interest in treatments that increase levels of HDL-cholesterol.
Preliminary data with novel treatments indicate that the reduction in cardiovascular disease achieved by increasing HDL-cholesterol is potentially greater than that produced by decreasing LDL-cholesterol.
The cardioprotective effect of HDL-cholesterol may not solely be related to its role in reverse cholesterol transport.
Conflict of interest: The author has lectured at Abbott-sponsored symposia.
Figure 1 Effect of simvastatin on major coronary event rates by subgroups defined by baseline HDL-c and triglycerides. (Adapted with permission from Ballantyne et al.2)
Figure 2 Reduction in risk of ischaemic heart disease events (relative odds reduction) according to number of years in trial and reduction in LDL-c from a meta-analysis of 49 trials. Fifty two per cent of patients had a history of coronary heart disease at entry. (Adapted from Law et al.3)
Figure 3 Small HDL particles are assembled within the mitochondria. Increased understanding of the process by which these particles are transported and metabolized has identified potential therapeutic targets for cardiovascular protection. Apo A1, apolipoprotein A1; SRB 1, scavenger receptor B-1; VLDL, very low density lipoprotein.
Figure 4 Action of ciprofibrate in patients with combined hyperlipidaemia.7 The drug induces preferential reduction in atherogenic apoB-containing lipoprotein subclasses, i.e. large VLDL-1, small VLDL-2, and dense LDL subspecies, and in CETP-mediated cholesteryl ester transfer from HDL to VLDL subfractions. There is also significant increase in HDL-3 levels. (Adapted with permission from Guerin et al.7)
Figure 5 The relation between HDL-c concentration after gemfibrozil treatment and coronary events in the VA-HIT trial. (Based on Rubins et al.8)
References
Colhoun HM, Betteridge DJ, Durrington PN et al.; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial.
Ballantyne CM, Olsson AG, Cook TJ et al. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S.
Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis.
Yusuf S, Hawken S, Ounpuu S et al.; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study.
Walldius G, Junger I, Holme I et al. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study.
Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials.
Guerin M, Le Goff W, Frisdal E et al. Action of ciprofibrate in type IIb hyperlipoproteinemia: modulation of the atherogenic lipoprotein phenotype and stimulation of high-density lipoprotein-mediated cellular cholesterol efflux.
Rubins HB, Robins SJ, Collins D et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group.
Sacks FM; Expert Group on HDL Cholesterol. The role of high-density lipoprotein (HDL) cholesterol in the prevention and treatment of coronary heart disease: expert group recommendations.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report.
Goldberg A, Alagona P Jr, Capuzzi DM et al. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia.
James WPT, Astrup A, Finer N et al. for the STORM study group. Effect of sibutramine on weight maintenance after weight loss: a randomised trial.
Jacobson TA. Combination lipid-altering therapy: an emerging treatment paradigm for the 21st century.
Brown BG, Zhao X-Q, Chait A et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease.
Freedman JE. Antioxidant versus lipid-altering therapy—some answers, more questions.
Brousseau ME, Schaefer EJ, Wolfe ML et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol.