-
PDF
- Split View
-
Views
-
Cite
Cite
François Mach, Colin Baigent, Alberico L Catapano, Konstantinos C Koskinas, Manuela Casula, Lina Badimon, M John Chapman, Guy G De Backer, Victoria Delgado, Brian A Ference, Ian M Graham, Alison Halliday, Ulf Landmesser, Borislava Mihaylova, Terje R Pedersen, Gabriele Riccardi, Dimitrios J Richter, Marc S Sabatine, Marja-Riitta Taskinen, Lale Tokgozoglu, Olov Wiklund, ESC Scientific Document Group , 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), European Heart Journal, Volume 41, Issue 1, 1 January 2020, Pages 111–188, https://doi.org/10.1093/eurheartj/ehz455
Close - Share Icon Share
 For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehz455#supplementary-data
For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehz455#supplementary-data
Table of contents
Abbreviations and acronyms 114
1 Preamble 116
2 Introduction 118
2.1 What is new in the 2019 Guidelines? 118
3 What is cardiovascular disease prevention? 118
3.1 Definition and rationale 118
3.2 Development of the Joint Task Force Guidelines for the management of dyslipidaemias 118
4 Total cardiovascular risk 118
4.1 Total cardiovascular risk estimation 118
4.1.1 Rationale for assessing total cardiovascular disease risk 121
4.1.2 How to use the risk estimation charts 124
4.2 Risk levels 125
4.2.1 Role of non-invasive cardiovascular imaging techniques in the assessment of total cardiovascular disease risk 126
4.2.2 Risk-based intervention strategies 127
5 Lipids and lipoproteins 127
5.1 Biological role of lipids and lipoproteins 127
5.2 Role of lipids and lipoproteins in the pathophysiology of atherosclerosis 127
5.3 Evidence for the causal effects of lipids and lipoproteins on the risk of atherosclerotic cardiovascular disease 128
5.3.1 Low-density lipoprotein cholesterol and risk of atherosclerosis 128
5.3.2 Triglyceride-rich lipoproteins and risk of atherosclerosis 128
5.3.3 High-density lipoprotein cholesterol and risk of atherosclerosis 129
5.3.4 Lipoprotein(a) and risk of atherosclerosis 129
5.4 Laboratory measurement of lipids and lipoproteins 129
5.4.1 Lipoprotein measurement 129
5.4.2 Lipid measurements 20
5.4.3 Fasting or non-fasting? 130
5.5 Recommendations for measuring lipids and lipoproteins to estimate risk of atherosclerotic cardiovascular disease 130
6 Treatment targets and goals 131
7 Lifestyle modifications to improve the plasma lipid profile 132
7.1 Influence of lifestyle on total cholesterol and low-density lipoprotein cholesterol levels 134
7.2 Influence of lifestyle on triglyceride levels 134
7.3 Influence of lifestyle on high-density lipoprotein cholesterol levels 135
7.4 Lifestyle recommendations to improve the plasma lipid profile 135
7.4.1 Body weight and physical activity 135
7.4.2 Dietary fat 135
7.4.3 Dietary carbohydrate and fibre 136
7.4.4 Alcohol 136
7.4.5 Smoking 136
7.5 Dietary supplements and functional foods for the treatment of dyslipidaemias 136
7.5.1 Phytosterols 136
7.5.2 Monacolin and red yeast rice 136
7.5.3 Dietary fibre 137
7.5.4 Soy 137
7.5.5 Policosanol and berberine 27
7.5.6 n-3 unsaturated fatty acids 137
8 Drugs for treatment of dyslipidaemias 137
8.1 Statins 137
8.1.1 Mechanism of action 137
8.1.2 Effects on lipids 137
8.1.2.1 Low-density lipoprotein cholesterol 137
8.1.2.2 Triglycerides 137
8.1.2.3 High-density lipoprotein cholesterol 137
8.1.2.4 Lipoprotein(a) 137
8.1.3 Other effects of statins 138
8.1.3.1 Effect on cardiovascular morbidity and mortality 138
8.1.4 Adverse effects and interactions of statins 138
8.1.4.1 Adverse effects on muscle 138
8.1.4.2 Adverse effects on the liver 139
8.1.4.3 Increased risk of new-onset diabetes mellitus 139
8.1.4.4 Increased risk of haemorrhagic stroke 139
8.1.4.5 Adverse effects on kidney function 139
8.1.4.6 Interactions 139
8.2 Cholesterol absorption inhibitors 140
8.2.1 Mechanism of action 140
8.2.2 Effects on lipids 140
8.2.3 Effect on cardiovascular morbidity and mortality 140
8.2.4 Adverse effects and interactions 140
8.3 Bile acid sequestrants 140
8.3.1 Mechanism of action 140
8.3.2 Effects on lipids 140
8.3.3 Effect on cardiovascular morbidity and mortality 140
8.3.4 Adverse effects and interactions 141
8.4 Proprotein convertase subtilisin/kexin type 9 inhibitors 141
8.4.1 Mechanism of action 141
8.4.2 Effects on lipids 141
8.4.2.1 Low-density lipoprotein cholesterol 141
8.4.2.2 Triglycerides and high-density lipoprotein cholesterol 141
8.4.2.3 Lipoprotein(a) 141
8.4.3 Effect on cardiovascular morbidity and mortality 141
8.4.4 Adverse effects and interactions 142
8.5 Lomitapide 142
8.6 Mipomersen 142
8.7 Fibrates 142
8.7.1 Mechanism of action 142
8.7.2 Effects on lipids 142
8.7.3 Effect on cardiovascular morbidity and mortality 143
8.7.4 Adverse effects and interactions 143
8.8 n-3 fatty acids 143
8.8.1 Mechanism of action 143
8.8.2 Effects on lipids 143
8.8.3 Effect on cardiovascular morbidity and mortality 143
8.8.4 Safety and interactions 144
8.9 Nicotinic acid 144
8.10 Cholesteryl ester transfer protein inhibitors 144
8.11 Future perspectives 144
8.11.1 New approaches to reduce low-density lipoprotein cholesterol 144
8.11.2 New approaches to reduce triglyceride-rich lipoproteins and their remnants 144
8.11.3 New approaches to increase high-density lipoprotein cholesterol 145
8.11.4 New approaches to reduce lipoprotein(a) levels 145
8.12 Strategies to control plasma cholesterol 145
8.13 Strategies to control plasma triglycerides 145
9 Management of dyslipidaemias in different clinical settings 148
9.1 Familial dyslipidaemias 148
9.1.1 Familial combined hyperlipidaemia 148
9.1.2 Familial hypercholesterolaemia 148
9.1.2.1 Heterozygous familial hypercholesterolaemia 148
9.1.2.2 Homozygous familial hypercholesterolaemia 151
9.1.2.3 Familial hypercholesterolaemia in children 151
9.1.3 Familial dysbetalipoproteinaemia 151
9.1.4 Genetic causes of hypertriglyceridaemia 151
9.1.4.1 Action to prevent acute pancreatitis in severe hypertriglyceridaemia 151
9.1.5 Other genetic disorders of lipoprotein metabolism 152
9.2 Women 152
9.2.1 Effects of statins in primary and secondary prevention 152
9.2.2 Non-statin lipid-lowering drugs 152
9.2.3 Hormone therapy 152
9.3 Older people 152
9.3.1 Effects of statins in primary and secondary prevention 153
9.3.2 Adverse effects, interactions, and adherence 153
9.4 Diabetes and metabolic syndrome 153
9.4.1 Specific features of dyslipidaemia in insulin resistance and type 2 diabetes 153
9.4.2 Evidence for lipid-lowering therapy 154
9.4.2.1 Low-density lipoprotein cholesterol 154
9.4.2.1 Triglycerides and high-density lipoprotein cholesterol 154
9.4.3 Type 1 diabetes 155
9.4.4 Management of dyslipidaemia for pregnant women with diabetes 155
9.5 Patients with acute coronary syndromes and patients undergoing percutaneous coronary intervention 156
9.5.1 Lipid-lowering therapy in patients with acute coronary syndromes 156
9.5.1.1 Statins 156
9.5.1.2 Ezetimibe 156
9.5.1.3 Proprotein convertase subtilisin/kexin type 9 inhibitors 156
9.5.1.4 n-3 polyunsaturated fatty acids 157
9.5.1.5 Cholesteryl ester transfer protein inhibitors 157
9.5.2 Lipid-lowering therapy in patients undergoing percutaneous coronary intervention 157
9.6 Stroke 158
9.7 Heart failure and valvular diseases 158
9.7.1 Prevention of incident heart failure in coronary artery disease patients 158
9.7.2 Chronic heart failure 158
9.7.3 Valvular heart diseases 158
9.8 Chronic kidney disease 159
9.8.1 Lipoprotein profile in chronic kidney disease 159
9.8.2 Evidence for risk reduction through statin-based therapy in patients with chronic kidney disease 159
9.8.3 Safety of lipid management in patients with chronic kidney disease 159
9.9 Transplantation 160
9.10 Peripheral arterial disease 160
9.10.1 Lower extremity arterial disease 160
9.10.2 Carotid artery disease 161
9.10.3 Retinal vascular disease 161
9.10.4 Secondary prevention in patients with abdominal aortic aneurysm 161
9.10.5 Renovascular atherosclerosis 161
9.11 Other special populations at risk of atherosclerotic cardiovascular disease 161
10 Inflammation 161
11 Monitoring of lipids and enzymes in patients on lipid-lowering therapy 162
12 Cost-effectiveness of cardiovascular disease prevention by lipid modification 162
13 Strategies to encourage adoption of healthy lifestyle changes and adherence to lipid-modifying therapies 166
14 Key messages 166
15 Gaps in evidence 167
16 Evidence-based ‘to do’ and ‘not to do’ messages from the Guidelines 168
17 Supplementary data 170
18 Appendix 170
19 References 171
Tables of Recommendations
Recommendations for cardiovascular imaging for risk assessment of atherosclerotic cardiovascular disease 127
Recommendations for cardiovascular disease risk estimation 127
Recommendations for lipid analyses for cardiovascular disease risk estimation 131
Recommendations for treatment goals for low-density lipoprotein cholesterol 132
Recommendations for pharmacological low-density lipoprotein cholesterol lowering 145
Recommendations for drug treatment of patients with hypertriglyceridaemia 148
Recommendations for the detection and treatment of patients with heterozygous familial hypercholesterolaemia 150
Recommendations for the treatment of dyslipidaemias in older people (aged >65 years) 153
Recommendations for the treatment of dyslipidaemias in diabetes mellitus 155
Recommendations for lipid-lowering therapy in very- high-risk patients with acute coronary syndromes 157
Recommendations for lipid-lowering therapy in very-high-risk patients undergoing percutaneous coronary intervention 157
Recommendations for lipid-lowering therapy for prevention of atherosclerotic cardiovascular disease events in patients with prior ischaemic stroke 158
Recommendations for the treatment of dyslipidaemias in chronic heart failure or valvular heart diseases 159
Recommendations for lipid management in patients with moderate to severe (Kidney Disease Outcomes Quality Initiative stages 3–5) chronic kidney disease 159
Recommendations for low-density lipoprotein lowering in solid organ transplant patients 160
Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease) 161
List of tables
Table 1 Classes of recommendations 117
Table 2 Levels of evidence 117
Table 3 New recommendations, and new and revised concepts 119
Table 4 Cardiovascular risk categories 125
Table 5 Intervention strategies as a function of total cardiovascular risk and untreated low-density lipoprotein cholesterol levels 126
Table 6 Physical and chemical characteristics of human plasma lipoproteins 128
Table 7 Treatment targets and goals for cardiovascular disease prevention 133
Table 8 Impact of specific lifestyle changes on lipid levels 134
Table 9 Food choices to lower low-density lipoprotein cholesterol and improve the overall lipoprotein profile 135
Table 10 Drugs potentially interacting with statins metabolized by cytochrome P450 3A4 leading to increased risk of myopathy and rhabdomyolysis 139
Table 11 Genetic disorders of lipoprotein metabolism 149
Table 12 Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolaemia 149
Table 13 Summary of recommendations for monitoring lipids and enzymes in patients, before and on lipid-lowering therapy 163
List of figures
Figure 1 Systematic Coronary Risk Estimation chart for European populations at high cardiovascular disease risk 122
Figure 2 Systematic Coronary Risk Estimation chart for European populations at low cardiovascular disease risk 123
Figure 3 Expected clinical benefit of low-density lipoprotein cholesterol-lowering therapies 146
Figure 4 Treatment goals and algorithm for low-density lipoprotein cholesterol-lowering according to cardiovascular disease risk 147
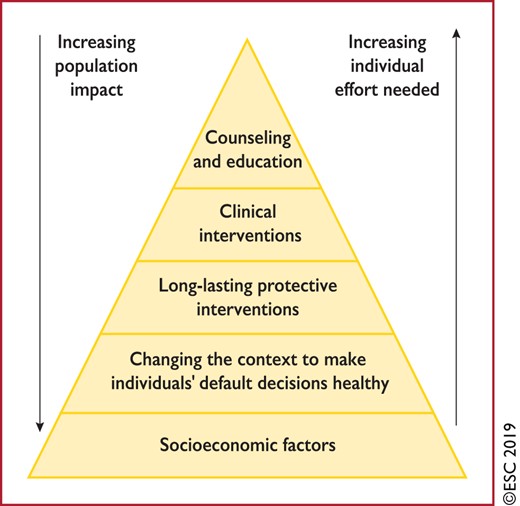
Figure 5 Health impact pyramid 164
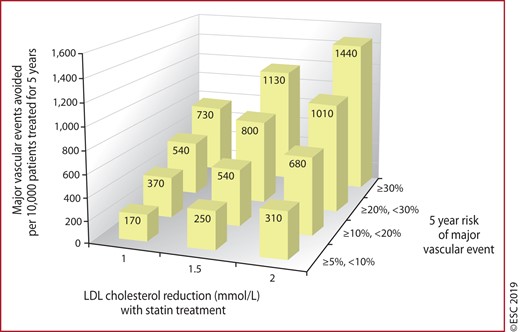
Figure 6 Absolute reductions in major vascular events with statin therapy 165
List of boxes
Box 1 How to use the risk estimation charts 124
Box 2 Risk estimation charts for different countries 124
Box 3 Qualifiers 124
Box 4 Factors modifying Systematic Coronary Risk Estimation risks 124
Box 5 Risk estimation: key messages 125
Box 6 Management of dyslipidaemia in women 152
Box 7 Summary of dyslipidaemia in metabolic syndrome and type 2 diabetes mellitus 154
Box 8 Key messages 165
Box 9 Gaps in the evidence 165
Box 10 Methods for enhancing adherence to lifestyle changes 166
Abbreviations and acronyms
- ABI
Ankle–brachial index
- ACCELERATE
Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High-Risk for Vascular Outcomes
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACS
Acute coronary syndrome
- ALT
Alanine aminotransferase
- ANGPTL3
Angiopoietin-like protein 3
- Apo
Apolipoprotein
- ART
Antiretroviral treatment
- ASCEND
A Study of Cardiovascular Events iN Diabetes
- ASCOT-LLA
Anglo-Scandinavian Cardiac Outcomes Trial – Lipid-Lowering Arm
- ASCVD
Atherosclerotic cardiovascular disease
- ASSIGN
CV risk estimation model from the Scottish Intercollegiate Guidelines Network
- AURORA
A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events
- b.i.d.
Twice a day (bis in die)
- BIOSTAT-CHF
BIOlogy Study to TAilored Treatment in Chronic Heart Failure
- BIP
Bezafibrate Infarction Prevention
- BMI
Body mass index
- BP
Blood pressure
- CABG
Coronary artery bypass graft surgery
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- CANTOS
Canakinumab Antiinflammatory Thrombosis Outcome Study
- CETP
Cholesteryl ester transfer protein
- CHD
Coronary heart disease
- CI
Confidence interval
- CIID
Chronic immune-mediated inflammatory diseases
- CIRT
Cardiovascular Inflammation Reduction Trial
- CK
Creatine kinase
- CKD
Chronic kidney disease
- COM-B
Capability, Opportunity and Motivation
- CORONA
Controlled Rosuvastatin Multinational Trial in Heart Failure
- CPG
Committee for Practice Guidelines
- CT
Computed tomography
- CTT
Cholesterol Treatment Trialists
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CYP
Cytochrome P450
- 4D
Die Deutsche Diabetes Dialyse Studie
- dal-OUTCOMES
Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome
- DASH
Dietary Approaches to Stop Hypertension
- DGAT-2
Diacylglycerol acyltransferase-2
- DHA
Docosahexaenoic acid
- DM
Diabetes mellitus
- EAPC
European Association of Preventive Cardiology
- EAS
European Atherosclerosis Society
- EBBINGHAUS
Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects
- eGFR
Estimated glomerular filtration rate
- EMA
European Medicines Agency
- EPA
Eicosapentaenoic acid
- ESC
European Society of Cardiology
- EVOLVE
EpanoVa fOr Lowering Very high triglyceridEs
- EVOPACS
EVOlocumab for early reduction of LDL-cholesterol levels in patients with Acute Coronary Syndromes
- FCH
Familial combined hyperlipidaemia
- FCS
Familial chylomicronaemia syndrome
- FDA
US Food and Drug Administration
- FH
Familial hypercholesterolaemia
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FOCUS
Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention
- FOURIER
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk
- GFR
Glomerular filtration rate
- GI
Gastrointestinal
- GISSI
Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico
- HbA1c
Glycated haemoglobin
- HeFH
Heterozygous familial hypercholesterolaemia
- HDL
High-density lipoprotein
- HDL-C
High-density lipoprotein cholesterol
- HF
Heart failure
- HHS
Helsinki Heart Study
- HIV
Human immunodeficiency virus
- HMG-CoA
Hydroxymethylglutaryl-coenzyme A
- HoFH
Homozygous familial hypercholesterolaemia
- HPS2-THRIVE
Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events
- HR
Hazard ratio
- HTG
Hypertriglyceridaemia
- IDEAL
Incremental Decrease In End-points Through Aggressive Lipid-lowering
- IDL
Intermediate-density lipoproteins
- IL
Interleukin
- ILLUMINATE
Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- IPD
Individual participant data
- JUPITER
Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- KDIGO
Kidney Disease: Improving Global Outcomes
- LCAT
Lecithin cholesterol acyltransferase
- LDL
Low-density lipoprotein
- LDL-C
Low-density lipoprotein cholesterol
- LDLR
Low-density lipoprotein receptor
- LEAD
Lower extremity arterial disease
- LEADER
Lower Extremity Arterial Disease Event Reduction
- LPL
Lipoprotein lipase
- Lp(a)
Lipoprotein(a)
- mAb
Monoclonal antibody
- MACE
Major adverse cardiovascular events
- MESA
Multi-Ethnic Study of Atherosclerosis
- MetS
Metabolic syndrome
- MI
Myocardial infarction
- mRNA
Messenger RNA
- MTP
Microsomal triglyceride transfer protein
- NAFLD
Non-alcoholic fatty liver disease
- NNT
Number needed to treat
- NPC1L1
Niemann-Pick C1-like protein 1
- NSTE-ACS
Non-ST elevation acute coronary syndrome
- o.d.
Once a day (omni die)
- ODYSSEY Outcomes
Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab
- PAD
Peripheral arterial disease
- PCI
Percutaneous coronary intervention
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- PPAR-α
Peroxisome proliferator-activated receptor-α
- PREDIMED
Prevención con Dieta Mediterránea
- PROCAM
Prospective Cardiovascular Munster Study
- PROMINENT
Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN PatiENts With DiabeTes
- PUFA
Polyunsaturated fatty acid
- PURE
Prospective Urban Rural Epidemiology
- RA
Rheumatoid arthritis
- RCT
Randomized controlled trial
- REDUCE-IT
Reduction of Cardiovascular Events with EPA-Intervention Trial
- REVEAL
Randomized EValuation of the Effects of Anacetrapib Through Lipid modification
- RR
Relative risk
- RYR
Red yeast rice
- SAMS
Statin-associated muscle symptoms
- SBP
Systolic blood pressure
- SCORE
Systematic Coronary Risk Estimation
- SEAS
Simvastatin and Ezetimibe in Aortic Stenosis
- SECURE-PCI
Statins Evaluation in Coronary Procedures and Revascularization
- SFA
Saturated fatty acid
- SHARP
Study of Heart and Renal Protection
- siRNA
Small interfering RNA
- SMI
Severe mental illness
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- STAREE
STAtin Therapy for Reducing Events in the Elderly
- STEMI
ST-elevation myocardial infarction
- STRENGTH
Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia
- TC
Total cholesterol
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TGs
Triglycerides
- TIA
Transient ischaemic attack
- TIMI
Thrombolysis In Myocardial Infarction
- TNF
Tumour necrosis factor
- TNT
Treating to New Targets
- TRL
Triglyceride-rich lipoprotein
- ULN
Upper limit of normal
- VA-HIT
Veterans Affairs High Density Lipoprotein Intervention Trial
- VITAL
VITamin D and OmegA-3 Trial
- VLDL
Very low-density lipoprotein
- WHO
World Health Organization
- WOSCOPS
West of Scotland Coronary Prevention Study
1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and its partners such as European Atherosclerosis Society (EAS), as well as by other societies and organisations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
The ESC carries out a number of registries which are essential to assess diagnostic/therapeutic processes, use of resources and adherence to Guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on data collected during routine clinical practice.
The guidelines are developed together with derivative educational material addressing the cultural and professional needs for cardiologists and allied professionals. Collecting high-quality observational data, at appropriate time interval following the release of ESC Guidelines, will help evaluate the level of implementation of the Guidelines, checking in priority the key end points defined with the ESC Guidelines and Education Committees and Task Force members in charge.
The Members of this Task Force were selected by the ESC and EAS, including representation from relevant ESC sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field from both societies undertook a comprehensive review of the published evidence for management of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined ESC scales, as outlined in the tables below.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period were notified to the ESC and EAS Chairpersons and updated. The Task Force received its entire financial support from the ESC and EAS without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG and EAS for publication in the European Heart Journal and Atherosclerosis Journal. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC/EAS Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, booklets with essential messages, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access the full text version of the Guidelines, which is freely available via the ESC and EAS websites and hosted on their journals’ websites (EHJ and Atherosclerosis Journal). The National Cardiac Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the ESC/EAS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC/EAS Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
The previous ESC/EAS lipid Guidelines were published in August 2016.1 The emergence of a substantial body of evidence over the last few years has required new, up-to-date Guidelines.
New evidence has confirmed that the key initiating event in atherogenesis is the retention of low-density lipoprotein (LDL) cholesterol (LDL-C) and other cholesterol-rich apolipoprotein (Apo) B-containing lipoproteins within the arterial wall.2 Several recent placebo-controlled clinical studies have shown that the addition of either ezetimibe or anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies (mAbs) to statin therapy provides a further reduction in atherosclerotic cardiovascular disease (ASCVD) risk, which is directly and positively correlated with the incrementally achieved absolute LDL-C reduction. Furthermore, these clinical trials have clearly indicated that the lower the achieved LDL-C values, the lower the risk of future cardiovascular (CV) events, with no lower limit for LDL-C values, or ‘J’-curve effect. In addition, studies of the clinical safety of these very low achieved LDL-C values have proved reassuring, albeit monitoring for longer periods is required. For raising high-density lipoprotein (HDL) cholesterol (HDL-C), recent studies have indicated that the currently available therapies do not reduce the risk of ASCVD. Finally, human Mendelian randomization studies have demonstrated the critical role of LDL-C, and other cholesterol-rich ApoB-containing lipoproteins, in atherosclerotic plaque formation and related subsequent CV events. Thus, there is no longer an ‘LDL-C hypothesis’, but established facts that increased LDL-C values are causally related to ASCVD, and that lowering LDL particles and other ApoB-containing lipoproteins as much as possible reduces CV events.
In order to be aligned with these new findings, the ESC/EAS Task Force members who have written these Guidelines have proposed new LDL-C goals, as well as a revised CV risk stratification, which are especially relevant to high- and very-high-risk patients.
These novel ESC/EAS Guidelines on lipids provide important new advice on patient management, which should enable more clinicians to efficiently and safely reduce CV risk through lipid modification.
2.1 What is new in the 2019 Guidelines?
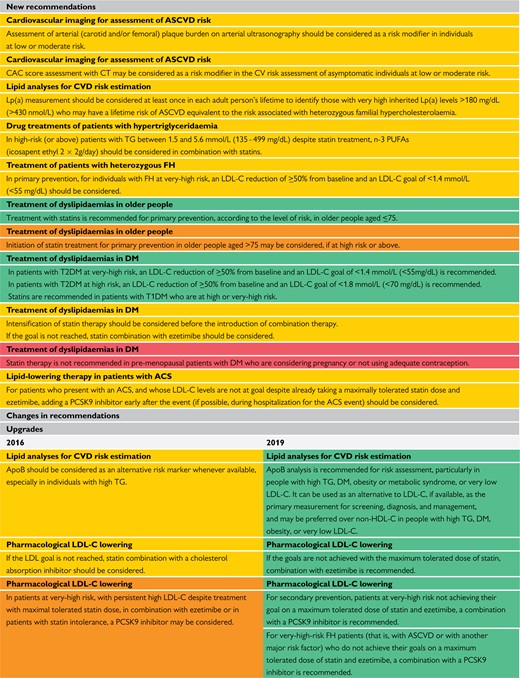
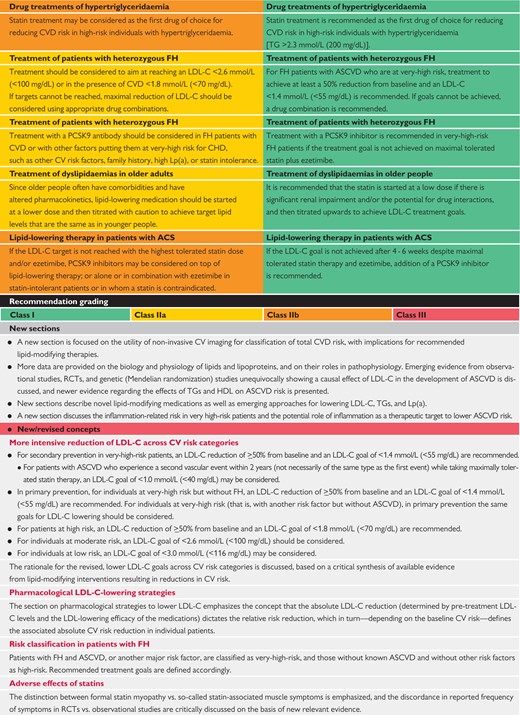
New recommendations, and new and revised concepts, are presented in Table 3.
New recommendations, and new and revised concepts
 |
 |
 |
 |
 |
 |
ACS = acute coronary syndrome; ApoB = apolipoprotein B; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CHD = coronary heart disease; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; HDL = high-density lipoprotein; LDL-C = low-density lipoproteins cholesterol; Lp(a) = lipoprotein(a); PCSK9 = proprotein convertase subtilisin/kexin type 9; PUFAs = polyunsaturated fatty acids; RCTs = randomized controlled trials; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus; TGs = triglycerides.
New recommendations, and new and revised concepts
 |
 |
 |
 |
 |
 |
ACS = acute coronary syndrome; ApoB = apolipoprotein B; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CHD = coronary heart disease; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; HDL = high-density lipoprotein; LDL-C = low-density lipoproteins cholesterol; Lp(a) = lipoprotein(a); PCSK9 = proprotein convertase subtilisin/kexin type 9; PUFAs = polyunsaturated fatty acids; RCTs = randomized controlled trials; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus; TGs = triglycerides.
3 What is cardiovascular disease prevention?
3.1 Definition and rationale
Cardiovascular disease (CVD), of which ASCVD is the major component, is responsible for >4 million deaths in Europe each year. It kills more women (2.2 million) than men (1.8 million), although CV deaths before the age of 65 years are more common in men (490 000 vs. 193 000).3 Prevention is defined as a co-ordinated set of actions, either at the population or individual level, aimed at eliminating or minimizing the impact of CV diseases and their related disabilities. More patients are surviving their first CVD event and are at high-risk of recurrences. In addition, the prevalence of some risk factors, notably diabetes (DM) and obesity, is increasing. The importance of ASCVD prevention remains undisputed and should be delivered at the general population level by promoting healthy lifestyle behaviour,4 and at the individual level by tackling unhealthy lifestyles and by reducing increased levels of causal CV risk factors, such as LDL cholesterol or blood pressure (BP) levels.
3.2 Development of the Joint Task Force Guidelines for the management of dyslipidaemias
The present Guidelines represent an evidence-based consensus of the European Task Force, including the ESC and the EAS.
By appraising the current evidence and identifying remaining knowledge gaps in the management of dyslipidaemias, the Task Force has formulated recommendations to guide action in clinical practice to prevent ASCVD by modifying plasma lipid levels.
This document has been developed for healthcare professionals to facilitate informed communication with individuals about their CV risk and the benefits of adopting and sustaining a healthy lifestyle, and of early modification of their lipid-related CV risk. In addition, the Guidelines provide tools for healthcare professionals to promote up-to-date intervention strategies, integrate these strategies into national or regional prevention frameworks, and to translate them into locally delivered healthcare services, in line with the recommendations of the World Health Organization (WHO) Global Status Report on Noncommunicable Diseases 2014.5
A lifetime approach to CV risk should be considered.1 This implies that—apart from improving lifestyle habits and reducing risk factor levels in patients with established ASCVD, and in those at increased risk of developing ASCVD—people of all ages should be encouraged to adopt or sustain a healthy lifestyle.
4 Total cardiovascular risk
4.1 Total cardiovascular risk estimation
CV risk in the context of these Guidelines means the likelihood of a person developing an atherosclerotic CV event over a defined period of time. Total CVD risk expresses the combined effect of a number of risk factors on this risk estimate. In these Guidelines, we address the lipid-related contribution to total CV risk and how to manage it at the clinical level.
4.1.1 Rationale for assessing total cardiovascular disease risk
All current guidelines on the prevention of ASCVD in clinical practice recommend the assessment of total CVD risk. Prevention of ASCVD in a given person should relate to his or her total CV risk: the higher the risk, the more intense the action should be.
Many risk assessment systems are available and have been comprehensively reviewed (Supplementary Table 1 in the Supplementary Data). Most guidelines use one of these risk assessment systems.6–8 Ideally, risk charts should be based on country-specific cohort data. These are not available for most countries. The SCORE (Systematic Coronary Risk Estimation) system can be recalibrated for use in different populations by adjusting for secular changes in CVD mortality and risk factor prevalence. Calibrated country-specific versions are available for many European countries and can be found at http://www.heartscore.org. These are now being updated to provide recalibrated, contemporaneous country-specific charts for all European countries. Other risk estimation systems—using both fatal and non-fatal events—can also be recalibrated, but the process is easier and scientifically more robust for mortality than for total events. The European Guidelines on CVD prevention in clinical practice (both the 20129 and 201610 versions) recommend the use of the SCORE system because it is based on large, representative European cohort data sets and because it is relatively straightforward to recalibrate for individual countries.
Persons with documented ASCVD, type 1 or type 2 DM (T1DM and T2DM, respectively), very high levels of individual risk factors, or chronic kidney disease (CKD) are generally at very-high or high total CV risk. No risk estimation models are needed for such persons; they all need active management of all risk factors. For other, apparently healthy people, the use of a risk estimation system such as SCORE, which estimates the 10 year cumulative risk of a first fatal atherosclerotic event, is recommended to estimate total CV risk, since many people have several risk factors that, in combination, may result in high levels of total CV risk.
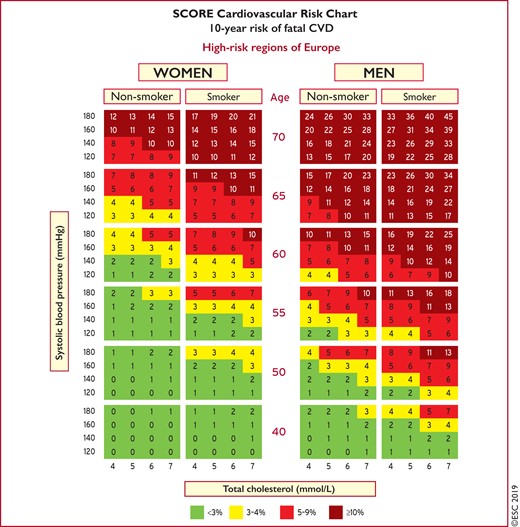
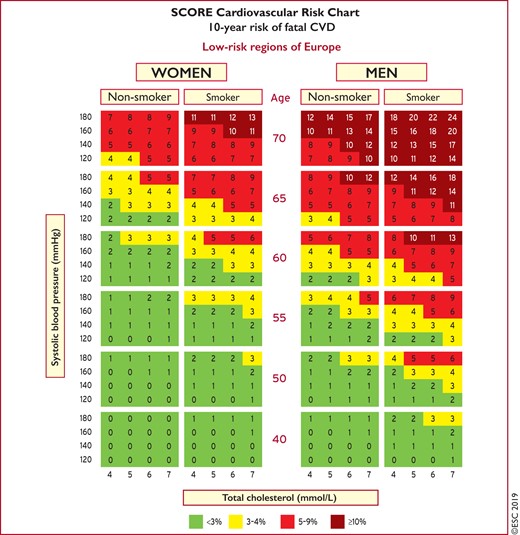
Risk estimates have been produced as charts for high- and low-risk regions in Europe (Figures 1 and 2).11 All International Classification of Diseases codes that are related to deaths from vascular origin caused by atherosclerosis are included. The reasons for retaining a system that estimates fatal as opposed to total fatal + non-fatal events are that non-fatal events are dependent on definition, developments in diagnostic tests, and methods of ascertainment, all of which can vary, resulting in very variable multipliers to convert fatal to total events. In addition, total event charts, in contrast to those based on mortality, are more difficult to recalibrate to suit different populations. That said, work is in progress to produce regional total event charts.
Systematic Coronary Risk Estimation chart for European populations at high cardiovascular disease risk. The 10-year risk of fatal cardiovascular disease in populations at high cardiovascular disease risk based on the following risk factors: age, gender, smoking, systolic blood pressure, and total cholesterol. To convert the risk of fatal cardiovascular disease to risk of total (fatal + non-fatal) cardiovascular disease, multiply by 3 in men and by 4 in women, and slightly less in older people. Note: the Systematic Coronary Risk Estimation chart is for use in people without overt cardiovascular disease, diabetes (type 1 and 2), chronic kidney disease, familial hypercholesterolaemia, or very high levels of individual risk factors because such people are already at high-risk and need intensive risk factor management. Cholesterol: 1 mmol/L = 38.67 mg/dL. The SCORE risk charts presented above differ slightly from those in the 2016 European Society of Cardiology/European Atherosclerosis Society Guidelines for the management of dyslipidaemias and the 2016 European Guidelines on cardiovascular disease prevention in clinical practice, in that: (i) age has been extended from age 65 to 70; (ii) the interaction between age and each of the other risk factors has been incorporated, thus reducing the overestimation of risk in older persons in the original Systematic Coronary Risk Estimation charts; and (iii) the cholesterol band of 8 mmol/L has been removed, since such persons will qualify for further evaluation in any event. SCORE = Systematic Coronary Risk Estimation.
Systematic Coronary Risk Estimation chart for European populations at low cardiovascular disease risk. The 10-year risk of fatal cardiovascular disease in populations at low cardiovascular disease risk based on the following risk factors: age, gender, smoking, systolic blood pressure, and total cholesterol. To convert the risk of fatal cardiovascular disease to risk of total (fatal + non-fatal) cardiovascular disease, multiply by 3 in men and by 4 in women, and slightly less in older people. Note: the Systematic Coronary Risk Estimation chart is for use in people without overt cardiovascular disease, diabetes (type 1 and 2), chronic kidney disease, familial hypercholesterolaemia, or very high levels of individual risk factors because such people are already at high-risk and need intensive risk factor management. Cholesterol: 1 mmol/L=38.67 mg/dL. The SCORE risk charts presented above differ slightly from those in the 2016 European Society of Cardiology/European Atherosclerosis Society Guidelines for the management of dyslipidaemias and the 2016 European Guidelines on cardiovascular disease prevention in clinical practice, in that: (i) age has been extended from age 65 to 70; (ii) the interaction between age and each of the other risk factors has been incorporated, thus reducing the overestimation of risk in older persons in the original Systematic Coronary Risk Estimation charts; and (iii) the cholesterol band of 8 mmol/L has been removed since such persons will qualify for further evaluation in any event. SCORE = Systematic Coronary Risk Estimation.
The SCORE data indicate that the total CVD event risk is about three times higher than the risk of fatal CVD for men, so a SCORE risk of 5% translates into a CVD risk of ∼15% of total (fatal + non-fatal) CVD endpoints; the multiplier is higher in women and lower in older people.
Clinicians often ask for thresholds to trigger certain interventions. This is problematic since risk is a continuum and there is no threshold at which, for example, a drug is automatically indicated. This is true for all continuous risk factors such as plasma cholesterol or systolic BP (SBP). Therefore, the goals that are proposed in this document reflect this concept.
A particular problem relates to young people with high levels of risk factors; a low absolute risk may conceal a very high relative risk requiring at least intensive lifestyle advice. To motivate young people (i.e. aged <40 years) not to delay changing their unhealthy lifestyle, an estimate of their relative risk—illustrating that lifestyle changes can reduce relative risk substantially—may be helpful (Supplementary Figure 1).
Another approach to this problem is to use CV risk age. The risk age of a person with several CV risk factors is the age of a person with the same level of risk but with ideal levels of risk factors. Thus, a high-risk 40-year-old would have a risk age ≥65 years. Risk age can be estimated visually by looking at the SCORE chart (as illustrated in Supplementary Figure 2). In this chart, the risk age of a person with risk factors is defined as the age at which a person with ideal risk factor levels would reach the same risk level. Ideal risk factors are non-smoking, total cholesterol (TC) ≤4 mmol/L (≤155 mg/dL), and SBP ≤120 mmHg. Risk age is also automatically calculated as part of the latest revision of HeartScore (http://www.HeartScore.org).
Risk age has been shown to be independent of the CV endpoint used,6,8 can be used in any population regardless of baseline risk or secular changes in mortality, and therefore avoids the need for recalibration.
Lifetime risk is another approach to illustrate the impact of risk factors that may be useful in younger people.12 The greater the burden of risk factors, the higher the lifetime risk. This approach produces higher risk figures for younger people because of their longer exposure times. Therefore, it is more useful as a way of illustrating risk than as a guide to treatment, because therapeutic trials have been based on a fixed follow-up period and not on lifetime risk.
Another problem relates to older people. In some age categories, the majority of people, especially males, will have estimated 10 year cumulative CV death risks exceeding the 5–10% level, based on age only, even when other CV risk factor levels are relatively low. Therefore, before initiating treatment in the elderly, clinicians should evaluate patients carefully. The relative strengths of risk factors vary with age and SCORE overestimates risk in older people (that is, those aged >65 years).11 These Guidelines include illustrative charts for older people (see Figures 1 and 2). While older people benefit from smoking cessation, and control of hypertension and hyperlipidaemia (see section 9.3), clinical judgement is required to avoid side effects from overmedication.
The additional impact of HDL-C on risk estimation is illustrated in Supplementary Figures 3 and 4; HDL-C can be used to increase the accuracy of the risk evaluation. In these charts, HDL-C is used categorically. The electronic version of SCORE, HeartScore (http://www.heartscore.org/en_GB/), has been modified to take HDL-C into consideration as a continuous variable. Clinicians should be aware that at extremely high values [above ∼2.3 mmol/L (90 mg/dL)] of HDL-C there appears to be an increased risk of ASCVD, so at such levels HDL-C cannot be used as a risk predictor.
4.1.2 How to use the risk estimation charts
Use of the low- or the high-risk SCORE charts will depend on the CVD mortality experience in each country. While any cut-off point is arbitrary and open to debate, in these Guidelines, the cut-off point for calling a country ‘low CVD risk’ is based on WHO data derived from the Global Burden of Disease Study.
Countries are categorized as low-risk if their age-adjusted 2016 CVD mortality rate was <150/100 000 (for men and women together) (http://www.who.int/healthinfo/global_burden_disease/estimates/en/). Countries with a CVD mortality rate of ≥150/100 000 or more are considered to be at high-risk.
Boxes 1 to 5 summarize the main points regarding the risk estimation charts and their use.
How to use the risk estimation charts
| To estimate a person’s 10-year risk of CVD death, find the table for his/her gender, smoking status, and age. Within the table, find the cell nearest to the person’s BP and TC. Risk estimates will need to be adjusted upwards as the person approaches the next age category. |
| Risk is initially assessed on the level of TC and systolic BP before treatment, if known. The longer the treatment and the more effective it is, the greater the reduction in risk, but in general it will not be more than about one-third of the baseline risk. For example, for a person on antihypertensive drug treatment in whom the pre-treatment BP is not known, if the total CV SCORE risk is 6%, then the pre-treatment total CV risk may have been 9%. |
| Low-risk persons should be offered advice to maintain their low-risk status. While no threshold is universally applicable, the intensity of advice should increase with increasing risk. |
| The charts may be used to give some indication of the effects of reducing risk factors, given that there is apparently a time lag before the risk reduces. In general, people who stop smoking halve their cumulative risk over a relatively short period of time. |
| To estimate a person’s 10-year risk of CVD death, find the table for his/her gender, smoking status, and age. Within the table, find the cell nearest to the person’s BP and TC. Risk estimates will need to be adjusted upwards as the person approaches the next age category. |
| Risk is initially assessed on the level of TC and systolic BP before treatment, if known. The longer the treatment and the more effective it is, the greater the reduction in risk, but in general it will not be more than about one-third of the baseline risk. For example, for a person on antihypertensive drug treatment in whom the pre-treatment BP is not known, if the total CV SCORE risk is 6%, then the pre-treatment total CV risk may have been 9%. |
| Low-risk persons should be offered advice to maintain their low-risk status. While no threshold is universally applicable, the intensity of advice should increase with increasing risk. |
| The charts may be used to give some indication of the effects of reducing risk factors, given that there is apparently a time lag before the risk reduces. In general, people who stop smoking halve their cumulative risk over a relatively short period of time. |
BP = blood pressure; CV = cardiovascular; CVD = cardiovascular disease; SCORE = Systematic Coronary Risk Estimation; TC = total cholesterol.
How to use the risk estimation charts
| To estimate a person’s 10-year risk of CVD death, find the table for his/her gender, smoking status, and age. Within the table, find the cell nearest to the person’s BP and TC. Risk estimates will need to be adjusted upwards as the person approaches the next age category. |
| Risk is initially assessed on the level of TC and systolic BP before treatment, if known. The longer the treatment and the more effective it is, the greater the reduction in risk, but in general it will not be more than about one-third of the baseline risk. For example, for a person on antihypertensive drug treatment in whom the pre-treatment BP is not known, if the total CV SCORE risk is 6%, then the pre-treatment total CV risk may have been 9%. |
| Low-risk persons should be offered advice to maintain their low-risk status. While no threshold is universally applicable, the intensity of advice should increase with increasing risk. |
| The charts may be used to give some indication of the effects of reducing risk factors, given that there is apparently a time lag before the risk reduces. In general, people who stop smoking halve their cumulative risk over a relatively short period of time. |
| To estimate a person’s 10-year risk of CVD death, find the table for his/her gender, smoking status, and age. Within the table, find the cell nearest to the person’s BP and TC. Risk estimates will need to be adjusted upwards as the person approaches the next age category. |
| Risk is initially assessed on the level of TC and systolic BP before treatment, if known. The longer the treatment and the more effective it is, the greater the reduction in risk, but in general it will not be more than about one-third of the baseline risk. For example, for a person on antihypertensive drug treatment in whom the pre-treatment BP is not known, if the total CV SCORE risk is 6%, then the pre-treatment total CV risk may have been 9%. |
| Low-risk persons should be offered advice to maintain their low-risk status. While no threshold is universally applicable, the intensity of advice should increase with increasing risk. |
| The charts may be used to give some indication of the effects of reducing risk factors, given that there is apparently a time lag before the risk reduces. In general, people who stop smoking halve their cumulative risk over a relatively short period of time. |
BP = blood pressure; CV = cardiovascular; CVD = cardiovascular disease; SCORE = Systematic Coronary Risk Estimation; TC = total cholesterol.
Risk estimation charts for different countries
| The low-risk charts should be considered for use in Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Malta, Portugal, Slovenia, Spain, Sweden, Switzerland, and the UK. |
| The high-risk charts should be considered for use in Albania, Algeria, Armenia, Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Lebanon, Libya, Lithuania, Montenegro, Morocco, Poland, Romania, Serbia, Slovakia, Tunisia, and Turkey. |
| Some countries have a cardiovascular disease mortality rate >350/100 000, and the high-risk chart may underestimate risk. These are Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kazakhstan, Kyrgyzstan, North Macedonia, Republic of Moldova, Russian Federation, Syria, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. |
| The low-risk charts should be considered for use in Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Malta, Portugal, Slovenia, Spain, Sweden, Switzerland, and the UK. |
| The high-risk charts should be considered for use in Albania, Algeria, Armenia, Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Lebanon, Libya, Lithuania, Montenegro, Morocco, Poland, Romania, Serbia, Slovakia, Tunisia, and Turkey. |
| Some countries have a cardiovascular disease mortality rate >350/100 000, and the high-risk chart may underestimate risk. These are Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kazakhstan, Kyrgyzstan, North Macedonia, Republic of Moldova, Russian Federation, Syria, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. |
Risk estimation charts for different countries
| The low-risk charts should be considered for use in Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Malta, Portugal, Slovenia, Spain, Sweden, Switzerland, and the UK. |
| The high-risk charts should be considered for use in Albania, Algeria, Armenia, Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Lebanon, Libya, Lithuania, Montenegro, Morocco, Poland, Romania, Serbia, Slovakia, Tunisia, and Turkey. |
| Some countries have a cardiovascular disease mortality rate >350/100 000, and the high-risk chart may underestimate risk. These are Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kazakhstan, Kyrgyzstan, North Macedonia, Republic of Moldova, Russian Federation, Syria, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. |
| The low-risk charts should be considered for use in Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Malta, Portugal, Slovenia, Spain, Sweden, Switzerland, and the UK. |
| The high-risk charts should be considered for use in Albania, Algeria, Armenia, Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Lebanon, Libya, Lithuania, Montenegro, Morocco, Poland, Romania, Serbia, Slovakia, Tunisia, and Turkey. |
| Some countries have a cardiovascular disease mortality rate >350/100 000, and the high-risk chart may underestimate risk. These are Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kazakhstan, Kyrgyzstan, North Macedonia, Republic of Moldova, Russian Federation, Syria, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. |
Qualifiers
| The charts can assist in risk assessment and management, but must be interpreted in light of the clinician’s knowledge and experience, and of the patient’s pre-test likelihood of CVD. |
| Risk will be overestimated in countries with decreasing CVD mortality, and underestimated in countries in which mortality is increasing. This is dealt with by recalibration (http://www.heartscore.org/en_GB/). |
| Risk estimates are lower in women than in men. However, risk is only deferred in women; the risk of a 60-year-old woman is similar to that of a 50-year-old man. Ultimately, more women die from CVD than men. |
| Relative risks may be unexpectedly high in young persons, even if absolute risk levels are low. The relative risk chart (Supplementary Figure 1) and the estimated risk age (Supplementary Figure 2) may be helpful in identifying and counselling such persons. |
| The charts can assist in risk assessment and management, but must be interpreted in light of the clinician’s knowledge and experience, and of the patient’s pre-test likelihood of CVD. |
| Risk will be overestimated in countries with decreasing CVD mortality, and underestimated in countries in which mortality is increasing. This is dealt with by recalibration (http://www.heartscore.org/en_GB/). |
| Risk estimates are lower in women than in men. However, risk is only deferred in women; the risk of a 60-year-old woman is similar to that of a 50-year-old man. Ultimately, more women die from CVD than men. |
| Relative risks may be unexpectedly high in young persons, even if absolute risk levels are low. The relative risk chart (Supplementary Figure 1) and the estimated risk age (Supplementary Figure 2) may be helpful in identifying and counselling such persons. |
CVD = cardiovascular disease.
Qualifiers
| The charts can assist in risk assessment and management, but must be interpreted in light of the clinician’s knowledge and experience, and of the patient’s pre-test likelihood of CVD. |
| Risk will be overestimated in countries with decreasing CVD mortality, and underestimated in countries in which mortality is increasing. This is dealt with by recalibration (http://www.heartscore.org/en_GB/). |
| Risk estimates are lower in women than in men. However, risk is only deferred in women; the risk of a 60-year-old woman is similar to that of a 50-year-old man. Ultimately, more women die from CVD than men. |
| Relative risks may be unexpectedly high in young persons, even if absolute risk levels are low. The relative risk chart (Supplementary Figure 1) and the estimated risk age (Supplementary Figure 2) may be helpful in identifying and counselling such persons. |
| The charts can assist in risk assessment and management, but must be interpreted in light of the clinician’s knowledge and experience, and of the patient’s pre-test likelihood of CVD. |
| Risk will be overestimated in countries with decreasing CVD mortality, and underestimated in countries in which mortality is increasing. This is dealt with by recalibration (http://www.heartscore.org/en_GB/). |
| Risk estimates are lower in women than in men. However, risk is only deferred in women; the risk of a 60-year-old woman is similar to that of a 50-year-old man. Ultimately, more women die from CVD than men. |
| Relative risks may be unexpectedly high in young persons, even if absolute risk levels are low. The relative risk chart (Supplementary Figure 1) and the estimated risk age (Supplementary Figure 2) may be helpful in identifying and counselling such persons. |
CVD = cardiovascular disease.
Factors modifying Systematic Coronary Risk Estimation risks
| Social deprivation: the origin of many of the causes of CVD. |
| Obesity and central obesity as measured by the body mass index and waist circumference, respectively. |
| Physical inactivity. |
| Psychosocial stress including vital exhaustion. |
| Family history of premature CVD (men: <55 years and women: <60 years). |
| Chronic immune-mediated inflammatory disorder. |
| Major psychiatric disorders. |
| Treatment for human immunodeficiency virus infection. |
| Atrial fibrillation. |
| Left ventricular hypertrophy. |
| Chronic kidney disease. |
| Obstructive sleep apnoea syndrome. |
| Non-alcoholic fatty liver disease. |
| Social deprivation: the origin of many of the causes of CVD. |
| Obesity and central obesity as measured by the body mass index and waist circumference, respectively. |
| Physical inactivity. |
| Psychosocial stress including vital exhaustion. |
| Family history of premature CVD (men: <55 years and women: <60 years). |
| Chronic immune-mediated inflammatory disorder. |
| Major psychiatric disorders. |
| Treatment for human immunodeficiency virus infection. |
| Atrial fibrillation. |
| Left ventricular hypertrophy. |
| Chronic kidney disease. |
| Obstructive sleep apnoea syndrome. |
| Non-alcoholic fatty liver disease. |
CVD = cardiovascular disease.
Factors modifying Systematic Coronary Risk Estimation risks
| Social deprivation: the origin of many of the causes of CVD. |
| Obesity and central obesity as measured by the body mass index and waist circumference, respectively. |
| Physical inactivity. |
| Psychosocial stress including vital exhaustion. |
| Family history of premature CVD (men: <55 years and women: <60 years). |
| Chronic immune-mediated inflammatory disorder. |
| Major psychiatric disorders. |
| Treatment for human immunodeficiency virus infection. |
| Atrial fibrillation. |
| Left ventricular hypertrophy. |
| Chronic kidney disease. |
| Obstructive sleep apnoea syndrome. |
| Non-alcoholic fatty liver disease. |
| Social deprivation: the origin of many of the causes of CVD. |
| Obesity and central obesity as measured by the body mass index and waist circumference, respectively. |
| Physical inactivity. |
| Psychosocial stress including vital exhaustion. |
| Family history of premature CVD (men: <55 years and women: <60 years). |
| Chronic immune-mediated inflammatory disorder. |
| Major psychiatric disorders. |
| Treatment for human immunodeficiency virus infection. |
| Atrial fibrillation. |
| Left ventricular hypertrophy. |
| Chronic kidney disease. |
| Obstructive sleep apnoea syndrome. |
| Non-alcoholic fatty liver disease. |
CVD = cardiovascular disease.
Social deprivation and psychosocial stress set the scene for increased risk.13 For those at moderate risk, other factors—including metabolic factors such as increased ApoB, lipoprotein(a) [Lp(a)], triglycerides (TGs), or C-reactive protein; the presence of albuminuria; the presence of atherosclerotic plaque in the carotid or femoral arteries; or the coronary artery calcium (CAC) score—may improve risk classification. Many other biomarkers are also associated with increased CVD risk, although few of these have been shown to be associated with appreciable reclassification. Total CV risk will also be higher than indicated in the SCORE charts in asymptomatic persons with abnormal markers of subclinical atherosclerotic vascular damage. Reclassification is of value in people identified as being at moderate CV risk by using markers such as CAC score >100 Agatston units, ankle–brachial index (ABI) <0.9 or >1.40, carotid–femoral pulse wave velocity >10 m/s, or the presence of plaques at carotid or femoral ultrasonography. In studies comparing these markers, CAC had the best reclassification ability.14–16
Some factors such as a high HDL-C up to 2.3 mmol/L (90mg/dL)17 or a family history of longevity can also be associated with lower risk.
Risk estimation: key messages
| In apparently healthy persons, CVD risk is most frequently the result of multiple, interacting risk factors. This is the basis for total CV risk estimation and management. |
| Risk factor screening including the lipid profile should be considered in men >40 years old, and in women >50 years of age or post-menopausal. |
| A risk estimation system such as SCORE can assist in making logical management decisions, and may help to avoid both under- and overtreatment. |
| Certain individuals declare themselves to be at high or very high CVD risk without needing risk scoring, and all risk factors require immediate attention. This is true for patients with documented CVD, older individuals with long-standing DM, familial hypercholesterolaemia, chronic kidney disease, carotid or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation. |
| All risk estimation systems are relatively crude and require attention to qualifying statements. |
| Additional factors affecting risk can be accommodated in electronic risk estimation systems such as HeartScore (www.heartscore.org). |
| The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk. |
| In apparently healthy persons, CVD risk is most frequently the result of multiple, interacting risk factors. This is the basis for total CV risk estimation and management. |
| Risk factor screening including the lipid profile should be considered in men >40 years old, and in women >50 years of age or post-menopausal. |
| A risk estimation system such as SCORE can assist in making logical management decisions, and may help to avoid both under- and overtreatment. |
| Certain individuals declare themselves to be at high or very high CVD risk without needing risk scoring, and all risk factors require immediate attention. This is true for patients with documented CVD, older individuals with long-standing DM, familial hypercholesterolaemia, chronic kidney disease, carotid or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation. |
| All risk estimation systems are relatively crude and require attention to qualifying statements. |
| Additional factors affecting risk can be accommodated in electronic risk estimation systems such as HeartScore (www.heartscore.org). |
| The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk. |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; SCORE = Systematic Coronary Risk Estimation.
Risk estimation: key messages
| In apparently healthy persons, CVD risk is most frequently the result of multiple, interacting risk factors. This is the basis for total CV risk estimation and management. |
| Risk factor screening including the lipid profile should be considered in men >40 years old, and in women >50 years of age or post-menopausal. |
| A risk estimation system such as SCORE can assist in making logical management decisions, and may help to avoid both under- and overtreatment. |
| Certain individuals declare themselves to be at high or very high CVD risk without needing risk scoring, and all risk factors require immediate attention. This is true for patients with documented CVD, older individuals with long-standing DM, familial hypercholesterolaemia, chronic kidney disease, carotid or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation. |
| All risk estimation systems are relatively crude and require attention to qualifying statements. |
| Additional factors affecting risk can be accommodated in electronic risk estimation systems such as HeartScore (www.heartscore.org). |
| The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk. |
| In apparently healthy persons, CVD risk is most frequently the result of multiple, interacting risk factors. This is the basis for total CV risk estimation and management. |
| Risk factor screening including the lipid profile should be considered in men >40 years old, and in women >50 years of age or post-menopausal. |
| A risk estimation system such as SCORE can assist in making logical management decisions, and may help to avoid both under- and overtreatment. |
| Certain individuals declare themselves to be at high or very high CVD risk without needing risk scoring, and all risk factors require immediate attention. This is true for patients with documented CVD, older individuals with long-standing DM, familial hypercholesterolaemia, chronic kidney disease, carotid or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation. |
| All risk estimation systems are relatively crude and require attention to qualifying statements. |
| Additional factors affecting risk can be accommodated in electronic risk estimation systems such as HeartScore (www.heartscore.org). |
| The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk. |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; SCORE = Systematic Coronary Risk Estimation.
4.2 Risk levels
A total CV risk estimate is part of a continuum. The cut-off points that are used to define high-risk are, in part, both arbitrary and based on the risk levels at which benefit is evident in clinical trials. In clinical practice, consideration should be given to practical issues in relation to the local healthcare systems. Not only should those at high risk be identified and managed, but those at moderate risk should also receive professional advice regarding lifestyle changes; in some cases, drug therapy will be needed to reduce atherosclerotic risk.
Low-risk people should be given advice to help them maintain this status. Thus, the intensity of preventive actions should be tailored to the patient’s total CV risk. The strongest driver of total CV risk is age, which can be considered as ‘exposure time’ to risk factors.
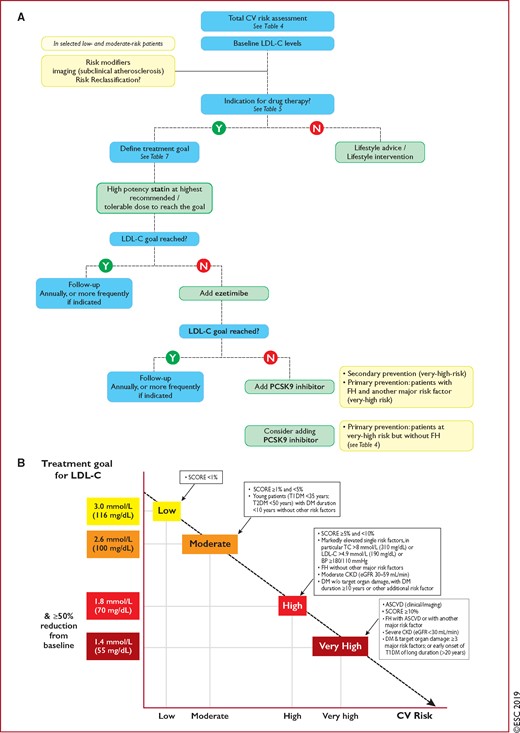
For these reasons, the Task Force suggests the following categories of risk and LDL-C goals, based on the best available evidence and in an ideal setting with unlimited resources. These categories represent a counsel of perfection, but these ideals are for guidance only and practical decision-making must be based on what is appropriate to the local situation.
With these considerations, we propose the levels of total CV risk presented in Table 4.
Cardiovascular risk categories
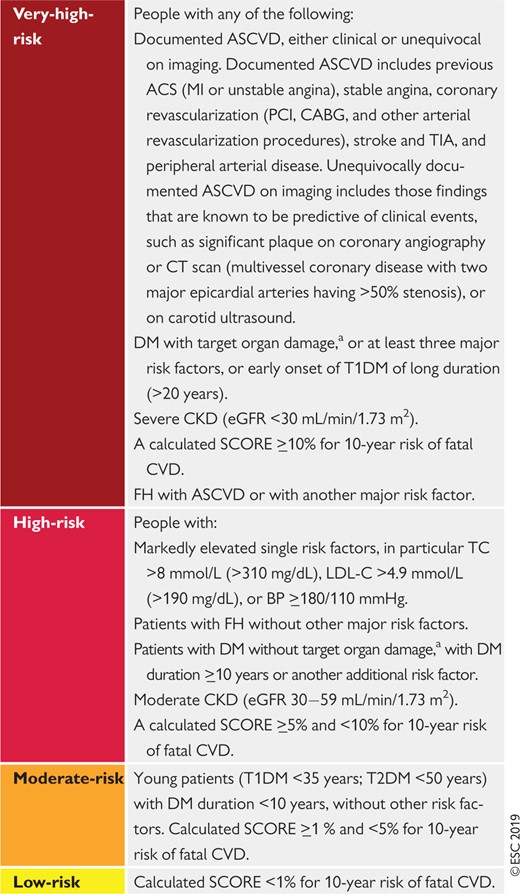 |
 |
ASCVD = atherosclerotic cardiovascular disease; ACS = acute coronary syndrome; BP = blood pressure; CABG = coronary artery bypass graft surgery; CKD = chronic kidney disease; CT = computed tomography; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; PCI = percutaneous coronary intervention; SCORE = Systematic Coronary Risk Estimation; T1DM = type 1 DM; T2DM = type 2 DM; TC = total cholesterol; TIA = transient ischaemic attack.
Target organ damage is defined as microalbuminuria, retinopathy, or neuropathy.
Cardiovascular risk categories
 |
 |
ASCVD = atherosclerotic cardiovascular disease; ACS = acute coronary syndrome; BP = blood pressure; CABG = coronary artery bypass graft surgery; CKD = chronic kidney disease; CT = computed tomography; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; PCI = percutaneous coronary intervention; SCORE = Systematic Coronary Risk Estimation; T1DM = type 1 DM; T2DM = type 2 DM; TC = total cholesterol; TIA = transient ischaemic attack.
Target organ damage is defined as microalbuminuria, retinopathy, or neuropathy.
4.2.1 Role of non-invasive cardiovascular imaging techniques in the assessment of total cardiovascular disease risk
Non-invasive imaging techniques can detect the presence, estimate the extent, and evaluate the clinical consequences of atherosclerotic vascular damage. Detection of coronary artery calcification with non-contrast computed tomography (CT) gives a good estimate of the atherosclerotic burden and is strongly associated with CV events.18 A recent meta-analysis from the US Preventive Services Task Force summarized the available evidence on the value of non-traditional risk factors for risk prediction, and found that, although there are no randomized trials showing that the use of CAC reduces health outcomes, nevertheless it improves both discrimination and reclassification.19 Assessment of carotid or femoral plaque burden with ultrasound has also been demonstrated to be predictive of CV events, comparable to CAC,20–23 while the measurement of the carotid intima–media thickness is inferior to CAC score and carotid plaque detection.16,24,25
In asymptomatic patients at low or moderate risk who would be eligible for statin therapy (see Table 5), assessment of ASCVD with imaging may have an impact on medical treatment, both from the physician’s and the patient’s points of view. Data from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that 41–57% of individuals who would be eligible for statin therapy had a CAC score of zero and the rate of atherosclerotic CVD events in the 10 year follow-up period was low (1.5–4.9%).26 In contrast, the rates of ASCVD and coronary heart disease (CHD) events in individuals with a CAC score >100 Agatston were 18.9 and 12.7 per 1000 person-years, respectively.18 Compared with a strategy of treating all patients, the use of CAC score to guide long-term statin therapy has been shown to be cost-effective.27 Note that CAC score is often very low in patients younger than 45 years of age with severe familial hypercholesterolaemia (FH), including homozygous FH (HoFH), and has low specificity in this population.
Assessment of coronary luminal stenosis >50% and plaque composition with coronary CT angiography also provides incremental prognostic value over traditional risk stratification models.28 As a result, in asymptomatic individuals with moderate risk, the presence of a CAC score >100 Agatston, and carotid or femoral plaque burden on ultrasonography, may reclassify them to a higher risk category. Therefore, the use of methods to detect these markers should be of interest in that group (see Recommendations for cardiovascular imaging for risk assessment of atherosclerotic cardiovascular disease below).14–16 Overall, CAC score assessment with CT may be considered in individuals at low or moderate risk in whom the respective LDL-C goal is not achieved with lifestyle intervention alone, and pharmacological therapy is an option (see Table 5). The use of imaging techniques to determine the presence and extent of atherosclerotic vascular damage in low-risk individuals not being considered for statin therapy is not justified due to low prognostic yield, and the associated costs and radiation hazards when measuring CAC score, particularly among low-risk women.29 Of note, CAC score is increased following statin treatment; therefore, the CAC scores of statin-treated patients should be interpreted with caution.
Recommendations for cardiovascular imaging for risk assessment of atherosclerotic cardiovascular disease
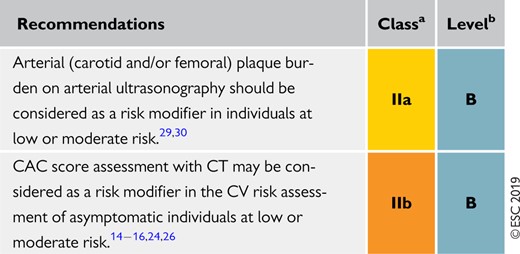 |
 |
CAC = coronary artery calcium; CT = computed tomography; CV = cardiovascular.
Class of recommendation.
Level of evidence.
Recommendations for cardiovascular imaging for risk assessment of atherosclerotic cardiovascular disease
 |
 |
CAC = coronary artery calcium; CT = computed tomography; CV = cardiovascular.
Class of recommendation.
Level of evidence.
4.2.2 Risk-based intervention strategies
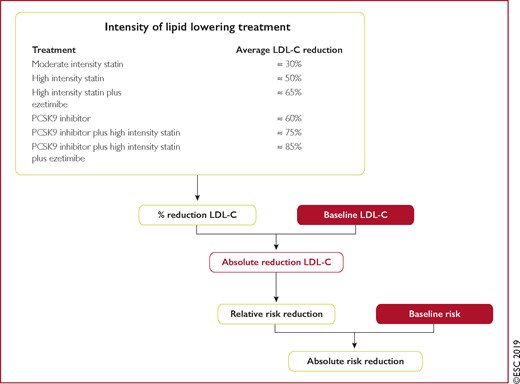
Table 5 presents suggested intervention strategies as a function of total CV risk and LDL-C level. This graded approach is based on evidence from multiple meta-analyses and individual randomized controlled trials (RCTs), which show a consistent and graded reduction in ASCVD risk in response to reductions in TC and LDL-C levels (see Recommendations for cardiovascular disease risk estimation below).31–41 These data are consistent in showing that, since the relative risk reduction is proportional to the absolute reduction in LDL-C and the absolute reduction in LDL-C resulting from a particular drug regimen depends only on baseline LDL-C, at any given level of baseline risk the higher the initial LDL-C level the greater the absolute reduction in risk. Advice on individual drug treatments is given in section 8.
Intervention strategies as a function of total cardiovascular risk and untreated low-density lipoprotein cholesterol levels
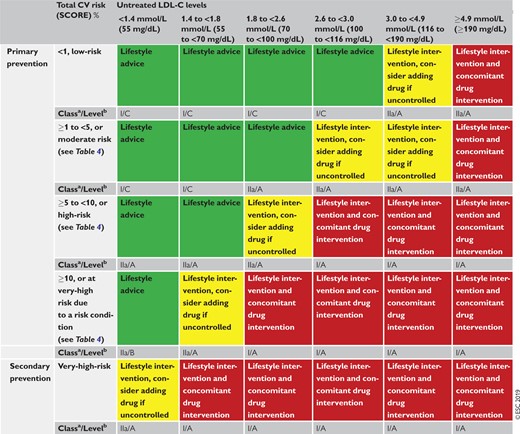 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
Class of recommendation.
Level of evidence.
Intervention strategies as a function of total cardiovascular risk and untreated low-density lipoprotein cholesterol levels
 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
Class of recommendation.
Level of evidence.
Recommendations for cardiovascular disease risk estimation
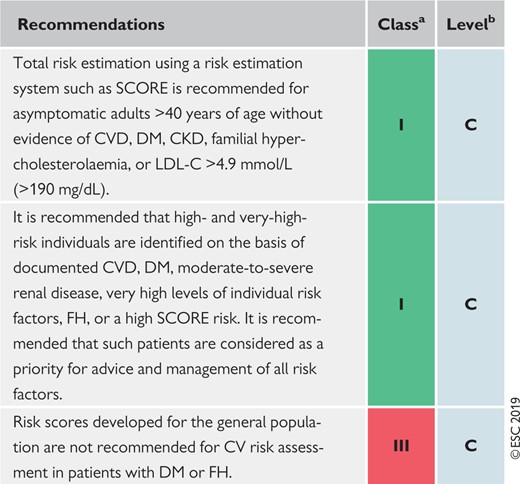 |
 |
CKD = chronic kidney disease; CV= cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
Class of recommendation.
Level of evidence.
Recommendations for cardiovascular disease risk estimation
 |
 |
CKD = chronic kidney disease; CV= cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
Class of recommendation.
Level of evidence.
5 Lipids and lipoproteins
5.1 Biological role of lipids and lipoproteins
Lipoproteins in plasma transport lipids to tissues for energy utilization, lipid deposition, steroid hormone production, and bile acid formation. Lipoproteins consist of esterified and unesterified cholesterol, TGs, and phospholipids and protein components named apolipoproteins that act as structural components, ligands for cellular receptor binding, and enzyme activators or inhibitors.
There are six major lipoproteins in blood: chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), LDL; Lp(a), and HDL (Table 6 and Supplementary Figure 5).
Physical and chemical characteristics of human plasma lipoproteins
| . | Density (g/mL) . | Diameter (nm) . | TGs (%) . | Cholesteryl esters (%) . | PLs (%) . | Cholesterol (%) . | Apolipoproteins . | |
|---|---|---|---|---|---|---|---|---|
| Major . | Others . | |||||||
| Chylomicrons | <0.95 | 80–100 | 90–95 | 2–4 | 2–6 | 1 | ApoB-48 | ApoA-I, A-II, A-IV, A-V |
| VLDL | 0.95–1.006 | 30–80 | 50–65 | 8–14 | 12–16 | 4–7 | ApoB-100 | ApoA-I, C-II, C-III, E, A-V |
| IDL | 1.006–1.019 | 25–30 | 25–40 | 20–35 | 16–24 | 7–11 | ApoB-100 | ApoC-II, C-III, E |
| LDL | 1.019–1.063 | 20–25 | 4–6 | 34–35 | 22–26 | 6–15 | ApoB-100 | |
| HDL | 1.063–1.210 | 8–13 | 7 | 10–20 | 55 | 5 | ApoA-I | ApoA-II, C-III, E, M |
| Lp(a) | 1.006–1.125 | 25–30 | 4–8 | 35–46 | 17–24 | 6–9 | Apo(a) | ApoB-100 |
| . | Density (g/mL) . | Diameter (nm) . | TGs (%) . | Cholesteryl esters (%) . | PLs (%) . | Cholesterol (%) . | Apolipoproteins . | |
|---|---|---|---|---|---|---|---|---|
| Major . | Others . | |||||||
| Chylomicrons | <0.95 | 80–100 | 90–95 | 2–4 | 2–6 | 1 | ApoB-48 | ApoA-I, A-II, A-IV, A-V |
| VLDL | 0.95–1.006 | 30–80 | 50–65 | 8–14 | 12–16 | 4–7 | ApoB-100 | ApoA-I, C-II, C-III, E, A-V |
| IDL | 1.006–1.019 | 25–30 | 25–40 | 20–35 | 16–24 | 7–11 | ApoB-100 | ApoC-II, C-III, E |
| LDL | 1.019–1.063 | 20–25 | 4–6 | 34–35 | 22–26 | 6–15 | ApoB-100 | |
| HDL | 1.063–1.210 | 8–13 | 7 | 10–20 | 55 | 5 | ApoA-I | ApoA-II, C-III, E, M |
| Lp(a) | 1.006–1.125 | 25–30 | 4–8 | 35–46 | 17–24 | 6–9 | Apo(a) | ApoB-100 |
Apo = apolipoprotein; HDL = high-density lipoprotein; IDL = intermediate-density lipoprotein; LDL = low-density lipoprotein; Lp(a) = lipoprotein(a); PLs = phospholipids; TGs = triglycerides; VLDL = very low-density lipoprotein.
Physical and chemical characteristics of human plasma lipoproteins
| . | Density (g/mL) . | Diameter (nm) . | TGs (%) . | Cholesteryl esters (%) . | PLs (%) . | Cholesterol (%) . | Apolipoproteins . | |
|---|---|---|---|---|---|---|---|---|
| Major . | Others . | |||||||
| Chylomicrons | <0.95 | 80–100 | 90–95 | 2–4 | 2–6 | 1 | ApoB-48 | ApoA-I, A-II, A-IV, A-V |
| VLDL | 0.95–1.006 | 30–80 | 50–65 | 8–14 | 12–16 | 4–7 | ApoB-100 | ApoA-I, C-II, C-III, E, A-V |
| IDL | 1.006–1.019 | 25–30 | 25–40 | 20–35 | 16–24 | 7–11 | ApoB-100 | ApoC-II, C-III, E |
| LDL | 1.019–1.063 | 20–25 | 4–6 | 34–35 | 22–26 | 6–15 | ApoB-100 | |
| HDL | 1.063–1.210 | 8–13 | 7 | 10–20 | 55 | 5 | ApoA-I | ApoA-II, C-III, E, M |
| Lp(a) | 1.006–1.125 | 25–30 | 4–8 | 35–46 | 17–24 | 6–9 | Apo(a) | ApoB-100 |
| . | Density (g/mL) . | Diameter (nm) . | TGs (%) . | Cholesteryl esters (%) . | PLs (%) . | Cholesterol (%) . | Apolipoproteins . | |
|---|---|---|---|---|---|---|---|---|
| Major . | Others . | |||||||
| Chylomicrons | <0.95 | 80–100 | 90–95 | 2–4 | 2–6 | 1 | ApoB-48 | ApoA-I, A-II, A-IV, A-V |
| VLDL | 0.95–1.006 | 30–80 | 50–65 | 8–14 | 12–16 | 4–7 | ApoB-100 | ApoA-I, C-II, C-III, E, A-V |
| IDL | 1.006–1.019 | 25–30 | 25–40 | 20–35 | 16–24 | 7–11 | ApoB-100 | ApoC-II, C-III, E |
| LDL | 1.019–1.063 | 20–25 | 4–6 | 34–35 | 22–26 | 6–15 | ApoB-100 | |
| HDL | 1.063–1.210 | 8–13 | 7 | 10–20 | 55 | 5 | ApoA-I | ApoA-II, C-III, E, M |
| Lp(a) | 1.006–1.125 | 25–30 | 4–8 | 35–46 | 17–24 | 6–9 | Apo(a) | ApoB-100 |
Apo = apolipoprotein; HDL = high-density lipoprotein; IDL = intermediate-density lipoprotein; LDL = low-density lipoprotein; Lp(a) = lipoprotein(a); PLs = phospholipids; TGs = triglycerides; VLDL = very low-density lipoprotein.
5.2 Role of lipids and lipoproteins in the pathophysiology of atherosclerosis
All ApoB-containing lipoproteins <70 nm in diameter, including smaller TG-rich lipoproteins and their remnant particles, can cross the endothelial barrier, especially in the presence of endothelial dysfunction, where they can become trapped after interaction with extracellular structures such as proteoglycans.42 ApoB-containing lipoproteins retained in the arterial wall provoke a complex process that leads to lipid deposition and the initiation of an atheroma.43
Continued exposure to ApoB-containing lipoproteins leads to additional particles being retained over time in the artery wall, and to the growth and progression of atherosclerotic plaques. On average, people with higher concentrations of plasma ApoB-containing lipoproteins will retain more particles and accumulate lipids faster, resulting in more rapid growth and the progression of atherosclerotic plaques.
Because atherosclerotic plaques grow over time as additional ApoB-containing lipoprotein particles are retained, the size of the total atherosclerotic plaque burden is likely to be determined by both the concentration of circulating LDL-C and other ApoB-containing lipoproteins, and by the total duration of exposure to these lipoproteins. Therefore, a person’s total atherosclerotic plaque burden is likely to be proportional to the cumulative exposure to these lipoproteins.44
Eventually, the increase of the atherosclerotic plaque burden along with changes in the composition of the plaque reaches a critical point at which disruption of a plaque can result, with the formation of an overlying thrombus that acutely obstructs blood flow resulting in unstable angina, myocardial infarction (MI), or death. Therefore, the risk of experiencing an acute ASCVD event rises rapidly as more ApoB-containing lipoproteins become retained and the atherosclerotic plaque burden increases. This provides the rationale for encouraging a healthy lifestyle to maintain low levels of ApoB-containing lipoproteins throughout life to slow the progression of atherosclerosis; it also explains the motivation to recommend treatment to lower LDL-C and other ApoB-containing lipoproteins, for both the primary prevention of ASCVD and the secondary prevention of recurrent CV events.44
5.3 Evidence for the causal effects of lipids and lipoproteins on the risk of atherosclerotic cardiovascular disease
5.3.1 Low-density lipoprotein cholesterol and risk of atherosclerosis
Plasma LDL-C is a measure of the cholesterol mass carried by LDL particles, by far the most numerous of the ApoB-containing lipoproteins, and is an estimate of the concentration of circulating LDL. Numerous epidemiological studies, Mendelian randomization studies, and RCTs have consistently demonstrated a log-linear relationship between the absolute changes in plasma LDL-C and the risk of ASCVD.34,45–50 The remarkable consistency among these studies, in addition to biological and experimental evidence, provides compelling evidence that LDL-C is causally associated with the risk of ASCVD, and that lowering LDL-C reduces the risk of ASCVD proportionally to the absolute achieved reduction in LDL-C.2,51
Furthermore, Mendelian randomization studies have demonstrated that long-term exposure to lower LDL-C levels is associated with a much lower risk of CV events as compared with shorter-term exposure to lower LDL-C (as achieved, for example, in randomized trials).48,52 These data provide strong support for the concept that LDL particles have both a causal and cumulative effect on the risk of ASCVD. Therefore, the effect of LDL-C on the risk of ASCVD appears to be determined by both the absolute magnitude and the total duration of exposure to LDL-C.2
The clinical benefit of lowering LDL-C is determined by the reduction in circulating LDL particles as estimated by ApoB, which is usually mirrored by a reduction of cholesterol carried by those particles.2,53 Therefore, the clinical benefit of therapies that lower LDL-C by reducing LDL particle mass will be proportional to the absolute reduction in LDL-C, because—on average—the reduction in LDL-C and LDL particles will be concordant.34,50,54,55 In contrast, the clinical benefit of therapies that lower LDL-C by a mechanism that may dramatically modify their composition may not be proportional to the observed absolute reduction in LDL-C, but instead would be expected to be proportional to the absolute change in LDL particle concentration as measured by a reduction in ApoB.2,53
5.3.2 Triglyceride-rich lipoproteins and risk of atherosclerosis
TG-rich VLDL particles and their remnants carry most of the circulating TGs. Therefore, the plasma TG concentration reflects the concentration of circulating ApoB-containing TG-rich lipoproteins.
Elevated plasma TG levels are associated with an increasing risk of ASCVD, but this association becomes null after adjusting for non-HDL-C, an estimate of the total concentration of all ApoB-containing lipoproteins.45 Similarly, lowering TG with fibrates reduces the risk of CV events by the same amount as LDL-C-lowering therapies when measured per unit change of non-HDL-C,50 suggesting that the effect of plasma TGs on ASCVD is mediated by changes in the concentration of TG-rich lipoproteins as estimated by non-HDL-C.
Mendelian randomization studies also suggest that the association between plasma TGs and the risk of CHD may be causal; however, this evidence must be interpreted with caution because nearly all variants associated with TGs are also associated with HDL-C, LDL-C, or Lp(a).56–59 A recent Mendelian randomization study demonstrated that TG-lowering lipoprotein lipase (LPL) variants and LDL-C-lowering LDL receptor variants had the same effect on the risk of ASCVD per unit change of ApoB, suggesting that all ApoB-containing lipoproteins have the same effect on the risk of CHD.53 Together, these studies strongly suggest that the causal effect of TG-rich lipoproteins and their remnants on the risk of ASCVD is determined by the circulating concentration of ApoB-containing particles, rather than by the TG content itself.
5.3.3 High-density lipoprotein cholesterol and risk of atherosclerosis
The inverse association between plasma HDL-C and the risk of ASCVD is among the most consistent and reproducible associations in observational epidemiology.45,60 In contrast, Mendelian randomization studies do not provide compelling evidence that HDL-C is causally associated with the risk of ASCVD.49,61,62 However, this evidence must be interpreted with caution because most genetic variants associated with HDL-C are also associated with directionally opposite changes in TGs, LDL-C, or both, thus making estimates of the effect of HDL-C on the risk of ASCVD very difficult using the Mendelian randomization study design. Furthermore, there is no evidence from randomized trials that therapeutically increasing plasma HDL-C reduces the risk of CV events.63–67 In the Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome (dal-OUTCOMES) trial, treatment with the cholesteryl ester transfer protein (CETP) inhibitor dalcetrapib increased HDL-C without any effect on LDL-C or ApoB, but did not reduce the risk of major CV events.65 Similarly, in the Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High-Risk for Vascular Outcomes (ACCELERATE) and Randomized Evaluation of the Effects of Anacetrapib Through Lipid Modification (REVEAL) trials, treatment with CETP inhibitors more than doubled HDL-C levels, but did not appear to reduce the risk of ASCVD events beyond that expected from the modest reductions in ApoB levels.2,63,64 Furthermore, several randomized trials have shown that directly infused HDL mimetics increase plasma HDL-C concentrations, but do not reduce the progression of atherosclerosis as measured by intravascular ultrasound.68,69
Therefore, there is currently no randomized trial or genetic evidence to suggest that raising plasma HDL-C is likely to reduce the risk of ASCVD events. Whether therapies that alter the function of HDL particles will reduce the risk of ASCVD is unknown.
5.3.4 Lipoprotein(a) and risk of atherosclerosis
Lp(a) is an LDL particle with an Apo(a) moiety covalently bound to its ApoB component.70 It is <70 nm in diameter and can freely flux across the endothelial barrier, where it can become—similarly to LDL—retained within the arterial wall and thus may increase the risk of ASCVD. Pro-atherogenic effects of Lp(a) have also been attributed to pro-coagulant effects as Lp(a) has a similar structure to plasminogen, and it has pro-inflammatory effects most likely related to the oxidized phospholipid load carried by Lp(a).71
Higher plasma Lp(a) concentrations are associated with an increased risk of ASCVD, but it appears to be a much weaker risk factor for most people than LDL-C.72,73 In contrast, Mendelian randomization studies have consistently demonstrated that lifelong exposure to higher Lp(a) levels is strongly and causally associated with an increased risk of ASCVD.74,75 While randomized trials evaluating therapies that lower Lp(a) by 20–30% (including niacin and CETP inhibitors) have not provided evidence that lowering Lp(a) reduces the risk of ASCVD beyond that which would be expected from the observed reduction in ApoB-containing lipoproteins, recent data with PCSK9 inhibitors have suggested a possible role for Lp(a) lowering in reducing CV risk.76
This conflicting evidence appears to have been reconciled by a recent Mendelian randomization study that showed that the causal effect of Lp(a) on the risk of ASCVD is proportional to the absolute change in plasma Lp(a) levels. Importantly, this study also suggested that people with extremely high Lp(a) levels >180 mg/dL (>430 nmol/L) may have an increased lifetime risk of ASCVD similar to that of people with heterozygous FH (HeFH). Because about 90% of a person’s Lp(a) level is inherited, extremely elevated Lp(a) may represent a new inherited lipid disorder that is associated with extremely high lifetime risk of ASCVD and is two-fold more prevalent than HeFH.77 However, this study77 and another based on the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial78 have shown that large absolute changes in Lp(a) may be needed to produce a clinically meaningful reduction in the risk of ASCVD events.
5.4 Laboratory measurement of lipids and lipoproteins
Measurement of lipids and lipoproteins is used to estimate the risk of ASCVD and guide therapeutic decision-making. Quantification of plasma lipids can be performed on whole plasma and quantification of lipoproteins can be achieved by measuring their protein component. Operationally, lipoproteins are classified based on their hydrated density (see Table 6).
5.4.1 Lipoprotein measurement
Given the central causal role of ApoB-containing lipoproteins in the initiation and progression of atherosclerosis, direct measurement of the circulating concentration of atherogenic ApoB-containing lipoproteins to both estimate risk and guide treatment decisions would be ideal. Because all ApoB-containing lipoproteins—including VLDL, TG-rich remnant particles, and LDL—contain a single ApoB molecule, quantitation of ApoB directly estimates the number of atherogenic particles in plasma.
Standardized, automated, accurate, and inexpensive methods to measure ApoB are available. Fasting is not required because even in the post-prandial state, ApoB48-containing chylomicrons typically represent <1% of the total concentration of circulating ApoB-containing lipoproteins. Furthermore, the analytical performances of ApoB measurement methods are superior to the measurement or calculation of LDL-C and non-HDL-C.79
5.4.2 Lipid measurements
In clinical practice, the concentration of plasma lipoproteins is not usually measured directly but is instead estimated by measuring their cholesterol content. TC in humans is distributed primarily among three major lipoprotein classes: VLDL, LDL, and HDL. Smaller amounts of cholesterol are also contained in two minor lipoprotein classes: IDL and Lp(a). A standard serum lipid profile measures the concentration of TC and HDL-C, as well as TG. With these values, the LDL-C concentration can be estimated.
Plasma LDL-C can be measured directly using enzymatic techniques or preparative ultracentrifugation, but in clinical medicine it is most often calculated using the Friedewald formula:
LDL-C = TC − HDL-C − (TG/2.2) in mmol/L
or
LDL-C = TC − HDL-C − (TG/5) in mg/dL
Although convenient, the Friedewald calculated value of LDL-C has several well-established limitations: (i) methodological errors may accumulate since the formula necessitates three separate analyses of TC, TGs, and HDL-C; and (ii) a constant cholesterol/TG ratio in VLDL is assumed. With high TG values (>4.5 mmol/L or >400 mg/dL) the formula cannot be used. This should especially be considered in non-fasting samples.
To overcome the problems associated with calculated LDL-C, direct enzymatic methods for the measurement of LDL-C have been developed. These methods are commercially available as ready to use tools for automatic analysis. The definition of LDL-C by the Friedewald equation and by direct measurement is the same: non-HDL-C – VLDL-C, representing the sum of the cholesterol carried by the biochemically defined LDL, IDL, and Lp(a) subfractions.
For the general population, calculated LDL-C and direct LDL-C show very strong correlations.80–83 However, calculated LDL-C has been found to underestimate LDL-C at concentrations of TG ≥2 mmol/L (177 mg/dL).81,82 Equally, at very low levels of LDL-C, calculated LDL-C may be misleading, especially in the presence of high TG.81,84–86 To avoid some of the problems with the Friedewald formula, a number of modifications for the calculation of LDL-C have been suggested, but it remains to be proved whether these modifications are superior to Friedewald’s formula for the estimation of CV risk.81,85–87 It is important to note that direct LDL-C measurements also have limitations, including systematic bias and inaccuracy in patients with dyslipidaemia, especially for high TG levels.88–90
As an alternative calculated LDL-C, non-HDL-C can be calculated as TC – HDL-C and is a measure of the TC carried by all atherogenic ApoB-containing lipoproteins, including TG-rich particles in VLDL and their remnants.100
Several methods for the determination of Lp(a) are available. The complex molecular structure of Lp(a) and the variation in size of Apo(a) has been a challenge in the development of analytical methods for Lp(a). Available methods are, to a varying degree, influenced by the Apo(a) isoform.91 Furthermore, the concentration of Lp(a) is reported as either a molar concentration (nmol/L) or as a mass (mg/dL) by the various assays, and conversion between molar and mass concentrations has been found to be both size- and concentration-dependent.91–93 Therefore, standardization between assays is needed to establish a reliable and reproducible method for the quantification of Lp(a) mass or particle number.92
5.4.3 Fasting or non-fasting?
Traditionally, blood sampling for lipid analyses has been recommended in the fasting state. Recent systematic studies comparing fasting and non-fasting samples have suggested that the difference is small for most lipid parameters.85,94–100 Non-fasting sampling has been used in large population-based studies.100 In most studies, non-fasting samples display a higher TG level of ∼0.3 mmol/L (27 mg/dL).100,101 On average, and for most individuals, this increment will be of no clinical significance. Indeed, a number of guidelines recommend non-fasting sampling.100,102,103
For general risk screening, non-fasting samples seem to have at least the same prognostic value as fasting samples.104 The practical advantages of non-fasting samples, including better patient acceptability, outweigh the potential imprecision in some patients, although the determination of some key analytes, such as fasting glucose, may be compromised. Furthermore, even if non-fasting sampling can be used in most cases, in patients with metabolic syndrome (MetS), DM, or hypertriglyceridaemia (HTG), calculated LDL-C should be interpreted with caution.
5.5 Recommendations for measuring lipids and lipoproteins to estimate risk of atherosclerotic cardiovascular disease
Measurement of plasma TC is needed to calculate risk using SCORE, while the inclusion of plasma HDL-C level can improve risk estimation using the online SCORE calculator. Therefore, both TC and HDL-C should be measured to estimate a person’s risk of ASCVD using SCORE, or one of the other risk calculators (almost all of which also include measurements of TC and HDL-C).
Plasma LDL-C should be measured to estimate the risk of ASCVD that can be modified with LDL-C-lowering therapies, and to identify whether markedly elevated LDL-C levels are present that may suggest a lifetime high-risk of ASCVD due to lifelong cumulative exposure to high levels of atherogenic lipoproteins, such as in FH. Plasma LDL-C can be either calculated or measured directly.
Plasma TG should be assessed to identify people who may have a greater modifiable risk of ASCVD than is reflected by LDL-C, due to the presence of an increased concentration of atherogenic ApoB-containing TG-rich lipoproteins and their remnants, and to identify people in whom calculated and directly measured LDL-C may underestimate the risk of ASCVD by underestimating either the concentration of circulating LDL particles or the cholesterol content carried by those particles, such as those with very low levels of LDL. This may be especially relevant in patients with DM or MetS.
In general, LDL-C, non-HDL-C, and ApoB concentrations are very highly correlated. As a result, under most circumstances, they provide very similar information about ASCVD risk.45,105–108 However, under certain circumstances—including among people with elevated TG levels, DM, obesity, or very low achieved LDL-C levels—the calculated or directly measured LDL-C level may underestimate both the total concentration of cholesterol carried by LDL and, more importantly, underestimate the total concentration of ApoB-containing lipoproteins, thus underestimating the risk of ASCVD. In around 20% of patients there may be discordance between measured LDL-C and ApoB levels.85,109
Considering the potential inaccuracy of LDL-C in dyslipidaemia, among patients with DM or high TG levels, and in patients with very low LDL-C levels, measurement of both ApoB and non-HDL-C is recommended as part of routine lipid analysis for risk evaluation in patients with elevated plasma TGs. Because ApoB provides an accurate estimate of the total concentration of atherogenic particles under all circumstances, it is the preferred measurement to further refine the estimate of ASCVD risk that is modifiable by lipid-lowering therapy.
Lp(a) has a similar structure to plasminogen and binds to the plasminogen receptor, leading to increased thrombosis (pro-thrombotic factor). Measurement of Lp(a) should be considered at least once in each person’s lifetime, if available, to identify people who have inherited an extremely elevated level of Lp(a) ≥180 mg/dL (≥430 nmol/L) and therefore have a very high lifetime risk of ASCVD that is approximately equivalent to the risk associated with HeFH. In addition, this strategy can identify people with less-extreme Lp(a) elevations who may be at a higher risk of ASCVD, which is not reflected by the SCORE system, or by other lipid or lipoprotein measurements. Measurement of Lp(a) has been shown to provide clinically significant improved risk reclassification under certain conditions, and therefore should be considered in patients who have an estimated 10-year risk of ASCVD that is close to the threshold between high and moderate risk.110–112
Recommendations for measuring lipids and lipoproteins to estimate the risk of ASCVD are summarized below.
Recommendations for lipid analyses for cardiovascular disease risk estimation
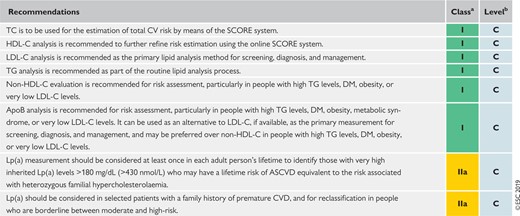 |
 |
Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; Lp(a) = lipoprotein(a); SCORE = Systematic Coronary Risk Estimation; TC = total cholesterol; TG = triglyceride.
Recommendations for lipid analyses for cardiovascular disease risk estimation
 |
 |
Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; Lp(a) = lipoprotein(a); SCORE = Systematic Coronary Risk Estimation; TC = total cholesterol; TG = triglyceride.
6 Treatment targets and goals
In previous EAS/ESC Guidelines for the management of dyslipidaemias1,113 and other major guidelines on the treatment of blood cholesterol to reduce atherosclerotic CV risk in adults,40,114 the importance of LDL-C lowering to prevent ASCVD is strongly emphasized. The European Task Force felt that limiting the current knowledge on CV prevention only to results from RCTs reduces the exploitation of the potential that is available for the prevention of ASCVD. It is the concordance of the conclusions from many different approaches (from basic science, clinical observations, genetics, epidemiology, RCTs, etc.) that contributes to the understanding of the causes of ASCVD and to the potential of prevention. The Task Force is aware of the limitations of some of the sources of evidence and accepts that RCTs have not examined different LDL-C goals systematically, but felt that it was appropriate to look at the totality of the evidence. Particular consideration was given to results from meta-analyses confirming the dose-dependent reduction in ASCVD with LDL-C-lowering agents; the greater the absolute LDL-C reduction, the greater the CV risk reduction.35,36,50,115 The benefits related to LDL-C reduction are not specific for statin therapy.33 No level of LDL-C below which benefit ceases or harm occurs has been defined.
There is considerable individual variability in the LDL-C response to dietary and drug treatments,31 which is traditionally taken to support a tailored approach to management. Total CV risk reduction should be individualized, and this can be more specific if goals are defined. The use of goals can also aid patient–doctor communication. It is judged that a goal approach may facilitate adherence to treatment, although this consensus opinion has not been fully tested. For all these reasons, the European Task Force retains a goal approach to lipid management and treatment goals are tailored to the total CV risk level. There is also evidence suggesting that lowering of LDL-C beyond the goals that were set in the previous EAS/ESC Guidelines is associated with fewer ASCVD events.34,116,117 Therefore, it seems appropriate to reduce LDL-C to as low a level as possible, at least in patients at very high CV risk, and for this reason a minimum 50% reduction is suggested for LDL reduction, together with reaching the tailored goal.
The lipid goals are part of a comprehensive CV risk reduction strategy and are summarized in Table 7. The rationales for the non-lipid targets are given in the 2016 ESC Joint Prevention Guidelines.10
Treatment targets and goals for cardiovascular disease prevention
| Smoking | No exposure to tobacco in any form. |
| Diet | Healthy diet low in saturated fat with a focus on wholegrain products, vegetables, fruit, and fish. |
| Physical activity | 3.5–7 h moderately vigorous physical activity per week or 30–60 min most days. |
| Body weight | BMI 20–25 kg/m2, and waist circumference <94 cm (men) and <80 cm (women). |
| Blood pressure | <140/90 mmHg.a |
| LDL-C |
|
| Non-HDL-C | Non-HDL-C secondary goals are <2.2, 2.6, and 3.4 mmol/L (<85, 100, and 130 mg/dL) for very-high-, high-, and moderate-risk people, respectively. |
| ApoB | ApoB secondary goals are <65, 80, and 100 mg/dL for very-high-, high-, and moderate-risk people, respectively. |
| Triglycerides | No goal, but <1.7 mmol/L (<150 mg/dL) indicates lower risk and higher levels indicate a need to look for other risk factors. |
| Diabetes | HbA1c: <7% (<53 mmol/mol). |
| Smoking | No exposure to tobacco in any form. |
| Diet | Healthy diet low in saturated fat with a focus on wholegrain products, vegetables, fruit, and fish. |
| Physical activity | 3.5–7 h moderately vigorous physical activity per week or 30–60 min most days. |
| Body weight | BMI 20–25 kg/m2, and waist circumference <94 cm (men) and <80 cm (women). |
| Blood pressure | <140/90 mmHg.a |
| LDL-C |
|
| Non-HDL-C | Non-HDL-C secondary goals are <2.2, 2.6, and 3.4 mmol/L (<85, 100, and 130 mg/dL) for very-high-, high-, and moderate-risk people, respectively. |
| ApoB | ApoB secondary goals are <65, 80, and 100 mg/dL for very-high-, high-, and moderate-risk people, respectively. |
| Triglycerides | No goal, but <1.7 mmol/L (<150 mg/dL) indicates lower risk and higher levels indicate a need to look for other risk factors. |
| Diabetes | HbA1c: <7% (<53 mmol/mol). |
Apo = apolipoprotein; BMI = body mass index; HbA1c = glycated haemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
Lower treatment targets are recommended for most treated hypertensive patients, provided that the treatment is well tolerated.118
The term ‘baseline’ refers to the LDL-C level in a person not taking any lipid-lowering medication, or to the extrapolated baseline value for those who are on current treatment.
Treatment targets and goals for cardiovascular disease prevention
| Smoking | No exposure to tobacco in any form. |
| Diet | Healthy diet low in saturated fat with a focus on wholegrain products, vegetables, fruit, and fish. |
| Physical activity | 3.5–7 h moderately vigorous physical activity per week or 30–60 min most days. |
| Body weight | BMI 20–25 kg/m2, and waist circumference <94 cm (men) and <80 cm (women). |
| Blood pressure | <140/90 mmHg.a |
| LDL-C |
|
| Non-HDL-C | Non-HDL-C secondary goals are <2.2, 2.6, and 3.4 mmol/L (<85, 100, and 130 mg/dL) for very-high-, high-, and moderate-risk people, respectively. |
| ApoB | ApoB secondary goals are <65, 80, and 100 mg/dL for very-high-, high-, and moderate-risk people, respectively. |
| Triglycerides | No goal, but <1.7 mmol/L (<150 mg/dL) indicates lower risk and higher levels indicate a need to look for other risk factors. |
| Diabetes | HbA1c: <7% (<53 mmol/mol). |
| Smoking | No exposure to tobacco in any form. |
| Diet | Healthy diet low in saturated fat with a focus on wholegrain products, vegetables, fruit, and fish. |
| Physical activity | 3.5–7 h moderately vigorous physical activity per week or 30–60 min most days. |
| Body weight | BMI 20–25 kg/m2, and waist circumference <94 cm (men) and <80 cm (women). |
| Blood pressure | <140/90 mmHg.a |
| LDL-C |
|
| Non-HDL-C | Non-HDL-C secondary goals are <2.2, 2.6, and 3.4 mmol/L (<85, 100, and 130 mg/dL) for very-high-, high-, and moderate-risk people, respectively. |
| ApoB | ApoB secondary goals are <65, 80, and 100 mg/dL for very-high-, high-, and moderate-risk people, respectively. |
| Triglycerides | No goal, but <1.7 mmol/L (<150 mg/dL) indicates lower risk and higher levels indicate a need to look for other risk factors. |
| Diabetes | HbA1c: <7% (<53 mmol/mol). |
Apo = apolipoprotein; BMI = body mass index; HbA1c = glycated haemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
Lower treatment targets are recommended for most treated hypertensive patients, provided that the treatment is well tolerated.118
The term ‘baseline’ refers to the LDL-C level in a person not taking any lipid-lowering medication, or to the extrapolated baseline value for those who are on current treatment.
The targeted approach to lipid management is primarily aimed at reducing atherosclerotic risk by substantially lowering LDL-C to levels that have been achieved in recent large-scale trials of PCSK-9 inhibitors. Therefore, for patients at very high CV risk, whether in secondary prevention or (rarely) in primary prevention, LDL-C reduction of ≥50% from baseline and an LDL-C goal of <1.4 mmol/L (<55 mg/dL) are recommended. For patients with ASCVD who experience a second vascular event within 2 years (not necessarily of the same type as the first event) while taking maximally tolerated statin-based therapy, an LDL-C goal <1.0 mmol/L (<40 mg/dL) may be considered.119,120 For people at high CV risk, an LDL-C reduction of ≥50% from baseline and an LDL-C goal <1.8 mmol/L (<70 mg/dL) are recommended. In patients at moderate CV risk, an LDL-C goal <2.6 mmol/L (<100 mg/dL) should be considered, while for low-risk individuals a goal of <3.0 mmol/L (<116 mg/dL) may be considered (see Recommendations for treatment goals for low-density lipoprotein cholesterol below and Supplementary Table 2).
Recommendations for treatment goals for low-density lipoprotein cholesterol
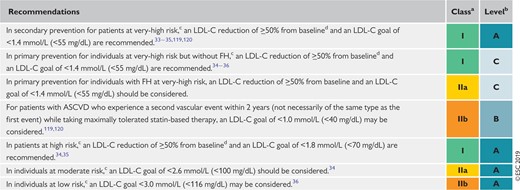 |
 |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol.
Class of recommendation.
Level of evidence.
For definitions see Table 4.
The term ‘baseline’ refers to the LDL-C level in a person not taking any LDL-C-lowering medication. In people who are taking LDL-C-lowering medication(s), the projected baseline (untreated) LDL-C levels should be estimated, based on the average LDL-C-lowering efficacy of the given medication or combination of medications.
Recommendations for treatment goals for low-density lipoprotein cholesterol
 |
 |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol.
Class of recommendation.
Level of evidence.
For definitions see Table 4.
The term ‘baseline’ refers to the LDL-C level in a person not taking any LDL-C-lowering medication. In people who are taking LDL-C-lowering medication(s), the projected baseline (untreated) LDL-C levels should be estimated, based on the average LDL-C-lowering efficacy of the given medication or combination of medications.
Secondary goals have also been defined by inference for non-HDL-C and for ApoB; they receive a moderate grading, as they have not been extensively studied in RCTs. The specific goal for non-HDL-C should be 0.8 mmol/L (30 mg/dL) higher than the corresponding LDL-C goal; the adjustment of lipid-lowering therapy in accordance with these secondary goals may be considered in patients at very high CV risk after achievement of an LDL-C goal, although the clinical advantages of this approach with respect to outcomes remain to be addressed. When secondary targets are used the recommendations are: (i) non-HDL-C <2.2 mmol/L (<85 mg/dL), <2.6 mmol/L (<100 mg/dL), and <3.4 mmol/L (<130 mg/dL) in people at very high, high, and moderate CV risk, respectively;121–123 and (ii) ApoB <65 mg/dL, <80 mg/dL, and <100 mg/dL in very-high, high, and moderate total CV risk, respectively.121,123,124
To date, no specific goals for HDL-C or TG levels have been determined in clinical trials, although increases in HDL-C predict atherosclerosis regression, and low HDL-C is associated with excess events and mortality in coronary artery disease (CAD) patients, even at low LDL levels. Clinicians should use clinical judgment when considering further treatment intensification in patients at high or very high total CV risk.
7 Lifestyle modifications to improve the plasma lipid profile
The pivotal role of nutrition in the prevention of ASCVD has been extensively reviewed.125–129 Dietary factors influence the development of CVD either directly or through their action on traditional risk factors, such as plasma lipids, BP, or glucose levels.
Convincing evidence of the causal association between diet and ASCVD risk is, nevertheless, available indirectly from randomized ‘metabolic ward’ studies showing that high saturated fat intake causes increased LDL-C concentrations, and from cohort studies, genetic epidemiological studies, and randomized trials showing that higher LDL-C levels cause ASCVD.
The lack of concordance between studies is due both to methodological problems (particularly inadequate sample sizes or short study durations) and the difficulties of evaluating the impact of a single dietary factor independently of any other changes in the diet.130 In fact, as foods are mixtures of different nutrients and other components, it is not appropriate to attribute the health effects of a food to only one of its components. Moreover, if energy intake must be kept constant, eating less of one macronutrient implies necessarily eating more of others. The quality of the replacement (for instance, unsaturated fat vs. highly refined grains) can influence the effect observed, significantly modifying the impact on health of the nutrient replaced. These limitations suggest caution in interpreting the results of RCTs or even meta-analyses of RCTs in relation to the effect of a single dietary change on ASCVD.130
To overcome, at least in part, these problems, in recent years nutrition research has focused on the relationship between ASCVD on the one hand, and foods and dietary patterns—rather than single nutrients—on the other. Consistent evidence from epidemiological studies indicates that higher consumption of fruit, non-starchy vegetables, nuts, legumes, fish, vegetable oils, yoghurt, and wholegrains, along with a lower intake of red and processed meats, foods higher in refined carbohydrates, and salt, is associated with a lower incidence of CV events.131 Moreover, it indicates that the replacement of animal fats, including dairy fat, with vegetable sources of fats and polyunsaturated fatty acids (PUFAs) may decrease the risk of CVD.132
Dietary patterns that have been more extensively evaluated are the Dietary Approaches to Stop Hypertension (DASH) diet—particularly in relation to BP control—and the Mediterranean diet; both have proved to be effective in reducing CV risk factors and, possibly, to contribute to ASCVD prevention.133 The most relevant difference between the Mediterranean and the DASH diet is the emphasis of the former on extra-virgin olive oil. The Mediterranean diet is associated with a reduced incidence of CV and other non-communicable diseases in epidemiological studies,134,135 and has been proved in RCTs to be effective in reducing CV events in primary and secondary prevention.136 In particular, the Prevención con Dieta Mediterránea (PREDIMED) trial indicated that participants allocated to a Mediterranean-type diet, supplemented with extra-virgin olive oil or nuts, had a significantly lower (around 30%) incidence of major CV events compared with those who were on a low-fat diet.137
In summary, despite the results of PREDIMED and a few other intervention studies with ASCVD endpoints that support a healthy lifestyle for ASCVD prevention, RCTs cannot represent the sole grounds on which dietary recommendations should rely. They also need to be based on the combination of large observational cohort studies and relatively short-term randomized trials having intermediate risk factors (such as blood lipids) as outcomes.
Table 8 summarizes the currently available evidence on the influences of lifestyle changes and functional foods on lipoproteins, indicating the magnitudes of the effects and the levels of evidence in relation to the impacts on the specific lipoprotein class; for the reasons outlined above, the levels of evidence are not based on RCTs with ASCVD endpoints. Moreover, within the Guidelines on the management of dyslipidaemias, information on the potential to improve plasma lipoprotein profiles by dietary means is clinically relevant, even in the absence of a clear demonstration of CV benefits.
Impact of specific lifestyle changes on lipid levels
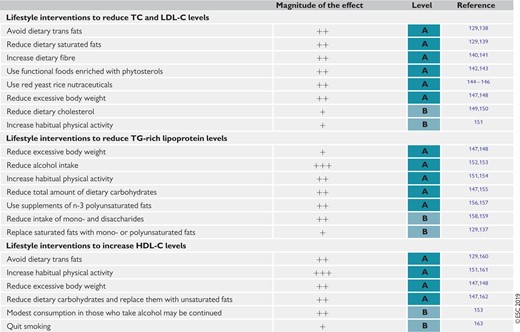 |
 |
The magnitude of the effect (+++ = >10%, ++ = 5–10%, + = <5%) and the level of evidence refer to the impact of each dietary modification on plasma levels of a specific lipoprotein class.
HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; TG = triglyceride.
Impact of specific lifestyle changes on lipid levels
 |
 |
The magnitude of the effect (+++ = >10%, ++ = 5–10%, + = <5%) and the level of evidence refer to the impact of each dietary modification on plasma levels of a specific lipoprotein class.
HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; TG = triglyceride.
7.1 Influence of lifestyle on total cholesterol and low-density lipoprotein cholesterol levels
Saturated fatty acids (SFAs) are the dietary factor with the greatest impact on LDL-C levels (0.02–0.04 mmol/L or 0.8–1.6 mg/dL of LDL-C increase for every additional 1% energy coming from saturated fat).164 Quantitatively, dietary trans fatty acids have a similar elevating effect on LDL-C to that of SFAs; however, while SFAs increase HDL-C levels, trans fats decrease them.137 Trans unsaturated fatty acids can be found in limited amounts (usually <5% of total fat) in dairy products and in meats from ruminants. ‘Partially hydrogenated fatty acids’ of industrial origin represent the major source of trans fatty acids in the diet; the average consumption of trans fatty acids ranges from 0.2–6.5% of the total energy intake in different populations.165 Unsaturated fat-rich oils from safflower, sunflower, rapeseed, flaxseed, corn, olives, or soybean were shown to reduce LDL-C levels (−0.42 to −0.20 mmol/L) when used in substitution of SFA-rich foods like butter or lard.166 The effects of carbohydrate consumption on LDL-C are described in section 7.4.3.
Body weight reduction also influences TC and LDL-C levels, but the magnitude of the effect is small: in obese people, a decrease in LDL-C concentration of 0.2 mmol/L (8 mg/dL) is observed for every 10 kg of weight loss.147,167 The reduction of LDL-C levels induced by regular physical exercise is even smaller.151,168 The benefits of weight reduction and physical exercise on the CV risk profile likely impact on other risk factors, especially hypertension and diabetes.
Table 9 summarizes the possible choices of foods to lower TC and LDL-C levels. Given the cultural diversity of the European populations, they should be translated into practical behaviours, considering local habits and socio-economic factors.
Food choices to lower low-density lipoprotein cholesterol and improve the overall lipoprotein profile
| . | To be preferred . | To be used in moderation . | To be chosen occasionally in limited amounts . |
|---|---|---|---|
| Cereals | Wholegrains | Refined bread, rice, and pasta, biscuits, corn flakes | Pastries, muffins, pies, croissants |
| Vegetables | Raw and cooked vegetables | Potatoes | Vegetables prepared in butter or cream |
| Legumes | Lentils, beans, fava beans, peas, chickpeas, soybean | ||
| Fruit | Fresh or frozen fruit | Dried fruit, jelly, jam, canned fruit, sorbets, ice lollies/popsicles, fruit juice | |
| Sweets and sweeteners | Non-caloric sweeteners | Sucrose, honey, chocolate, sweets/candies | Cakes, ice creams, fructose, soft drinks |
| Meat and fish | Lean and oily fish, poultry without skin | Lean cuts of beef, lamb, pork, and veal, seafood, shellfish | Sausages, salami, bacon, spare ribs, hot dogs, organ meats |
| Dairy food and eggs | Skimmed milk and yoghurt | Low-fat milk, low-fat cheese and other milk products, eggs | Regular cheese, cream, whole milk and yoghurt |
| Cooking fat and dressings | Vinegar, mustard, fat-free dressings | Olive oil, non-tropical vegetable oils, soft margarines, salad dressing, mayonnaise, ketchup | Trans fats and hard margarines (better to avoid them), palm and coconut oils, butter, lard, bacon fat |
| Nuts/seeds | All, unsalted (except coconut) | Coconut | |
| Cooking procedures | Grilling, boiling, steaming | Stir-frying, roasting | Frying |
| . | To be preferred . | To be used in moderation . | To be chosen occasionally in limited amounts . |
|---|---|---|---|
| Cereals | Wholegrains | Refined bread, rice, and pasta, biscuits, corn flakes | Pastries, muffins, pies, croissants |
| Vegetables | Raw and cooked vegetables | Potatoes | Vegetables prepared in butter or cream |
| Legumes | Lentils, beans, fava beans, peas, chickpeas, soybean | ||
| Fruit | Fresh or frozen fruit | Dried fruit, jelly, jam, canned fruit, sorbets, ice lollies/popsicles, fruit juice | |
| Sweets and sweeteners | Non-caloric sweeteners | Sucrose, honey, chocolate, sweets/candies | Cakes, ice creams, fructose, soft drinks |
| Meat and fish | Lean and oily fish, poultry without skin | Lean cuts of beef, lamb, pork, and veal, seafood, shellfish | Sausages, salami, bacon, spare ribs, hot dogs, organ meats |
| Dairy food and eggs | Skimmed milk and yoghurt | Low-fat milk, low-fat cheese and other milk products, eggs | Regular cheese, cream, whole milk and yoghurt |
| Cooking fat and dressings | Vinegar, mustard, fat-free dressings | Olive oil, non-tropical vegetable oils, soft margarines, salad dressing, mayonnaise, ketchup | Trans fats and hard margarines (better to avoid them), palm and coconut oils, butter, lard, bacon fat |
| Nuts/seeds | All, unsalted (except coconut) | Coconut | |
| Cooking procedures | Grilling, boiling, steaming | Stir-frying, roasting | Frying |
Food choices to lower low-density lipoprotein cholesterol and improve the overall lipoprotein profile
| . | To be preferred . | To be used in moderation . | To be chosen occasionally in limited amounts . |
|---|---|---|---|
| Cereals | Wholegrains | Refined bread, rice, and pasta, biscuits, corn flakes | Pastries, muffins, pies, croissants |
| Vegetables | Raw and cooked vegetables | Potatoes | Vegetables prepared in butter or cream |
| Legumes | Lentils, beans, fava beans, peas, chickpeas, soybean | ||
| Fruit | Fresh or frozen fruit | Dried fruit, jelly, jam, canned fruit, sorbets, ice lollies/popsicles, fruit juice | |
| Sweets and sweeteners | Non-caloric sweeteners | Sucrose, honey, chocolate, sweets/candies | Cakes, ice creams, fructose, soft drinks |
| Meat and fish | Lean and oily fish, poultry without skin | Lean cuts of beef, lamb, pork, and veal, seafood, shellfish | Sausages, salami, bacon, spare ribs, hot dogs, organ meats |
| Dairy food and eggs | Skimmed milk and yoghurt | Low-fat milk, low-fat cheese and other milk products, eggs | Regular cheese, cream, whole milk and yoghurt |
| Cooking fat and dressings | Vinegar, mustard, fat-free dressings | Olive oil, non-tropical vegetable oils, soft margarines, salad dressing, mayonnaise, ketchup | Trans fats and hard margarines (better to avoid them), palm and coconut oils, butter, lard, bacon fat |
| Nuts/seeds | All, unsalted (except coconut) | Coconut | |
| Cooking procedures | Grilling, boiling, steaming | Stir-frying, roasting | Frying |
| . | To be preferred . | To be used in moderation . | To be chosen occasionally in limited amounts . |
|---|---|---|---|
| Cereals | Wholegrains | Refined bread, rice, and pasta, biscuits, corn flakes | Pastries, muffins, pies, croissants |
| Vegetables | Raw and cooked vegetables | Potatoes | Vegetables prepared in butter or cream |
| Legumes | Lentils, beans, fava beans, peas, chickpeas, soybean | ||
| Fruit | Fresh or frozen fruit | Dried fruit, jelly, jam, canned fruit, sorbets, ice lollies/popsicles, fruit juice | |
| Sweets and sweeteners | Non-caloric sweeteners | Sucrose, honey, chocolate, sweets/candies | Cakes, ice creams, fructose, soft drinks |
| Meat and fish | Lean and oily fish, poultry without skin | Lean cuts of beef, lamb, pork, and veal, seafood, shellfish | Sausages, salami, bacon, spare ribs, hot dogs, organ meats |
| Dairy food and eggs | Skimmed milk and yoghurt | Low-fat milk, low-fat cheese and other milk products, eggs | Regular cheese, cream, whole milk and yoghurt |
| Cooking fat and dressings | Vinegar, mustard, fat-free dressings | Olive oil, non-tropical vegetable oils, soft margarines, salad dressing, mayonnaise, ketchup | Trans fats and hard margarines (better to avoid them), palm and coconut oils, butter, lard, bacon fat |
| Nuts/seeds | All, unsalted (except coconut) | Coconut | |
| Cooking procedures | Grilling, boiling, steaming | Stir-frying, roasting | Frying |
7.2 Influence of lifestyle on triglyceride levels
Weight reduction improves insulin sensitivity and decreases TG levels. Regular physical exercise reduces plasma TG levels over and above the effect of weight reduction.151,168,169 Alcohol intake has a major impact on TG levels, particularly in individuals with HTG.153,170 The detrimental effects of a high-carbohydrate diet on TGs occur mainly when refined carbohydrate-rich foods are consumed, while they are much less prominent if the diet is based largely on fibre-rich, low-glycaemic index foods. This applies particularly to people with DM or MetS.171,172
Habitual consumption of significant amounts (>10% energy) of dietary fructose contributes to TG elevation, particularly in people with HTG or abdominal obesity. These effects are dose-dependent; with a habitual fructose consumption between 15–20% of total energy intake, plasma TG increases by as much as 30–40%. Sucrose, a disaccharide containing glucose and fructose, represents an important source of fructose in the diet.159,173,174
7.3 Influence of lifestyle on high-density lipoprotein cholesterol levels
Weight reduction increases HDL-C levels; a 0.01 mmol/L (0.4 mg/dL) increase is observed for every kilogram decrease in body weight when weight reduction has stabilized. Aerobic physical activity, such as 25–30 km of brisk walking per week (or any equivalent activity), may increase HDL-C levels by 0.08–0.15 mmol/L (3.1–6 mg/dL).169 Smoking cessation may also contribute to HDL-C elevation, provided that weight gain is prevented.163
7.4 Lifestyle recommendations to improve the plasma lipid profile
LDL-C lowering represents the primary target for reducing CV risk and therefore deserves special emphasis in the evaluation of lifestyle measures. The diet recommended to the general population, and particularly to people at increased CV risk, may also be able to modify plasma TG and HDL-C levels (Table 9). This section focuses on dietary and other lifestyle factors that may be implemented to improve the overall lipoprotein profile.
7.4.1 Body weight and physical activity
Since overweight, obesity, and—in particular—abdominal adiposity often contribute to dyslipidaemia, caloric intake should be reduced and energy expenditure increased in those with excessive weight and/or abdominal adiposity.
In the case of excess weight, body weight reduction, even if modest (5–10% of basal body weight), improves lipid abnormalities and favourably affects the other CV risk factors often present in dyslipidaemic individuals.148 While the beneficial effects of weight reduction on metabolic and surrogate markers have been demonstrated, the benefits of weight loss on mortality and CV outcome are less clear.175
Weight reduction can be achieved by decreasing the consumption of energy-dense foods, inducing a caloric deficit of 300–500 kcal/day. The intervention should combine diet and exercise; this approach also leads to the greatest improvement in physical performance and quality of life, and mitigates reductions in muscle and bone mass, particularly in older people.176 It is always appropriate to advise people with dyslipidaemia to engage in regular physical exercise of moderate intensity for ≥30 min/day, even if they are not overweight.168
7.4.2 Dietary fat
Avoiding any consumption of trans fat is a key measure of the dietary prevention of CVD. The trans fatty acids produced in the partial hydrogenation of vegetable oils account for 80% of total intake. Thanks to efforts made in different parts of the world, the intake of trans fatty acids has decreased substantially over the past 10–15 years.
As for saturated fat, its consumption should be <10% of the total caloric intake and should be further reduced (<7% of energy) in the presence of hypercholesterolaemia. For most individuals, a wide range of total fat intakes is acceptable, and will depend upon individual preferences and characteristics. However, fat intakes >35–40% of calories are generally associated with increased intakes of both saturated fat and calories. Conversely, low intakes of fats and oils increase the risk of inadequate intakes of vitamin E and of essential fatty acids, and may contribute to a reduction of HDL-C.164
Fat intake should predominantly come from sources of monounsaturated fatty acids, including both n-6 and n-3 PUFAs. Not enough data are available to make a recommendation regarding the optimal n-3:n-6 fatty acid ratio.177,178 The cholesterol intake in the diet should be reduced (<300 mg/day), particularly in people with high plasma cholesterol levels.
7.4.3 Dietary carbohydrate and fibre
Dietary carbohydrate has a ‘neutral’ effect on LDL-C, although excessive consumption is represented by untoward effects on plasma TGs and HDL-C levels.164 Dietary fibre (particularly of the soluble type)—which is present in legumes, fruits, vegetables, and wholegrain cereals (e.g. oats and barley)—has a hypocholesterolaemic effect and represents a good dietary substitute for saturated fat to maximize the effects of the diet on LDL-C levels, and to minimize the untoward effects of a high-carbohydrate diet on other lipoproteins.140,179
Carbohydrate intake should range between 45–55% of total energy intake, since both higher and lower percentages of carbohydrate diets are associated with increased mortality.180,181 A fat-modified diet that provides 25–40 g per day of total dietary fibre, including ≥7–13 g of soluble fibre, is well tolerated, effective, and recommended for plasma lipid control; conversely, there is no justification for the recommendation of very low-carbohydrate diets.182
Intake of added sugar should not exceed 10% of total energy (in addition to the amount present in natural foods such as fruits and dairy products); more restrictive advice concerning sugars may be useful for those needing to lose weight or with high plasma TG values, MetS, or DM. Soft drinks should be used with moderation by the general population, and should be drastically limited in those individuals with elevated TG values or visceral adiposity.158,159,174 The Prospective Urban Rural Epidemiology (PURE) study was a large, epidemiological cohort study of 135 335 individuals enrolled in 18 countries with food frequency questionnaires recorded. Total fat and types of fat were not associated with CVD, MI, or CVD mortality, whereas saturated fat had an inverse association with stroke.181 However, a meta-analysis of epidemiological studies including the PURE study showed a U-shaped relationship between carbohydrate intake and mortality: diets associated with the highest mortality rate had carbohydrate intakes >70% and <40% of energy, with minimal risk observed when carbohydrate intake was between 45–55% of total energy intake.180
7.4.4 Alcohol
Moderate alcohol consumption [≤10 g/day (1 unit) for men and women] is acceptable for those who drink alcoholic beverages, if TG levels are not elevated.183,184
7.4.5 Smoking
Smoking cessation has clear benefits regarding overall CV risk, and specifically on HDL-C levels.163
7.5 Dietary supplements and functional foods for the treatment of dyslipidaemias
Nutritional evaluation of functional foods includes not only the search for clinical evidence of beneficial effects relevant to improved health or the reduction of disease risk, but also the demonstration of good tolerability. Overall, the available evidence on functional foods so far identified in this field is incomplete; the major gap is an absence of diet-based intervention trials of enough duration to be relevant for the natural history of dyslipidaemia and CVD.
7.5.1 Phytosterols
The principal phytosterols are sitosterol, campesterol, and stigmasterol; they occur naturally in vegetable oils and in smaller amounts in vegetables, fresh fruits, nuts, grains, and legumes. The dietary intake of plant sterols ranges between an average of 250 mg/day in Northern Europe to ∼500 mg/day in Mediterranean countries. Phytosterols compete with cholesterol for intestinal absorption, thereby modulating TC levels.
Daily consumption of 2 g of phytosterols can effectively lower TC and LDL-C levels by 7–10% in humans (with a certain degree of heterogeneity among individuals), while it has little or no effect on HDL-C and TG levels.143 However, to date no studies have been performed on the subsequent effect on CVD. Based on LDL-C lowering and the absence of adverse signals, functional foods with plant sterols/stanols (≥2 g/day with the main meal) may be considered: (i) in individuals with high cholesterol levels at intermediate or low global CV risk who do not qualify for pharmacotherapy; (ii) as an adjunct to pharmacological therapy in high- and very-high-risk patients who fail to achieve LDL-C goals on statins or could not be treated with statins; and (iii) in adults and children (aged >6 years) with FH, in line with current guidance.142
7.5.2 Monacolin and red yeast rice
Red yeast rice (RYR) is a source of fermented pigment that has been used in China as a food colorant and flavour enhancer for centuries. Hypocholesterolaemic effects of RYR are related to a statin-like mechanism—inhibition of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase—of monacolins, which represent the bioactive ingredient. Different commercial preparations of RYR have different concentrations of monacolins, and lower TC and LDL-C levels to variable extents, but the consumer is not able to make that distinction.144,185 Moreover, the long-term safety of the regular consumption of these products has not been fully documented and safety issues due to the possible presence of contaminants in some preparations have been raised. Side effects like those observed with statins have also been reported.
In the only available RCT in patients with ASCVD, a partially purified extract of RYR reduced recurrent events by 45%.146 A clinically relevant hypocholesterolaemic effect (up to a 20% reduction) has been observed with RYR preparations providing an o.d. [once daily (omni die)] dose of 2.5–10 mg monacolin K.145 Nutraceuticals containing purified RYR may be considered in people with elevated plasma cholesterol concentrations who do not qualify for treatment with statins in view of their global CV risk. However, there is a clear need for better regulation of RYR supplements. Information regarding the precise composition of these products, the quantities of their components, and their purity should be implemented.185
7.5.3 Dietary fibre
Available evidence consistently demonstrates a TC- and LDL-C-lowering effect of β-glucan, a viscous fibre from oat and barley. Foods enriched with these fibres or supplements are well tolerated, effective, and recommended for LDL-C lowering.186 However, the dosage needed to achieve a clinically relevant reduction in levels of LDL-C of 3–5% varies from 3–10 g per day according to the specific type of fibre.187
7.5.4 Soy
The cholesterol-lowering effect of soy is generally attributed to its isoflavone and phytoestrogen content, which decreases progressively with the increasing degree of soybean processing. Soy protein has also been indicated as being able to induce a modest LDL-C-lowering effect when replacing animal protein foods. However, this was not confirmed when changes in other dietary components were taken into account.187,188
7.5.5 Policosanol and berberine
Policosanol is a natural mixture of long-chain aliphatic alcohols extracted primarily from sugarcane wax.189 Studies show that policosanol from sugarcane, rice or wheat germ has no significant effect on LDL-C, HDL-C, TG, ApoB, Lp(a), homocysteine, high-sensitivity C-reactive protein, fibrinogen, or blood coagulation factor levels.190
As for berberine, a recent meta-analysis evaluated its effects on plasma lipids in humans.191 The comparative evaluation of berberine and lifestyle intervention or placebo indicated that in the berberine group, LDL-C and plasma TG levels were more effectively reduced than in the control group. However, due to the lack of high-quality randomized clinical trials, the efficacy of berberine for treating dyslipidaemia needs to be further validated. Moreover, the bioavailability of the different berberine preparations is a matter of debate.187
7.5.6 n-3 unsaturated fatty acids
Observational evidence indicates that consumption of fish (at least twice a week) and vegetable foods rich in n-3 fatty acids (α-linoleic acid is present in walnuts, some vegetables, and some seed oils) is associated with lower risk of CV death and stroke, but has no major effects on plasma lipoprotein metabolism.178,192 Pharmacological doses of long-chain n-3 fatty acids (2–3 g/day) reduce TG levels by about 30% and also reduce the post-prandial lipaemic response, but a higher dosage may increase LDL-C levels. α-Linolenic acid is less effective at altering TG levels.156,193 Recently, a significantly lower risk of ischaemic events, including CV death, was observed in patients with elevated TG levels despite the use of statins treated with 2 g of icosapent ethyl b.i.d. [twice a day].194
Other features of a healthy diet contributing to CVD prevention are presented in the Supplementary Data.
8 Drugs for treatment of dyslipidaemias
8.1 Statins
8.1.1 Mechanism of action
Statins reduce the synthesis of cholesterol in the liver by competitively inhibiting the enzyme HMG-CoA reductase, the rate-limiting step in cholesterol biosynthesis. The reduction in intracellular cholesterol promotes increased LDL receptor (LDLR) expression at the surface of the hepatocytes, which in turn results in increased uptake of LDL from the blood, and decreased plasma concentrations of LDL- and other ApoB-containing lipoproteins, including TG-rich particles.
8.1.2 Effects on lipids
8.1.2.1 Low-density lipoprotein cholesterol.
The degree of LDL-C reduction is dose-dependent and varies between the different statins. A high-intensity regimen is defined as the dose of a statin that, on average, reduces LDL-C by ≥50%; moderate-intensity therapy is defined as the dose expected to reduce LDL-C by 30–50%. Notably, there is considerable interindividual variation in LDL-C reduction with the same dose of drug.31 Poor responses to statin treatment in clinical studies are to some extent caused by poor compliance, but may also be explained by genetic backgrounds.195,196 Interindividual variations in statin responses warrant monitoring of responses on initiation of therapy.
Among patients who cannot tolerate the recommended intensity of a statin because of adverse effects or those who do not reach their goal, the addition of a non-statin lipid-modifying agent to a maximally tolerated statin is recommended.197,198
8.1.2.2 Triglycerides.
Statins usually reduce TG levels by 10–20% from baseline values.199 More potent statins (atorvastatin, rosuvastatin, and pitavastatin) demonstrate robust lowering of TG levels, especially at high doses and in patients with elevated TGs (HTG), in whom the absolute risk, and therefore the absolute risk reduction, is larger.
The mechanism of the TG-lowering effect has not been fully elucidated, but it seems to be partly independent of the LDLR pathway. It may involve the upregulation of VLDL uptake by hepatocytes, as well as a reduction of the production rate of VLDLs; these effects seem to be dependent on pre-treatment VLDL concentrations.200
8.1.2.3 High-density lipoprotein cholesterol.
In a meta-analysis,201 elevations in HDL-C levels varied with dose among the respective statins; such elevations ranged from 1–10%. However, given the marked statin-mediated decrement in atherogenic ApoB-containing lipoproteins, the extent to which the very modest effect on HDL-C levels might contribute to the overall observed reductions in CV risk consistently observed in statin intervention trials cannot reliably be disentangled.
8.1.2.4 Lipoprotein(a).
Statins only marginally affect Lp(a) plasma levels. Previous studies have reported either no effect on or an increase of Lp(a) levels after statin treatment.202,203 The mechanisms by which statins raise oxidized phospholipids on Lp(a) require further investigation.
8.1.3 Other effects of statins
Although reduction of LDL-C levels is the major effect of statins, a number of other, potentially important effects have been suggested (pleiotropic effects of statins).204,205 Among such effects that are potentially relevant for the prevention of CVD are the anti-inflammatory and antioxidant effects of statin treatment. These effects have been shown in vitro and in experimental systems, but their clinical relevance remains unproven.18,206
8.1.3.1 Effect on cardiovascular morbidity and mortality.
A large number of meta-analyses have been performed to analyse the effects of statins in populations and in subgroups.34–36,38,51,207–214 In the Cholesterol Treatment Trialists (CTT) meta-analysis of individual participant data (IPD) from >170 000 participants in 26 RCTs of a statin vs. control or a more vs. less intensive statin regimen,34 for each 1 mmol/L reduction in LDL-C, statin/more statin reduced major vascular events (MI, CAD death, or any stroke or coronary revascularization) by ∼22%, major coronary events by 23%, CAD death by 20%, total stroke by 17%, and total mortality by 10% over 5 years. The proportional effects (per mmol/L reduction in LDL-C) on major vascular events were similar in all subgroups examined, so the absolute risk reduction was proportional to the absolute baseline risk. The relative benefits were half as large in the first year as compared with subsequent years. There was no increased risk for any non-CV cause of death, including cancer, in those allocated statins. The absolute benefit from statin treatment was lower in people in primary prevention, who are typically at lower risk.36,38,214,215 In the CTT meta-analysis of treatment in people with a low-risk of vascular disease,36 the relative risk reduction of major vascular events per mmol/L reduction in LDL-C was at least as large in low-risk individuals (i.e. in primary prevention). In those without a history of vascular disease, statin therapy reduced the risk of all-cause mortality by 9% per mmol/L reduction in LDL cholesterol. Similar results were reported in a Cochrane review in 2013.213 The West of Scotland Coronary Prevention Study (WOSCOPS) data were recently reanalysed, and demonstrated that even people without DM and a 10 year predicted ASCVD risk of <7.5% benefit from statin treatment. There was also a legacy effect with a mortality benefit of 18% in all-cause death over 20 years.216 Statins are effective for the prevention of ASCVD in the elderly, including those aged >75 years.217 Statins are not effective in a few specific groups, notably those with heart failure (HF) or patients receiving haemodialysis.214,218–222
Current available evidence from meta-analyses suggests that the clinical benefit of statin treatment is largely a class effect, driven by the absolute LDL-C reduction; therefore, the type of statin used should reflect the treatment goals for a given patient.
The following scheme may be proposed.
Evaluate the total CV risk of the individual.
Determine the treatment goals (depending on current risk).
Involve the patient in decisions on CV risk management.
Choose a statin regimen and, where necessary, additional treatments (e.g. ezetimibe or PCSK9 inhibitors) that can meet the treatment goals (per cent and absolute value).
Response to statin treatment is variable, therefore uptitration of the statin dose may be required before additional LDL-lowering treatments are started.
These are general criteria for the choice of drug. Factors such as the clinical condition of the patient, concomitant medications, drug tolerability, local treatment tradition, and drug cost will play major roles in determining the final choice of drug and dose.
Furthermore, the effects of statins on a number of other clinical conditions have been evaluated. For cancer, a meta-analysis of IPD from randomized trials has shown that statins do not have any significant effect on cancer, at least over a period of ∼5 years.223 Other conditions, such as dementia,224 hepatic steatosis,225 venous thromboembolism,226 atrial fibrillation,227,228 and polycystic ovary syndrome229 have also been studied, and no effect of statins on these conditions has been reliably demonstrated.
The suggested effect on Alzheimer's disease was recently reviewed in a Cochrane analysis reporting no conclusive effect from statins.230 Furthermore, neurocognitive functions were extensively investigated in the Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects (EBBINGHAUS) study231 and no excess risk was observed among patients on a statin regimen randomized to a PCSK9 mAb.
8.1.4 Adverse effects and interactions of statins
Statins differ in their absorption, bioavailability, plasma protein binding, excretion, and lipophilicity. Evening administration is usually recommended. Lovastatin and simvastatin are prodrugs, whereas the other available statins are administered in their active form. Their bioavailability is relatively low, owing to a first-pass effect in the liver, and many statins undergo significant hepatic metabolism via cytochrome P450 (CYP) isoenzymes, except pravastatin, rosuvastatin, and pitavastatin. These enzymes are expressed mainly in the liver and gut wall. Although statins are generally very well tolerated, they do have some specific adverse effects on muscle, glucose haemostasis, and haemorrhagic stroke. However, there is also widespread misinformation about potential adverse effects, as reviewed recently.232,233
8.1.4.1 Adverse effects on muscle.
Myopathy is the most clinically relevant adverse effect of statins. Among the risk factors for myopathy, it is particularly important that interaction with concomitant drug therapy is considered (see below). Rhabdomyolysis is the most severe form of statin-induced muscle damage, characterized by severe muscular pain, muscle necrosis, and myoglobinuria potentially leading to renal failure and death. In rhabdomyolysis, creatine kinase (CK) levels are elevated by ≥10 times, and often ≥40 times, the upper limit of normal (ULN).234 The frequency of rhabdomyolysis has been estimated to represent 1–3 cases/100 000 patient-years.235 Patients taking statin therapy frequently report muscle symptoms [so-called ‘statin-associated muscle symptoms’ (SAMS)], and in non-randomized, observational studies, statins are associated with muscular pain and tenderness (myalgia) without CK elevation or major functional loss, with the reported frequency of SAMS in such studies varying between 10–15% among statin-treated individuals.236–238 However, in part because individuals in observational studies are not blind to the treatment they are receiving, such studies are unreliable when used to assess the adverse effects of statins.233 In contrast, in blinded randomized trials of statins vs. placebo there is no, or only a slightly, increased frequency of muscle symptoms in statin-allocated groups.239,240 The Anglo-Scandinavian Cardiac Outcomes Trial – Lipid-Lowering Arm (ASCOT-LLA) study addressed this issue by comparing the incidence of four different adverse events, including muscle-related symptoms, during both the blinded, placebo-controlled trial and its open-label extension study.238 They concluded that a nocebo effect (i.e. one caused by negative expectations) may partly explain the higher frequency of SAMS in observational studies compared with in trials. Suggested practical management of muscular symptoms is shown in Supplementary Figure 6.198,234,241 Several studies have shown a considerable LDL-C-lowering effect of alternative dosing, such as every other day or twice a week, with atorvastatin or rosuvastatin.242 Although no clinical endpoint trials are available, this strategy should be considered in high-risk patients in whom statin treatment with daily doses is not possible.
8.1.4.2 Adverse effects on the liver.
The activity of alanine aminotransferase (ALT) in plasma is commonly used to assess hepatocellular damage. Mild elevation of ALT occurs in 0.5–2.0% of patients on statin treatment, more commonly with potent statins or high doses. The common definition of clinically relevant ALT elevation has been an increase of three times the ULN on two consecutive occasions. Mild elevation of ALT has not been shown to be associated with true hepatotoxicity or changes in liver function. Progression to liver failure is exceedingly rare, therefore routine monitoring of ALT during statin treatment is no longer recommended.243 Patients with mild ALT elevation due to steatosis have been studied during statin treatment and there is no indication that statins cause any worsening of liver disease.244–246
8.1.4.3 Increased risk of new-onset diabetes mellitus.
Patients on statin treatment have been shown to exhibit an increased risk of dysglycaemia and development of type 2 diabetes mellitus (T2DM). Several studies have shown that this is a consistent, dose-related effect.232 A minor, not clinically relevant elevation of glycated haemoglobin (HbA1c) has also been observed. The number needed to cause one case of diabetes has been estimated as 255 over 4 years of statin treatment.247 However, the risk is higher with the more potent statins at high doses,248 and is also higher in the elderly, and in the presence of other risk factors for diabetes such as overweight or insulin resistance.249 Overall, the absolute reduction in the risk of CVD in high-risk patients clearly outweighs the possible adverse effects of a small increase in the incidence of diabetes.233 This effect is probably related to the mechanism of action of statins, as Mendelian randomization studies have confirmed the increased risk of DM in individuals with HMG-CoA reductase polymorphisms that reduce cholesterol synthesis.250
8.1.4.4 Increased risk of haemorrhagic stroke.
In observational studies, TC is negatively associated with haemorrhagic stroke, and in the CTT meta-analysis, there was a 21% [95% confidence interval (CI) 5–41%; P=0.01] relative increase per mmol/L lower LDL cholesterol in haemorrhagic stroke.34,251,252 However, other meta-analyses have yielded conflicting findings and there is a need for further exploration of the risk of haemorrhagic stroke in particular types of patients. Note, however, that the overall benefit on other stroke subtypes greatly outweighs this small (and uncertain) hazard.34,36
8.1.4.5 Adverse effects on kidney function.
There is no clear evidence that statins have a clinically significant beneficial or adverse effect on renal function.253 An increased frequency of proteinuria has been reported for all statins, but has been analysed in more detail for rosuvastatin. With a dose of 80 mg, a frequency of 12% was reported. With the approved doses of <40 mg, the frequency is much lower and in line with the frequency for other statins. The proteinuria induced by statins is of tubular origin, usually transitory, and is believed to be due to reduced tubular reabsorption and not to glomerular dysfunction.254,255 In clinical trials, the frequency of proteinuria is generally low and, in most cases, is not higher than for placebo.256
8.1.4.6 Interactions.
A number of important drug interactions with statins have been described that may increase the risk of adverse effects. Inhibitors and inducers of enzymatic pathways involved in statin metabolism are summarized in Table 10. All currently available statins—except pravastatin, rosuvastatin, and pitavastatin—undergo major hepatic metabolism via the CYPs. These isoenzymes are mainly expressed in the liver and intestine. Pravastatin does not undergo metabolism through the CYP system, but is metabolized by sulfation and conjugation. CYP3A4 isoenzymes are the most abundant, but other isoenzymes such as CYP2C8, CYP2C9, CYP2C19, and CYP2D6 are frequently involved in the metabolism of statins. Thus, other pharmacological substrates of these CYPs may interfere with statin metabolism. Conversely, statin therapy may interfere with the catabolism of other drugs that are metabolized by the same enzymatic system.
Drugs potentially interacting with statins metabolized by cytochrome P450 3A4 leading to increased risk of myopathy and rhabdomyolysis
| Anti-infective agents . | Calcium antagonists . | Other . |
|---|---|---|
| Itraconazole | Verapamil | Ciclosporin |
| Ketoconazole | Diltiazem | Danazol |
| Posaconazole | Amlodipine | Amiodarone |
| Erythromycin | Ranolazine | |
| Clarithromycin | Grapefruit juice | |
| Telithromycin | Nefazodone | |
| HIV protease inhibitors | Gemfibrozil |
| Anti-infective agents . | Calcium antagonists . | Other . |
|---|---|---|
| Itraconazole | Verapamil | Ciclosporin |
| Ketoconazole | Diltiazem | Danazol |
| Posaconazole | Amlodipine | Amiodarone |
| Erythromycin | Ranolazine | |
| Clarithromycin | Grapefruit juice | |
| Telithromycin | Nefazodone | |
| HIV protease inhibitors | Gemfibrozil |
Drugs potentially interacting with statins metabolized by cytochrome P450 3A4 leading to increased risk of myopathy and rhabdomyolysis
| Anti-infective agents . | Calcium antagonists . | Other . |
|---|---|---|
| Itraconazole | Verapamil | Ciclosporin |
| Ketoconazole | Diltiazem | Danazol |
| Posaconazole | Amlodipine | Amiodarone |
| Erythromycin | Ranolazine | |
| Clarithromycin | Grapefruit juice | |
| Telithromycin | Nefazodone | |
| HIV protease inhibitors | Gemfibrozil |
| Anti-infective agents . | Calcium antagonists . | Other . |
|---|---|---|
| Itraconazole | Verapamil | Ciclosporin |
| Ketoconazole | Diltiazem | Danazol |
| Posaconazole | Amlodipine | Amiodarone |
| Erythromycin | Ranolazine | |
| Clarithromycin | Grapefruit juice | |
| Telithromycin | Nefazodone | |
| HIV protease inhibitors | Gemfibrozil |
Combination of statins with gemfibrozil enhances the risk of myopathy, and its association with statins must be avoided. There is no or very little increased risk for myopathy when combining statins with other fibrates, such as fenofibrate, bezafibrate, or ciprofibrate.259,260
8.2 Cholesterol absorption inhibitors
8.2.1 Mechanism of action
Ezetimibe inhibits intestinal uptake of dietary and biliary cholesterol at the level of the brush border of the intestine [by interacting with the Niemann-Pick C1-like protein 1 (NPC1L1)] without affecting the absorption of fat-soluble nutrients. By inhibiting cholesterol absorption, ezetimibe reduces the amount of cholesterol delivered to the liver. In response to reduced cholesterol delivery, the liver reacts by upregulating LDLR expression, which in turn leads to increased clearance of LDL from the blood.
8.2.2 Effects on lipids
In clinical studies, ezetimibe in monotherapy at 10 mg/day reduces LDL-C in hypercholesterolaemic patients by 15–22% with relatively high interindividual variation.261 A meta-analysis of RCTs that included over 2700 people showed an 18.5% reduction in LDL-C as compared with placebo.262 In addition, there was a significant 3% increase in HDL-C, a significant 8% reduction in TGs, and a 13% reduction in TC with ezetimibe as compared with placebo.
Ezetimibe added to ongoing statin therapy reduces LDL-C levels by an additional 21–27% compared with placebo in patients with hypercholesterolaemia with or without established CHD. In statin-naïve patients, ezetimibe and statin combination therapy has resulted in around a 15% greater reduction in LDL-C when compared with the same statins and doses in monotherapy. In other studies, this combination has also significantly improved reductions in LDL-C levels when compared with doubling of the statin dose (13–20%), and after switching from statin monotherapy to ezetimibe and statin combination therapy (11–15%).263
Co-administration of ezetimibe and bile acid sequestrants (colesevelam, colestipol, or cholestyramine) has been reported to result in an additional reduction of LDL-C levels by 10–20% when compared with the stable bile acid sequestrant regimen alone.264 Co-administration of ezetimibe with PCSK9 inhibitors also results in an additional effect.265
8.2.3 Effect on cardiovascular morbidity and mortality
The efficacy of ezetimibe in association with simvastatin has been addressed in people with aortic stenosis in the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial,266 and in patients with CKD in the Study of Heart and Renal Protection (SHARP) trial.222 In both the SEAS and SHARP trials, a reduction in CV events was demonstrated in the simvastatin–ezetimibe arm vs. placebo.266,267
In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), ezetimibe was added to simvastatin (40 mg) in patients after acute coronary syndrome (ACS).33 A total of 18 144 patients were randomized to statin or statin plus ezetimibe, and 5314 patients over 7 years experienced a CV event; 170 fewer events (32.7 vs. 34.7%) were recorded in the group taking simvastatin plus ezetimibe (P=0.016). The average LDL-C during the study was 1.8 mmol/L (70 mg/dL) in the simvastatin group and 1.4 mmol/L (55 mg/dL) in patients taking ezetimibe plus simvastatin. Also, ischaemic stroke was reduced by 21% in this trial (P=0.008). There was no evidence of harm caused by ezetimibe or the further LDL-C reduction. In this group of patients already treated with statins to reach goals, the absolute CV benefit from added ezetimibe was small, although significant and in line with the CTT expectations.268 Therefore, the study supports the proposition that LDL-C lowering by means other than statins is beneficial and safe. The beneficial effect of ezetimibe is also supported by genetic studies of mutations in NPC1L1; naturally occurring mutations that inactivate the protein were found to be associated with reduced plasma LDL-C and reduced risk for CAD.55,269,270
Taken together with other studies,271 IMPROVE-IT supports the proposal that ezetimibe should be used as second-line therapy in association with statins when the therapeutic goal is not achieved at the maximal tolerated statin dose, or in cases where a statin cannot be prescribed.272,273
8.2.4 Adverse effects and interactions
Ezetimibe is rapidly absorbed and extensively metabolized to pharmacologically active ezetimibe glucuronide. The recommended dose of ezetimibe of 10 mg/day can be administered in the morning or evening irrespective of food intake. There are no clinically significant effects of age, sex, or race on ezetimibe pharmacokinetics, and no dosage adjustment is necessary in patients with mild hepatic impairment or mild-to-severe renal insufficiency. Life-threatening liver failure with ezetimibe as monotherapy or in combination with statins is extremely rare. The addition of ezetimibe to statin therapy does not appear to increase the incidence of elevated CK levels beyond what is noted with statin treatment alone.261
8.3 Bile acid sequestrants
8.3.1 Mechanism of action
Bile acids are synthesized in the liver from cholesterol and are released into the intestinal lumen, but most of the bile acid is returned to the liver from the terminal ileum via active absorption. The two older bile acid sequestrants, cholestyramine and colestipol, are both bile acid-binding exchange resins. The synthetic drug colesevelam is also available in some countries. As bile acid sequestrants are not systemically absorbed or altered by digestive enzymes, the beneficial clinical effects are indirect. By binding the bile acids, the drugs prevent the reabsorption of both the drug and cholesterol into the blood, and thereby remove a large portion of the bile acids from the enterohepatic circulation. The liver, depleted of bile, synthesizes more from hepatic cholesterol, therefore increasing the hepatic demand for cholesterol and increasing LDLR expression, which results in a decrease of circulating LDL.
8.3.2 Effects on lipids
At the top daily dose of 24 g of cholestyramine, 20 g of colestipol, or 4.5 g of colesevelam, a reduction in LDL-C of 18–25% has been observed. No major effect on HDL-C has been reported, while TGs may increase in some predisposed patients.274 Colesevelam can also reduce glucose levels in hyperglycaemic patients.275
8.3.3 Effect on cardiovascular morbidity and mortality
In clinical trials, bile acid sequestrants have contributed greatly to the demonstration of the efficacy of LDL-C lowering in reducing CV events in hypercholesterolaemic people, with a benefit proportional to the degree of LDL-C lowering. Of note, these studies were performed before many of the modern treatment options were available.276–278
8.3.4 Adverse effects and interactions
Gastrointestinal (GI) adverse effects (most commonly flatulence, constipation, dyspepsia, and nausea) are often present with these drugs, even at low doses, which limits their practical use. These adverse effects can be attenuated by beginning treatment at low doses and ingesting ample fluid with the drug. The dose should be increased gradually. Reduced absorption of fat-soluble vitamins has been reported. Furthermore, these drugs may increase circulating TG levels in certain patients.
Bile acid sequestrants have major drug interactions with several commonly prescribed drugs, and must therefore be administered either 4 h before or 1 h after other drugs. Colesevelam is better tolerated and has fewer interactions with other drugs, and can be taken together with statins and several other drugs.279
8.4 Proprotein convertase subtilisin/kexin type 9 inhibitors
8.4.1 Mechanism of action
Recently, a new class of drugs, PCSK9 inhibitors, has become available that targets a protein (PCSK9) involved in the control of the LDLR.280 Elevated concentration or function of this protein in plasma reduces LDLR expression by promoting, upon binding, LDLR lysosomal catabolism and a subsequent increase in plasma LDL concentrations, while lower concentration or function of PCSK9 is related to lower plasma LDL-C levels.281 Therapeutic strategies have been developed mainly using mAbs; the mechanism of action relates to the reduction of the plasma level of PCSK9, which in turn is not available to bind the LDLR. Since this interaction triggers the intracellular degradation of the LDLR, lower levels of circulating PCSK9 will result in increased expression of LDLRs at the cell surface and therefore in a reduction of circulating LDL-C levels.281 Currently, the only approved PCSK9 inhibitors are two fully human mAbs, alirocumab and evolocumab. Statin treatment increases circulating PCSK9 serum levels,282 and thus the best effect of these mAbs has been demonstrated in combination with statins.
8.4.2 Effects on lipids
8.4.2.1 Low-density lipoprotein cholesterol.
In clinical trials, alirocumab and evolocumab—either alone or in combination with statins, and/or other lipid-lowering therapies—have been shown to significantly reduce LDL-C levels on average by 60%, depending on dose. The efficacy appears to be largely independent of any background therapy. In combination with high-intensity or maximally tolerated statins, alirocumab and evolocumab reduced LDL-C by 46–73% more than placebo, and by 30% more than ezetimibe. Among patients in whom statins cannot be prescribed, PCSK9 inhibition reduced LDL-C when administered in combination with ezetimibe.283 Both alirocumab and evolocumab have also been shown to effectively lower LDL-C levels in patients who are at high CV risk, including those with DM.284
Given the mechanism of action, these drugs are effective at reducing LDL-C in all patients capable of expressing LDLR in the liver. Therefore, this pharmacological approach is effective in the vast majority of patients, including those with HeFH and, albeit to a lower level, those with HoFH with residual LDLR expression. Receptor-deficient HoFH responds poorly to the therapy.285
8.4.2.2 Triglycerides and high-density lipoprotein cholesterol.
These highly efficacious LDL-lowering agents also lower TG levels, and increase those of HDL-C and ApoA-I as a function of the dosing regimen. In phase II trials, evolocumab lowered TG levels by 26%, and raised HDL-C and ApoA-I by 9 and 4%, respectively; similar findings have been reported for alirocumab.286,287 However, the TG effect must be confirmed in populations with higher starting plasma TG levels.
8.4.2.3 Lipoprotein(a).
In contrast to statins, inhibiting PCSK9 with mAbs also reduces Lp(a) plasma levels. Pooled results of phase II trials have shown that treatment leads to an Lp(a) reduction of about 30–40%.288,289 While recent investigations have attempted to unravel the mechanism, it remains unclear. However, it appears to be distinct from that of statins, which also enhance LDLR function but do not lower circulating Lp(a) levels in humans. The relative contribution of this effect to the reduction of risk remains to be addressed in appropriately designed studies.
8.4.3 Effect on cardiovascular morbidity and mortality
Early preliminary data from phase III trials suggests a reduction of CV events in line with the LDL-C reduction achieved.286,290,291
Recently, two major studies were completed: the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) and the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY Outcomes) trials.119,120 The designs of the trials were similar with regard to the settings of secondary prevention and background therapy; however, the populations enrolled had either stable CHD, peripheral arterial disease (PAD), or stroke; or a recent (median 2.6 months) ACS, respectively. The relative benefit ranged from 15–20% reductions in the risk of the primary endpoints. Both studies had relatively short follow-up periods and the evidence from statin trials indicates that the clinical benefits of LDL-lowering may only emerge after about 1 year,51 so these trials may have underestimated the potential impact of longer-term treatment.120,290
In the FOURIER trial,119 27 564 patients with atherosclerotic CVD, and LDL-C levels of 1.8 mmol/L (70 mg/dL) or higher who were receiving statin therapy, were randomly assigned to receive evolocumab or placebo. Allocation to evolocumab reduced median LDL-C from 2.38 mmol/L (92 mg/dL) at baseline to a mean of 0.78 mmol/L (30 mg/dL) at 48 weeks. After a median follow-up of 2.2 years, evolocumab treatment significantly reduced the risk of the primary endpoint (composite of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) by 15% [hazard ratio (HR) 0.85, 95% CI 0.79–0.92]. An analysis of the time to benefit also showed that there was a lower benefit in the first year than in subsequent years, consistent with the effects of statins observed within the CTT meta-analysis.268 In the FOURIER trial, allocation to evolocumab did not reduce the risk of CV mortality (HR 1.05, 95% CI 0.88–1.25) or all-cause mortality.
The ODYSSEY Outcomes trial randomized 18 924 patients after hospitalization for acute MI or unstable angina, treated with statins, and with LDL-C ≥1.8 mmol/L (≥70 mg/dL), non-HDL cholesterol ≥2.6 mmol/L (≥100 mg/dL), or ApoB ≥80 mg/dL, to receive injections of alirocumab or matching placebo. Allocation to alirocumab reduced the mean baseline LDL-C from 2.38 mmol/L (92 mg/dL) to 1.24 mmol/L (48 mg/dL) at 12 months. There was a 15% relative reduction in the primary outcome (composite of CHD death, non-fatal MI, ischaemic stroke, or unstable angina requiring hospitalization) (HR 0.85, 95% CI 0.78–0.93) after a median follow-up of 2.8 years.120 Although there was a significant reduction in all-cause mortality in the ODYSSEY trial, this was an exploratory outcome and was not supported by a significant effect on CV death.
8.4.4 Adverse effects and interactions
Anti-PCSK9 mAbs are injected subcutaneously, every other week or once a month, at different doses depending on the agent used. The potential for interaction with orally absorbed drugs is absent, as they will not interfere with pharmacokinetics or pharmacodynamics. Among the most frequently reported side effects are itching at the site of injection and flu-like symptoms.292 In some studies, an increase of patient-reported neurocognitive effects has been described.293 However, the EBBINGHAUS trial,231 which was specifically designed to detect neurocognitive function changes, was reassuring, as were the safety reports in both the FOURIER and ODYSSEY trials. Mendelian randomization studies have also suggested that PCSK9 inhibition may increase the risk of DM with an LDL-C-related effect, as apparently occurs for statins.294 To date, no signal has emerged from RCTs.295–297 Although large long-term trials of PCSK9 inhibitors are needed to rule out these and other potential side effects of inhibition of PCSK9,298 7 year data from the IMPROVE-IT study have shown that prolonged low LDL-C concentrations are not associated with any clear adverse effects.299
A potential problem of long-term antibody treatment is the occurrence of autoantibodies. Evolocumab and alirocumab are fully human antibodies and, therefore, theoretically less likely to induce autoantibodies. To date, only very few cases of antidrug antibodies have been reported, and no reduction of LDL-C lowering has been observed, but long-term use needs to be monitored. Indeed, the development programme for a third PCSK9 inhibitor, bococizumab, a humanized antibody, was discontinued because of an increase of neutralizing antibodies, which resulted in the attenuation of the LDL-C-lowering effect over time, as well as a higher rate of injection site reactions.300 However, although PCSK9 inhibitors are very effective drugs that can reduce LDL-C and CV events on top of statin and/or ezetimibe treatment, considering the costs of the treatments and the limited data on long-term safety, these drugs are likely to be considered cost-effective only in those patients at very high-risk of ASCVD, and their use may not be possible in some countries with limited healthcare resources.
8.5 Lomitapide
The microsomal TG transfer protein (MTP) transfers TGs and phospholipids from the endoplasmic reticulum to ApoB, as a necessary step in the formation of VLDL. MTP inhibition thus prevents the formation of VLDL in the liver and of chylomicrons in the intestine.
Lomitapide is an MTP inhibitor designed for o.d. oral treatment of HoFH. In an open-label, single-arm titration study evaluating lomitapide as adjunct therapy to statins, with or without apheresis and a low-fat diet,301 LDL-C was reduced by 50% from baseline at 26 weeks and by 44% at 56 weeks. Lomitapide was also shown to decrease the frequency of apheresis in HoFH patients. It should be noted that the drug’s effect on CV outcomes has not yet been determined.
As a consequence of its mechanism of action, lomitapide has been shown to be associated with increased aminotransferase levels, which most likely reflects the increased fat in the liver, as well as poor GI tolerability.301,302 The GI side effects were the most frequent reasons preventing a further increase in the dose of lomitapide in clinical trials.301 However, it has been noted that the frequency and intensity of GI side effects generally decrease with time. Therefore, prescription of lomitapide requires careful patient education and liver function monitoring during therapy.
8.6 Mipomersen
Mipomersen is an antisense oligonucleotide able to bind the messenger RNA (mRNA) of ApoB-100, which triggers the selective degradation of mRNA molecules. After subcutaneous injection, the oligonucleotide is preferentially transported to the liver, where it binds to a specific mRNA preventing the translation of ApoB protein and, consequently, reducing the production of atherogenic lipids and lipoproteins, including LDL and Lp(a).303 An adjunct to lipid-lowering medications and diet, mipomersen is indicated to reduce LDL-C in patients with HoFH. Mipomersen is currently approved by the US Food and Drug Administration (FDA), but not by the European Medicines Agency (EMA).
Reactions at the injection site are the most common adverse effects observed in patients treated with mipomersen.304 However, the main concerns regarding mipomersen’s safety are related to liver toxicity. Mipomersen may lead to the development of steatosis. Treated patients have shown a higher increase of liver fat from baseline compared with patients randomized to placebo.303 The efficacy and safety of long-term mipomersen treatment are currently under evaluation in patients with severe HeFH, and in statin-‘intolerant’ patients.
8.7 Fibrates
8.7.1 Mechanism of action
Fibrates are agonists of peroxisome proliferator-activated receptor-α (PPAR-α), acting via transcription factors regulating, among other things, various steps in lipid and lipoprotein metabolism. As a consequence, fibrates have good efficacy in lowering fasting TG levels, as well as post-prandial TGs and TG-rich lipoprotein (TRL) remnant particles.
8.7.2 Effects on lipids
Clinical impacts on lipid profiles vary among members of the fibrate class, but are estimated to reach a 50% reduction of the TG level, a ≤20% reduction of the LDL-C level (but a paradoxical small LDL-C increase may be observed with high TG levels), and an increase of the HDL-C level of ≤20%. The magnitude of effect is highly dependent on the baseline lipid levels.305 Both the HDL-raising and TG-lowering effects of fibrates have been reported to be markedly less (∼5 and ∼20%, respectively) in long-term intervention trials in people with T2DM but without elevated levels of TGs.306,307
8.7.3 Effect on cardiovascular morbidity and mortality
The clinical effects of fibrates have been primarily illustrated by six RCTs: the Helsinki Heart Study (HHS), Veterans Affairs High Density Lipoprotein Intervention Trial (VA-HIT), Bezafibrate Infarction Prevention (BIP), Lower Extremity Arterial Disease Event Reduction (LEADER), Fenofibrate Intervention and Event Lowering in Diabetes (FIELD), and Action to Control Cardiovascular Risk in Diabetes (ACCORD) trials; in the latter trial, fenofibrate was added to statin therapy.306–311 In CV outcome trials of fibrates, the risk reduction appeared to be proportional to the degree of non-HDL-C lowering.50
Although the HHS reported a significant reduction in CVD outcomes with gemfibrozil, neither the FIELD nor the ACCORD studies involving fenofibrate showed a reduction in total CVD outcomes. The LEADER trial included male participants with lower-extremity arterial disease and failed to show that bezafibrate could lead to a clinically important reduction in CVD combined endpoints. Decreases in the rates of non-fatal MI were reported, although often as a result of post hoc analyses. The effect was most evident in people with elevated TG/low HDL-C levels. However, the data on other outcome parameters have remained equivocal. Only one study, ACCORD, has analysed the effect of a fibrate as an add-on treatment to a statin. No overall benefit was reported in two recent meta-analyses.312,313 Results from other meta-analyses suggest reduced major CVD events in patients with high TGs and low HDL-C in fibrate-treated patients, but no decrease in CVD or total mortality.314–316 Thus, the overall efficacy of fibrates on CVD outcomes is much less robust than that of statins. Recently, a new selective PPAR-α modulator (pemafibrate) has been reported to have marked efficacy in reducing TRLs.317 The study, Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN PatiENts With DiabeTes (PROMINENT), is an ongoing CVD outcome trial designed to evaluate the efficacy of pemafibrate in some 10 000 high-risk diabetic patients with high TG and low HDL-C levels.318 Overall, the potential CV benefits of fibrates require further confirmation.
8.7.4 Adverse effects and interactions
Fibrates are generally well tolerated with mild adverse effects, GI disturbances being reported in <5% of patients, and skin rashes in 2%.319 In general, myopathy, liver enzyme elevations, and cholelithiasis represent the most well-known adverse effects associated with fibrate therapy.319 The risk of myopathy has been reported to be 5.5-fold greater with fibrate monotherapy (mainly with gemfibrozil) compared with a statin, and it varies with different fibrates and statins used in combination. This is explained by the pharmacological interactions between the metabolism of different fibrates and pathways of glucuronidation of statins. Gemfibrozil inhibits the metabolism of statins via the glucuronidation pathway, which leads to marked increases in plasma concentrations of statins.320 As fenofibrate does not share the same pharmacokinetic pathways as gemfibrozil, the risk of myopathy is much less with this combination therapy.319
As a class, fibrates have been reported to raise both serum creatinine and homocysteine levels in both short- and long-term studies. The increase of serum creatinine by fibrate therapy seems to be fully reversible when the drug is stopped. Data from meta-analyses suggest that a reduction of calculated glomerular filtration rate (GFR) does not reflect any adverse effects on kidney function.315 Fibrates are associated with a slightly increased risk of pancreatitis.321 The increase in homocysteine levels caused by fibrates has been considered to be relatively neutral with respect to CVD risk. However, a fibrate-induced increase in homocysteine may blunt elevation of both HDL-C and ApoA1 levels, and this effect may contribute to the smaller than estimated benefits of fenofibrate in CV outcome parameters.322
8.8 n-3 fatty acids
8.8.1 Mechanism of action
The n-3 (or omega-3) fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] can be used at pharmacological doses to lower TGs. n-3 fatty acids (2–4 g/day) affect serum lipids and lipoproteins, in particular VLDL concentrations. The underlying mechanism is poorly understood, although it may be related, at least in part, to their ability to interact with PPARs and to decreased secretion of ApoB.
8.8.2 Effects on lipids
n-3 fatty acids reduce TGs, but their effects on other lipoproteins are trivial. More detailed data on clinical outcomes are needed to justify wide use of prescription n-3 fatty acids.323 The recommended doses of total EPA and DHA to lower TGs have varied between 2–4 g/day. Three recent studies in people with high TGs using EPA reported a significant reduction in serum TG levels of up to 45% in a dose-dependent manner.324–326 The efficacy of omega-3 fatty acids in lowering serum TGs has also been reported in meta-analyses.157 Recently, the EpanoVa fOr Lowering Very high triglyceridEs II (EVOLVE II) trial confirmed the efficacy of omega-3 fatty acids in lowering serum TGs.327
8.8.3 Effect on cardiovascular morbidity and mortality
A Cochrane meta-analysis, including 112 059 people from 79 trials, reported no overall effect of omega-3 PUFAs on total mortality (relative risk 0.98, 95% CI 0.90–1.03) or CV events (relative risk 0.99, 95% Cl 0.94–1.04), with only a suggestion that omega-3 fatty acids reduced CHD events (relative risk 0.93, 95% Cl 0.88–0.97).328 Recently, the A Study of Cardiovascular Events iN Diabetes (ASCEND) trial,329 which randomly assigned 15 480 patients with DM but without atherosclerotic CV disease to n-3 fatty acids or placebo, showed no significant difference in the risk of serious vascular events after a mean follow-up of 7.4 years (relative risk 1.00, 95% Cl 0.91–1.09).
The data remain inconclusive and the clinical efficacy of omega-3 fatty acids appears to be related to non-lipid effects.330,331 Moreover, studies with omega-3 fatty acids have suffered from the dose used (1 g/day), which does not affect plasma lipids to a large extent, as the dose required to decrease plasma TGs is >2 g/day. The Reduction of Cardiovascular Events with EPA-Intervention Trial (REDUCE-IT)195 aimed to evaluate the potential benefits of omega-3 oil (EPA) on ASCVD outcomes in people with elevated serum TGs; the trial enrolled ∼8000 patients on statin therapy, with LDL-C levels between 1.0–2.6 mmol/L (41–100 mg/dL) and various CV risk factors, including persistent elevated TGs between 1.7–5.6 mmol/L (150–499 mg/dL), and either established ASCVD or DM, and at least one other CV risk factor. Use of high doses (2 g b.i.d.) of EPA as compared with placebo (mineral oil) resulted in a ∼25% relative risk reduction (P < 0.001) in major adverse CV events (MACE). Another randomized placebo-controlled trial, Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia (STRENGTH),332 which aims to determine whether reduction of TRLs and remnants in statin-treated patients will provide additional ASCVD risk reduction, is ongoing. The VITamin D and OmegA-3 TriaL (VITAL), which reported recently, was a 2×2 factorial design study in which healthy participants were randomized in a 1:1 fashion to either vitamin D3 (at a dose of 2000 IU per day) vs. matching placebo, and n-3 fatty acids (1 g per day as a fish-oil capsule containing 840 mg of n-3 fatty acids, including 460 mg of EPA and 380 mg of DHA) vs. matching placebo. It showed that supplementation with either n-3 fatty acids at a dose of 1 g/day, or vitamin D3 at a dose of 2000 IU/day, was not effective for primary prevention of CV or cancer events among healthy middle-aged men and women over 5 years of follow-up.333
8.8.4 Safety and interactions
The administration of n-3 fatty acids appears to be safe and devoid of clinically significant interactions. The most common side effect is GI disturbance. The antithrombotic effects may increase the propensity for bleeding, especially when given in addition to aspirin/clopidogrel. Recently, data from one study have associated a risk of prostate cancer with high dietary intake of n-3 PUFAs.334
8.9 Nicotinic acid
Nicotinic acid has key action sites in both the liver and adipose tissue. In the liver, nicotinic acid inhibits diacylglycerol acyltransferase-2 resulting in decreased secretion of VLDL particles, which is also reflected in reductions of plasma levels of both IDL and LDL particles.335 Nicotinic acid primarily raises HDL-C and ApoA1 by stimulating ApoA1 production in the liver.335 Two large randomized trials with nicotinic acid—one with extended-release niacin66 and one with niacin plus laropiprant67—have shown no beneficial effect and an increased frequency of serious adverse effects. No medication containing nicotinic acid is currently approved in Europe.
8.10 Cholesteryl ester transfer protein inhibitors
To date, the pharmacological approach that has led to the greatest elevations in HDL-C levels has been direct inhibition of CETP by small-molecule inhibitors, which may induce an increase in HDL-C by ≥100% on a dose-dependent basis. Torcetrapib was studied in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial, which was stopped early due to increased mortality.336 Dalcetrapib raises HDL-C levels by 30–40% with no appreciable effect on LDL-C, offering specific insight into pure HDL-C raising. However, dalcetrapib failed to show any benefit in ACS patients in the dal-OUTCOMES trial. Evacetrapib, which raises HDL-C levels by 130% and lowers LDL-C by 37%, was studied in the ACCELERATE trial,63 which was terminated due to futility. Recently, anacetrapib, which raises HDL-C and ApoA-I levels (by 104 and 36%, respectively), and lowers LDL-C and ApoB (by 17 and 18%, respectively), was studied in the REVEAL trial. Anacetrapib reduced major coronary events by 9% over a median of 4.1 years.64 The magnitude of the relative risk reduction appeared to be consistent with the magnitude of LDL-C- or non-HDL-C-level lowering.337 This drug has not been submitted for regulatory approval.
8.11 Future perspectives
8.11.1 New approaches to reduce low-density lipoprotein cholesterol
An alternative approach targeting PCSK9 consists of RNA interference. In a phase I and a phase II trial, the small interfering RNA (siRNA) molecule inclisiran—which inhibits the synthesis of PCSK9—reduced LDL-C by up to 50% and the reduction was dose-dependent. Reductions in PCSK9 and LDL-C levels were maintained for ≤6 months.338,339 No specific serious adverse events were observed. HPS4/TIMI65/ORION4, with a planned mean duration of 5 years, is currently comparing inclisiran vs. placebo among 15 000 patients with a prior MI or stroke.
Bempedoic acid is a novel, first-in-class, oral small molecule that inhibits cholesterol synthesis by inhibiting the action of ATP citrate lyase, a cytosolic enzyme upstream of 3-hydroxy-3-methylglutaryl-coenzyme A reductase.340 So far, it has been tested in diabetic patients, and patients with or without statin ‘intolerance’. In monotherapy, bempedoic acid reduces LDL-C levels by ∼30% and by about 50% in combination with ezetimibe. Bempedoic acid is currently being tested in phase III trials and some trials have been completed.341,342
8.11.2 New approaches to reduce triglyceride-rich lipoproteins and their remnants
As genetic studies indicate that angiopoietin-like protein 3 (ANGPTL3) deficiency protects against atherosclerotic disease and that this relationship is causal,343 an ANGPTL3 antibody (evinacumab) is being developed. Evinacumab has been shown to decrease TGs, LDL-C, and Lp(a) levels in HoFH patients.344 Another approach that is currently being investigated is the inhibition of ANGPTL3 production by antisense oligonucleotides.345 IONIS-ANGPTL3-LRx, an antisense oligonucleotide targeting ANGPTL3, another critical protein in the clearance of TRLs, reduces plasma TGs by about 85%. Thus, the future may yield tools to improve TRL clearance that will be reflected in the atherogenic load of the remnant particles.
The rapid development of gene-silencing technology has allowed proteins (ApoC-III) that are critical in the regulation of TRL clearance processes to be targeted. A second-generation antisense oligonucleotide targeting ApoC-III mRNA has been developed.346 Two phase III trials have evaluated the safety and efficacy of volanesorsen in patients with elevated TG levels.347,348 Volanesorsen reduced plasma TGs by ∼70% and ApoC-III by 80–90%.349 The EMA recently issued a marketing authorization for Waylivra (volanesorsen) as an adjunct to diet in adult patients with genetically confirmed familial chylomicronaemia syndrome (FCS) who are at high-risk for pancreatitis, in whom response to diet and TG-lowering therapy has been inadequate.
8.11.3 New approaches to increase high-density lipoprotein cholesterol
Although genetic studies suggest that low HDL-C levels are not a cause of ASCVD, casting doubt on the possibilities of future treatment options to raising HDL-C levels with attenuation of CVD, major developments in the search for efficacious agents to raise HDL-C and ApoA1 levels with concomitant benefits on atherosclerosis and CV events are on the horizon. On the one hand, interest is focused on ApoA1 mimetic peptides and recombinant forms of HDL possessing potential for in vivo HDL particle remodelling and enhanced cardioprotective activity.350 On the other, agents that enhance catabolism of TG-rich lipoproteins, such as the antisense oligonucleotide to ApoC-III, and which lead to a concomitant reduction in TGs (∼70%) and a marked elevation in HDL-C (∼40%) in hypertriglyceridemia, are under development.351 Importantly, however, we currently lack understanding of the relationship between the modality of raising HDL/ApoA-I levels and a possible antiatherogenic function of HDL particles.
8.11.4 New approaches to reduce lipoprotein(a) levels
Another approach under study is the selective decrease of Lp(a) concentrations. RNA-based therapies are now being evaluated in clinical settings. Results from studies of an antisense oligonucleotide in patients with normal Lp(a) values as well as in patients with elevated Lp(a) concentrations have shown a reduction of >90%.352 These approaches are currently being evaluated in phase II–III studies and an outcome trial is planned to study whether Lp(a) reduction translates into risk reduction.
8.12 Strategies to control plasma cholesterol
Recommendations for pharmacological low-density lipoprotein cholesterol lowering
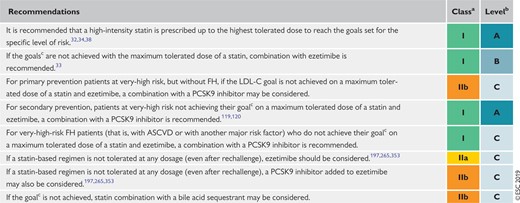 |
 |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
For definitions see Table 7.
Recommendations for pharmacological low-density lipoprotein cholesterol lowering
 |
 |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
For definitions see Table 7.
Although LDL-C goals are attained with monotherapy in many patients, a significant proportion of patients at high-risk or with very high LDL-C levels need additional treatment. In this case, combination therapy is reasonable. In patients at very-high risk and with persistent high-risk despite being treated with a maximally tolerated statin, combination with ezetimibe is recommended and, if still not at goal, the addition of a PCSK9 inhibitor is recommended (see Figure 4 and Recommendations for pharmacological low-density lipoprotein cholesterol lowering). Of note, addition of a PCSK9 inhibitor directly to a statin is also feasible120,290 (Figure 4).
Expected clinical benefits of low-density lipoprotein cholesterol-lowering therapies. The expected clinical benefits of treatment to lower low-density lipoprotein cholesterol for any person can be estimated; it depends on the intensity of therapy, the baseline low-density lipoprotein cholesterol level, the expected absolute achieved reduction in low-density lipoprotein cholesterol, and the baseline estimated risk of atherosclerotic cardiovascular disease. The intensity of therapy should be selected to achieve the recommended proportional reduction in low-density lipoprotein cholesterol based on the person’s estimated risk of atherosclerotic cardiovascular disease. Multiplying the proportional reduction in low-density lipoprotein cholesterol by a person’s baseline low-density lipoprotein cholesterol level estimates the expected absolute reduction in low-density lipoprotein cholesterol that is likely to be achieved with that therapy. Because each 1.0 mmol/L absolute reduction in low-density lipoprotein cholesterol is associated with a 20% reduction in the risk of cardiovascular events, larger absolute reductions in low-density lipoprotein cholesterol lead to larger proportional reductions in risk. Multiplying the proportional reduction in risk expected for the achieved absolute reduction in low-density lipoprotein cholesterol by a person’s estimated baseline risk of atherosclerotic cardiovascular disease determines the expected absolute risk reduction for that person. LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
(A) Treatment algorithm for pharmacological low-density lipoprotein cholesterol lowering. (B) Treatment goals for low-density lipoprotein cholesterol across categories of total cardiovascular disease risk. ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CKD = chronic kidney disease; CV = cardiovascular; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; SCORE = Systematic Coronary Risk Estimation; T1DM = type 1 DM; T2DM = type 2 DM; TC = total cholesterol.
As shown in Figure 3, the expected clinical benefit of treatment to lower the LDL-C level of any person can be estimated; it depends on the intensity of therapy, the baseline LDL-C level, and the baseline estimated risk of ASCVD. This simple algorithm can be used to help clinicians select the appropriate therapy and quantify the expected benefits of LDL-C-lowering therapy to help inform discussions with patients. For ease of reference, Supplementary Table 3 provides a summary of the absolute LDL-C reductions that can be achieved with various therapeutic approaches at particular baseline levels of LDL-C.
8.13 Strategies to control plasma triglycerides
Although CVD risk is increased when fasting TGs are >1.7 mmol/L (>150 mg/dL),56 the use of drugs to lower TG levels may only be considered in high-risk patients when TGs are >2.3 mmol/L (>200 mg/dL) and TGs cannot be lowered by lifestyle measures. The available pharmacological interventions include statins, fibrates, PCSK9 inhibitors, and n-3 PUFAs. A meta-analysis of 10 trials included people treated with various agents that reduce serum TGs (fibrates, niacin, and n-3 PUFAs) and reported a 12% reduction in CV outcomes.354 Recently, the REDUCE-IT trial194 demonstrated that in statin-treated patients with high CV risk with fasting TG levels between 135–499 mg/dL (1.52–1.63 mmol/L), high-dose icosapent ethyl, a highly purified and stable EPA (2 g) taken b.i.d., significantly reduced the risk of ischaemic events, including CV death, by about one-quarter over a median follow-up of 4.9 years. In addition, the VITAL trial showed that n-3 fatty acids at the lower dose of 1 g/day were not effective for primary prevention of CV or cancer events among healthy middle-aged men and women over 5 years of follow-up.333 Recommendations for the treatment of HTG are shown below.
Recommendations for drug treatment of patients with hypertriglyceridaemia
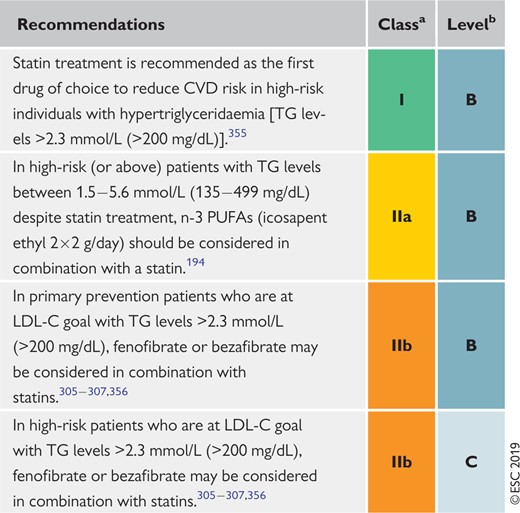 |
 |
CVD = cardiovascular disease; LDL-C = low-density lipoprotein cholesterol; PUFA = polyunsaturated fatty acids; TG = triglyceride.
Class of recommendation.
Level of evidence.
Recommendations for drug treatment of patients with hypertriglyceridaemia
 |
 |
CVD = cardiovascular disease; LDL-C = low-density lipoprotein cholesterol; PUFA = polyunsaturated fatty acids; TG = triglyceride.
Class of recommendation.
Level of evidence.
9 Management of dyslipidaemias in different clinical settings
9.1 Familial dyslipidaemias
Plasma lipid levels are, to a very large extent, determined by genetic factors. In its more extreme forms this is manifested as familial dyslipidaemias. A number of monogenic lipid disorders have been identified; among these, FH is the most common and is strongly related to CVD (Table 11). In general, in a patient with dyslipidaemia, the pattern of inheritance commonly does not suggest that there is a major single gene (monogenic) disorder causing the abnormality; rather, it stems from the inheritance of more than one gene variant affecting lipoprotein metabolism that, on its own, might have relatively little effect, but in combination with another or others has a greater influence on TC, TGs, or HDL-C. The pattern of inheritance is polygenic.357 It is common to find that high LDL-C, high TG, or low HDL-C levels affect several family members.
Genetic disorders of lipoprotein metabolism
| Disorder . | Prevalence . | Gene(s) . | Effect on lipoproteins . |
|---|---|---|---|
| HeFH | 1 in 200–250 |
| ↑LDL-C |
| HoFH | 1 in 160 000–320 000 |
| ↑↑LDL-C |
| FCH | 1 in 100/200 | USF1 + modifying genes | ↑LDL-C ↑VLDL-C ↑ApoB |
| Familial dysbetalipoproteinaemia | 1 in 5000 | APO E | ↑↑IDL and chylomicron remnants (βVLDL) |
| Familial lipoprotein lipase deficiency (familial chylomicron syndrome) | 2 in 106 |
| ↑↑chylomicrons and VLDL-C |
| Tangier disease (analphalipoproteinaemia) | 1 in 106 | ABCA1 | ↓↓HDL-C |
| Familial LCAT deficiency | 1 in 106 | LCAT | ↓HDL-C |
| Disorder . | Prevalence . | Gene(s) . | Effect on lipoproteins . |
|---|---|---|---|
| HeFH | 1 in 200–250 |
| ↑LDL-C |
| HoFH | 1 in 160 000–320 000 |
| ↑↑LDL-C |
| FCH | 1 in 100/200 | USF1 + modifying genes | ↑LDL-C ↑VLDL-C ↑ApoB |
| Familial dysbetalipoproteinaemia | 1 in 5000 | APO E | ↑↑IDL and chylomicron remnants (βVLDL) |
| Familial lipoprotein lipase deficiency (familial chylomicron syndrome) | 2 in 106 |
| ↑↑chylomicrons and VLDL-C |
| Tangier disease (analphalipoproteinaemia) | 1 in 106 | ABCA1 | ↓↓HDL-C |
| Familial LCAT deficiency | 1 in 106 | LCAT | ↓HDL-C |
Apo = apolipoprotein; FCH = familial combined hyperlipidaemia; HDL-C = high-density lipoprotein cholesterol; HeFH = heterozygous familial hypercholesterolaemia; HoFH = homozygous familial hypercholesterolaemia; IDL = intermediate-density lipoprotein; LCAT = lecithin cholesterol acyltransferase; LDL-C = low-density lipoprotein cholesterol; VLDL = very low-density lipoprotein cholesterol.
Genetic disorders of lipoprotein metabolism
| Disorder . | Prevalence . | Gene(s) . | Effect on lipoproteins . |
|---|---|---|---|
| HeFH | 1 in 200–250 |
| ↑LDL-C |
| HoFH | 1 in 160 000–320 000 |
| ↑↑LDL-C |
| FCH | 1 in 100/200 | USF1 + modifying genes | ↑LDL-C ↑VLDL-C ↑ApoB |
| Familial dysbetalipoproteinaemia | 1 in 5000 | APO E | ↑↑IDL and chylomicron remnants (βVLDL) |
| Familial lipoprotein lipase deficiency (familial chylomicron syndrome) | 2 in 106 |
| ↑↑chylomicrons and VLDL-C |
| Tangier disease (analphalipoproteinaemia) | 1 in 106 | ABCA1 | ↓↓HDL-C |
| Familial LCAT deficiency | 1 in 106 | LCAT | ↓HDL-C |
| Disorder . | Prevalence . | Gene(s) . | Effect on lipoproteins . |
|---|---|---|---|
| HeFH | 1 in 200–250 |
| ↑LDL-C |
| HoFH | 1 in 160 000–320 000 |
| ↑↑LDL-C |
| FCH | 1 in 100/200 | USF1 + modifying genes | ↑LDL-C ↑VLDL-C ↑ApoB |
| Familial dysbetalipoproteinaemia | 1 in 5000 | APO E | ↑↑IDL and chylomicron remnants (βVLDL) |
| Familial lipoprotein lipase deficiency (familial chylomicron syndrome) | 2 in 106 |
| ↑↑chylomicrons and VLDL-C |
| Tangier disease (analphalipoproteinaemia) | 1 in 106 | ABCA1 | ↓↓HDL-C |
| Familial LCAT deficiency | 1 in 106 | LCAT | ↓HDL-C |
Apo = apolipoprotein; FCH = familial combined hyperlipidaemia; HDL-C = high-density lipoprotein cholesterol; HeFH = heterozygous familial hypercholesterolaemia; HoFH = homozygous familial hypercholesterolaemia; IDL = intermediate-density lipoprotein; LCAT = lecithin cholesterol acyltransferase; LDL-C = low-density lipoprotein cholesterol; VLDL = very low-density lipoprotein cholesterol.
9.1.1 Familial combined hyperlipidaemia
Familial combined hyperlipidaemia (FCH) is a highly prevalent mixed dyslipidaemia (1:100–200) characterized by elevated levels of LDL-C, TGs, or both, and is an important cause of premature CAD. FCH is a complex disease, and the phenotype is determined by the interaction of multiple susceptibility genes and the environment. It has considerable overlap with the dyslipidaemic phenotypes of T2DM and MetS. Even within a family, the phenotype shows high inter- and intrapersonal variability based on lipid values (TGs, LDL-C, HDL-C, and ApoB). FCH has no monogenic component and is not linked to a single genetic cause, but the phenotype is high LDL-C and/or high TGs.358,359 Therefore, the diagnosis is commonly missed in clinical practice; the combination of ApoB >120 mg/dL and TGs >1.5 mmol/L (>133 mg/dL) with a family history of premature CVD can be used to identify people who most probably have FCH.360
The concept of mixed dyslipidaemia is also valuable clinically in assessing CV risk. It emphasizes both the importance of considering family history in deciding how rigorously to treat dyslipidaemia and that elevated LDL-C levels portend a higher risk when HTG is also present. Statin treatment decreases CV risk by the same relative amount in people with HTG as in those without. Because the absolute risk is often greater in those with HTG, they may therefore benefit greatly from LDL-lowering therapy.
9.1.2 Familial hypercholesterolaemia
9.1.2.1 Heterozygous familial hypercholesterolaemia.
FH is a common codominant monogenic dyslipidaemia causing premature CVD due to lifelong elevation of plasma levels of LDL-C. If left untreated, men and women with HeFH typically develop early CAD before the ages of 55 and 60 years respectively. The risk of CHD among individuals with definite or probable HeFH is estimated to be increased at least 10-fold. However, early diagnosis and appropriate treatment can dramatically reduce the risk for CAD.
The prevalence of HeFH in the population is estimated to be 1/200–250,361 translating to a total number of cases between 14–34 million worldwide.362,363 Only a minor fraction of these cases is identified and properly treated.
FH is a monogenic disease caused by loss-of-function mutations in the LDLR or apoB genes, or a gain-of-function mutation in the PCSK9 gene; around 95% of FH cases are caused by mutations in LDLR. More than 1000 different mutations that cause FH have been identified in LDLR. The different mutations cause reduced function or complete loss-of-function, the latter being associated with more severe hypercholesterolaemia and CVD.
The diagnosis of FH is usually based on clinical presentation. The commonly used criteria from the Dutch Lipid Clinic Network are shown in Table 12. Other criteria are the Simon Broome register or the WHO criteria.364,365
Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolaemia
| Criteria . | Points . |
|---|---|
| 1) Family history | |
| First-degree relative with known premature (men aged <55 years; women <60 years) coronary or vascular disease, or first-degree relative with known LDL-C above the 95th percentile | 1 |
| First-degree relative with tendinous xanthomata and/or arcus cornealis, or children aged <18 years with LDL-C above the 95th percentile | 2 |
| 2) Clinical history | |
| Patient with premature (men aged <55 years; women <60 years) CAD | 2 |
| Patient with premature (men aged <55 years; women <60 years) cerebral or peripheral vascular disease | 1 |
| 3) Physical examinationa | |
| Tendinous xanthomata | 6 |
| Arcus cornealis before age 45 years | 4 |
| 4) LDL-C levels (without treatment) | |
| LDL-C ≥8.5 mmol/L (≥325 mg/dL) | 8 |
| LDL-C 6.5–8.4 mmol/L (251–325 mg/dL) | 5 |
| LDL-C 5.0–6.4 mmol/L (191–250 mg/dL) | 3 |
| LDL-C 4.0–4.9 mmol/L (155–190 mg/dL) | 1 |
| 5) DNA analysis | |
| Functional mutation in the LDLR, apoB, or PCSK9 genes | 8 |
| Choose only one score per group, the highest applicable; diagnosis is based on the total number of points obtained | |
| A ‘definite’ FH diagnosis requires >8 points | |
| A ‘probable’ FH diagnosis requires 6–8 points | |
| A ‘possible’ FH diagnosis requires 3–5 points | |
| Criteria . | Points . |
|---|---|
| 1) Family history | |
| First-degree relative with known premature (men aged <55 years; women <60 years) coronary or vascular disease, or first-degree relative with known LDL-C above the 95th percentile | 1 |
| First-degree relative with tendinous xanthomata and/or arcus cornealis, or children aged <18 years with LDL-C above the 95th percentile | 2 |
| 2) Clinical history | |
| Patient with premature (men aged <55 years; women <60 years) CAD | 2 |
| Patient with premature (men aged <55 years; women <60 years) cerebral or peripheral vascular disease | 1 |
| 3) Physical examinationa | |
| Tendinous xanthomata | 6 |
| Arcus cornealis before age 45 years | 4 |
| 4) LDL-C levels (without treatment) | |
| LDL-C ≥8.5 mmol/L (≥325 mg/dL) | 8 |
| LDL-C 6.5–8.4 mmol/L (251–325 mg/dL) | 5 |
| LDL-C 5.0–6.4 mmol/L (191–250 mg/dL) | 3 |
| LDL-C 4.0–4.9 mmol/L (155–190 mg/dL) | 1 |
| 5) DNA analysis | |
| Functional mutation in the LDLR, apoB, or PCSK9 genes | 8 |
| Choose only one score per group, the highest applicable; diagnosis is based on the total number of points obtained | |
| A ‘definite’ FH diagnosis requires >8 points | |
| A ‘probable’ FH diagnosis requires 6–8 points | |
| A ‘possible’ FH diagnosis requires 3–5 points | |
CAD = coronary artery disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Exclusive of each other (i.e. maximum 6 points if both are present).
Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolaemia
| Criteria . | Points . |
|---|---|
| 1) Family history | |
| First-degree relative with known premature (men aged <55 years; women <60 years) coronary or vascular disease, or first-degree relative with known LDL-C above the 95th percentile | 1 |
| First-degree relative with tendinous xanthomata and/or arcus cornealis, or children aged <18 years with LDL-C above the 95th percentile | 2 |
| 2) Clinical history | |
| Patient with premature (men aged <55 years; women <60 years) CAD | 2 |
| Patient with premature (men aged <55 years; women <60 years) cerebral or peripheral vascular disease | 1 |
| 3) Physical examinationa | |
| Tendinous xanthomata | 6 |
| Arcus cornealis before age 45 years | 4 |
| 4) LDL-C levels (without treatment) | |
| LDL-C ≥8.5 mmol/L (≥325 mg/dL) | 8 |
| LDL-C 6.5–8.4 mmol/L (251–325 mg/dL) | 5 |
| LDL-C 5.0–6.4 mmol/L (191–250 mg/dL) | 3 |
| LDL-C 4.0–4.9 mmol/L (155–190 mg/dL) | 1 |
| 5) DNA analysis | |
| Functional mutation in the LDLR, apoB, or PCSK9 genes | 8 |
| Choose only one score per group, the highest applicable; diagnosis is based on the total number of points obtained | |
| A ‘definite’ FH diagnosis requires >8 points | |
| A ‘probable’ FH diagnosis requires 6–8 points | |
| A ‘possible’ FH diagnosis requires 3–5 points | |
| Criteria . | Points . |
|---|---|
| 1) Family history | |
| First-degree relative with known premature (men aged <55 years; women <60 years) coronary or vascular disease, or first-degree relative with known LDL-C above the 95th percentile | 1 |
| First-degree relative with tendinous xanthomata and/or arcus cornealis, or children aged <18 years with LDL-C above the 95th percentile | 2 |
| 2) Clinical history | |
| Patient with premature (men aged <55 years; women <60 years) CAD | 2 |
| Patient with premature (men aged <55 years; women <60 years) cerebral or peripheral vascular disease | 1 |
| 3) Physical examinationa | |
| Tendinous xanthomata | 6 |
| Arcus cornealis before age 45 years | 4 |
| 4) LDL-C levels (without treatment) | |
| LDL-C ≥8.5 mmol/L (≥325 mg/dL) | 8 |
| LDL-C 6.5–8.4 mmol/L (251–325 mg/dL) | 5 |
| LDL-C 5.0–6.4 mmol/L (191–250 mg/dL) | 3 |
| LDL-C 4.0–4.9 mmol/L (155–190 mg/dL) | 1 |
| 5) DNA analysis | |
| Functional mutation in the LDLR, apoB, or PCSK9 genes | 8 |
| Choose only one score per group, the highest applicable; diagnosis is based on the total number of points obtained | |
| A ‘definite’ FH diagnosis requires >8 points | |
| A ‘probable’ FH diagnosis requires 6–8 points | |
| A ‘possible’ FH diagnosis requires 3–5 points | |
CAD = coronary artery disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Exclusive of each other (i.e. maximum 6 points if both are present).
The diagnosis can be verified by showing causative mutations in the pathogenic genes. However, in most studies, the frequency of detectable mutations in patients with a clinically definite or probable HeFH is between 60–80%. This suggests that a considerable proportion of patients with FH have either a polygenic cause of the disease or that other genes, yet to be identified, are involved.
Genetic testing and cascade screening. Probands (index cases) should be identified according to the following criteria:
TC ≥8 mmol/L (≥310 mg/dL) without treatment in an adult or adult family member (or >95th percentile by age and gender for country);
Premature CHD in the patient or a family member;
Tendon xanthomas in the patient or a family member; or
Sudden premature cardiac death in a family member.
Cascade screening of family members of a known index case allows for the efficient identification of new cases. Cascade screening is best performed by a lipid clinic. In most families, the cases may be identified with TC or LDL-C analysis; however, genetic testing is recommended when the causative mutation is known.
Cholesterol-lowering treatment should be initiated as soon as possible after a diagnosis has been made. To improve risk assessment, the use of imaging techniques to detect asymptomatic atherosclerosis is recommended. The concept of cumulative cholesterol burden illustrates the importance of early treatment (for children, see below). Treatment should be initiated with high-intensity statin therapy, in most cases in combination with ezetimibe. In FH patients at very-high risk of ASCVD due to a prior history of ASCVD or another major risk factor, LDL-C goals are a ≥50% reduction of LDL-C from baseline and an LDL-C <1.4 mmol/L (<55 mg/dL). In the absence of ASCVD or another major risk factor, patients with FH are categorized as high-risk, and LDL-C goals are a ≥50% reduction of LDL-C from baseline and an LDL-C <1.8 mmol/L (<70 mg/dL).
PCSK9 inhibitors lower LDL-C levels by up to 60% on top of statins. Two RCTs have reported a beneficial effect on clinical endpoints in ASCVD patients without FH.119,120 PCSK9 inhibitors are recommended in very-high-risk patients with FH if the treatment goal is not achieved on maximal tolerated statin plus ezetimibe. PCSK9 inhibitors are also recommended in FH patients who cannot tolerate statins.366,367
Recommendations for the detection and treatment of patients with HeFH are shown below.
Recommendations for the detection and treatment of patients with heterozygous familial hypercholesterolaemia
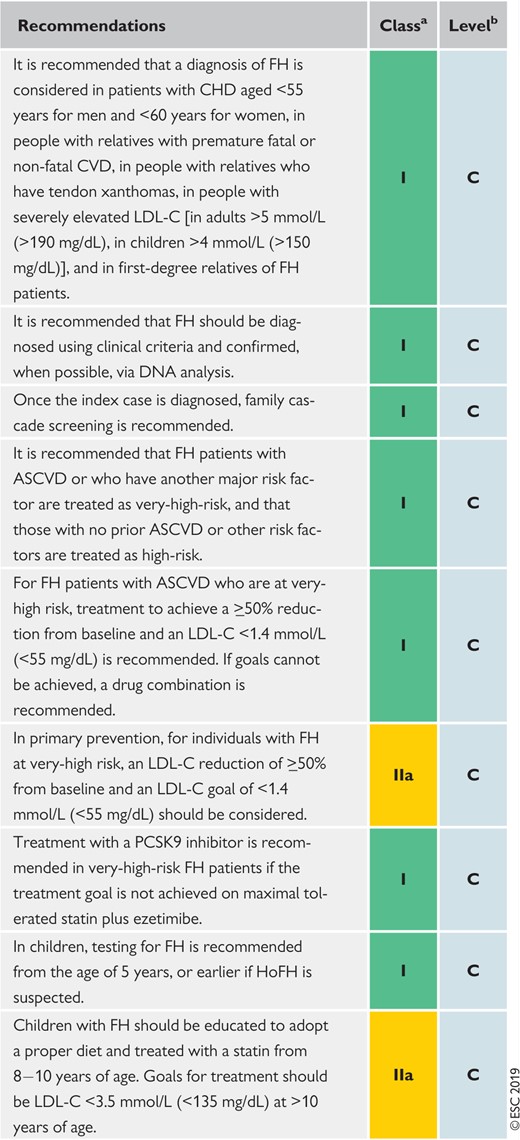 |
 |
ASCVD = atherosclerotic cardiovascular disease; CHD = coronary heart disease; CVD = cardiovascular disease; FH = familial hypercholesterolaemia; HoFH = homozygous FH; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
Recommendations for the detection and treatment of patients with heterozygous familial hypercholesterolaemia
 |
 |
ASCVD = atherosclerotic cardiovascular disease; CHD = coronary heart disease; CVD = cardiovascular disease; FH = familial hypercholesterolaemia; HoFH = homozygous FH; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
9.1.2.2 Homozygous familial hypercholesterolaemia.
HoFH is a rare and life-threatening disease. The clinical picture is characterized by extensive xanthomas, marked premature and progressive CVD, and TC >13 mmol/L (>500 mg/dL). Most patients develop CAD and aortic stenosis before the age of 20 years and die before 30 years of age. The frequency of HoFH is estimated to be 1/160 000–1/320 000. The early identification of these children and prompt referral to a specialized clinic is crucial. The patients should be treated with intensive LDL-lowering drug therapy and, when available, with lipoprotein apheresis. This treatment (every 1–2 weeks) can decrease plasma LDL-C levels by 55–70%. The procedure frequency may be adjusted for each patient as lipid levels, symptoms, and other disease-related parameters change. Maximally tolerated pharmacological therapy must be maintained.368 For a more detailed discussion on HoFH, see the EAS consensus statements.366,368
9.1.2.3 Familial hypercholesterolaemia in children.
FH is diagnosed in children based on phenotypic criteria including elevated LDL-C plus a family history of elevated LDL-C, premature CAD, and/or positive genetic testing.369 Testing during childhood is optimal to discriminate between FH and non-FH using LDL-C. In children with a family history of high cholesterol or premature CHD, an accepted cut-off is ≥4.0 mmol/L (≥160 mg/dL). If a parent has a known genetic defect, the diagnostic level for the child is ≥3.5 mmol/L (≥130 mg/dL). If possible, genetic testing of the child is suggested.
Although there have been no placebo-controlled trials in children, observational studies have suggested that early treatment can reduce LDL-C burden, improve endothelial function, substantially attenuate the development of atherosclerosis, and improve coronary outcomes.369–371 Treatment of children with FH includes a healthy lifestyle and statin treatment. A heart-healthy diet should be adopted early in life and statin treatment should be considered at 6–10 years of age. Statin treatment should be started with low doses and the dose should be increased to reach goals.372 The goal in children >10 years of age is an LDL-C <3.5 mmol/L (<135 mg/dL) and at younger ages a ≥50% reduction of LDL-C.
9.1.3 Familial dysbetalipoproteinaemia
Familial dysbetalipoproteinaemia (i.e. type III hyperlipoproteinaemia; remnant removal disease) is rare and is generally inherited as an autosomal recessive disorder with variable penetrance. Familial dysbetalipoproteinaemia produces a characteristic clinical syndrome in which both TC and TGs are elevated before treatment, usually both in the range of 7–10 mmol/L. In severe cases, patients develop tuberoeruptive xanthomas, particularly over the elbows and knees, and palmar xanthomata in the skin creases of the hands and wrists. The risk of CAD is very high, and accelerated atherosclerosis of the femoral and tibial arteries is also prevalent. The syndrome is usually not expressed at a young age or in women before menopause. The majority of cases are homozygous for the E2 isoform of ApoE. ApoE is important for the hepatic clearance of chylomicron remnants and IDL. ApoE2 binds less readily than the E3 and E4 isoforms to hepatic receptors. However, without some coincidental cause of dyslipidaemia such as dyslipidaemia associated with HTG, DM, obesity, or hypothyroidism,373–375 ApoE2 homozygosity does not generally cause familial dysbetalipoproteinaemia.
The detection of ApoE2 homozygosity in a dyslipidaemic patient is diagnostic and analysis of ApoE isoforms is now available in most clinical laboratories. The presence of cholesterol remnants characteristic of familial dysbetalipoproteinaemia can be reliably predicted on the basis of plasma levels of cholesterol, TGs, and ApoB.376 If suspicion is confirmed, ApoE genotyping can be performed. In older patients with xanthomata resembling those of familial dysbetalipoproteinaemia who prove not to be homozygous for ApoE2, a paraprotein should be sought. The treatment of familial dysbetalipoproteinaemia should be undertaken in a specialist clinic. Most cases respond well to treatment with a statin or, if dominated by high TGs, a fibrate; often a combination of a statin and a fibrate may be needed.
9.1.4 Genetic causes of hypertriglyceridaemia
Although the genetic aetiology for HTG seems to be very complex, recent data have extended our genetic understanding of HTG, in particular that of chylomicronaemia.37,226,377 Moderate elevation of TG levels (between 2.0–10.0 mmol/L) is caused by the polygenic effect of multiple genes influencing both VLDL production and removal. Monogenic severe HTG causes chylomicronaemia, pancreatitis, and lipid deposits. Thus far, mutations in six genes (LPL, apoC2, apoA5, LMF1, GPIHBP1, and GPD1) with monogenic effects have been recognized to lead to severe elevation of serum TGs due to disruption of the chylomicron removal pathways. These mutations are inherited as autosomal recessive traits and are rare. The profound defect in the catabolism of chylomicrons and VLDL results in chylomicronaemia and TG levels >11.2 mmol/L (>1000 mg/dL), with turbid and milky serum. Severe HTG is seen in patients who are homozygous or compound heterozygous for mutations of the enzyme LPL, and in other genes linked to the catabolism of TG-rich lipoproteins. Heterozygous carriers of these same gene mutations commonly express moderate elevations of serum TG levels that expose them to increased CVD risk.378 Recently, gene therapy for LPL deficiency has been developed and tested in clinical trials,379 and the alipogene tiparvovec was approved by the EMA in 2013. However, this therapy is no longer available. A gain-of-function mutation in apoC3 that leads to high ApoC-III levels can also cause severe HTG by inhibiting the activity of LPL, whereas loss-of-function mutations are associated with a favourable lipid profile with low TG levels.380 These findings have raised the possibility of ApoC-III being a novel lipid drug target.
9.1.4.1 Action to prevent acute pancreatitis in severe hypertriglyceridaemia.
The risk of pancreatitis is clinically significant if TGs are >10 mmol/L (880 mg/dL), particularly when occurring in association with familial chylomicronaemia, and actions to prevent acute pancreatitis are mandatory.381,382 Notably, HTG is the cause of ∼10% of all cases with pancreatitis, and patients can develop pancreatitis even when their TG concentration is 5–10 mmol/L (440–880 mg/dL). Recent data from a prospective cohort study reported that the risk of acute pancreatitis increased significantly over the quartiles of serum TGs, highlighting the fact that, as a risk factor, serum TGs may have been underestimated.383 Any factor that increases VLDL production can aggravate the risk of pancreatitis, with alcohol consumption being the most common contributing factor. Either a patient should be admitted to hospital if symptomatic, or careful and close follow-up of the patient’s TG values should be undertaken. Restriction of calories and fat content (10–15% recommended) in the diet, and alcohol abstinence are obligatory. Fibrate therapy (fenofibrate) should be initiated, with n-3 fatty acids (2–4 g/day) as adjunct therapy. Lomitapide may also be considered in severe cases.37 In patients with DM, insulin therapy should be initiated to achieve good glycaemic control. In general, a sharp decrease of TG values is seen within 2–5 days. In the acute setting, plasmapheresis is able to rapidly lower TG levels.384 Volanesorsen has been recently approved by the EMA as an adjunct to diet in adult patients with genetically confirmed FCS who are at high-risk for pancreatitis.
9.1.5 Other genetic disorders of lipoprotein metabolism
Sometimes patients are encountered with extremely low levels of LDL-C or HDL-C. The most common form of genetic hypolipidaemia is hypobetalipoproteinaemia, which is dominantly inherited and often due to truncation of ApoB. Serum LDL-C is typically between 0.5–1.5 mmol/L (20–60 mg/dL). A more profound deficiency of ApoB occurs in abetalipoproteinaemia when steatorrhoea, and neurological or other complications require specialist treatment. Almost absent levels of HDL-C occur in Tangier disease (analphalipoproteinaemia) and very low levels of HDL-C occur in lecithin cholesterol acyltransferase (LCAT) deficiency. Both these conditions are associated with distinct clinical syndromes and require specialist investigation. Very high levels of HDL-C are detected in patients with CETP deficiency. In the heterozygous form, levels of 2.0–2.3 mmol/L (80–90 mg/dL) are typically observed, and levels ≥5 mmol/L (≥200 mg/dL) are observed in homozygotes. This is not associated with atherosclerotic disease and may be associated with reduced risk.
Lysosomal acid lipase deficiency or cholesterol ester storage disease (in children with Wolman disease) is a rare cause (recessive transmission) of elevated LDL-C and low HDL-C, accompanied by hepatomegaly and microvesicular hepatosteatosis. Statin treatment does decrease LDL-C levels and could therefore prevent ASCVD in these patients, but it cannot stop the progression of liver damage. Treatment with a PCSK9 inhibitor may lead to an even greater overload of lysosomes.385 Enzyme replacement therapy with sebelipase alfa might offer a treatment solution in the near future.386
9.2 Women
Few randomized trials of statin therapy have reported independently significant CV benefits in women,387,389 chiefly because women have not been adequately represented in statin trials.
9.2.1 Effects of statins in primary and secondary prevention
There has previously been controversy over whether statins are effective for primary prevention in women. Using published data, a 2013 Cochrane analysis showed that statin therapy reduced all-cause mortality, vascular events, and revascularizations in primary prevention, and the proportional effects in women were similar to those in men.213 The CTT collaboration has provided a more complete assessment of the evidence through a comprehensive analysis of IPD from 22 trials of statins vs. control and five trials of more- vs. less-intensive statin therapy.35 Overall, 46 675 (27%) of 174 149 participants were women, and after adjustment for non-gender differences, the proportional reductions per mmol/L reduction in LDL-C in major vascular events, major coronary events, coronary revascularization, and stroke were similar in women and men.35
9.2.2 Non-statin lipid-lowering drugs
Definitive evidence of the cardioprotective effects of non-statin drugs that lower LDL-C is now available, and the beneficial effects are similar in both women and men. In the IMPROVE-IT study,33 the relative benefit of adding ezetimibe to simvastatin was similar in women and men.33 In the ACCORD lipid study, there was no evidence that fenofibrate added to the effects of simvastatin in patients with T2DM,306 but an analysis of the FIELD study showed consistent CV event reduction in both women and men.389 Several outcome trials assessing the effects of adding a PCSK9 inhibitor to high-intensity statin therapy have now been reported, with similar proportional reductions in major vascular events in women and men.120,286,290
9.2.3 Hormone therapy
Currently prescribed third-generation, low-dose oestrogen–progestin oral contraceptives do not appear to increase adverse coronary events390 and can be used, after baseline lipid profile assessment, in women with acceptable TC levels. In contrast, alternative contraceptive measures should be recommended in women with hypercholesterolaemia [LDL-C >4 mmol/L (>160 mg/dL)] or with multiple risk factors, and in those at high-risk of thrombotic events.391 Oestrogen replacement therapy, despite some favourable effects on lipid profiles, has not been demonstrated to reduce CV risk and cannot be recommended for CVD prevention in women.392 No lipid-lowering drugs should be administered during pregnancy and the period of breastfeeding because data on possible adverse effects are lacking. However, bile acid sequestrants may be considered.
Box 6 lists the main measures in the management of dyslipidaemia in women.
Management of dyslipidaemia in women
| Statin treatment is recommended for primary prevention of ASCVD in high-risk women.34,35 |
| Statins are recommended for secondary prevention in women with the same indications and goals as in men.34,35 |
| Lipid-lowering drugs should not be given when pregnancy is planned, during pregnancy, or during the breastfeeding period. However, for severe FH patients, bile acid sequestrants (which are not absorbed) and/or LDL apheresis may be considered. |
| Statin treatment is recommended for primary prevention of ASCVD in high-risk women.34,35 |
| Statins are recommended for secondary prevention in women with the same indications and goals as in men.34,35 |
| Lipid-lowering drugs should not be given when pregnancy is planned, during pregnancy, or during the breastfeeding period. However, for severe FH patients, bile acid sequestrants (which are not absorbed) and/or LDL apheresis may be considered. |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL = low-density lipoprotein.
Management of dyslipidaemia in women
| Statin treatment is recommended for primary prevention of ASCVD in high-risk women.34,35 |
| Statins are recommended for secondary prevention in women with the same indications and goals as in men.34,35 |
| Lipid-lowering drugs should not be given when pregnancy is planned, during pregnancy, or during the breastfeeding period. However, for severe FH patients, bile acid sequestrants (which are not absorbed) and/or LDL apheresis may be considered. |
| Statin treatment is recommended for primary prevention of ASCVD in high-risk women.34,35 |
| Statins are recommended for secondary prevention in women with the same indications and goals as in men.34,35 |
| Lipid-lowering drugs should not be given when pregnancy is planned, during pregnancy, or during the breastfeeding period. However, for severe FH patients, bile acid sequestrants (which are not absorbed) and/or LDL apheresis may be considered. |
ASCVD = atherosclerotic cardiovascular disease; FH = familial hypercholesterolaemia; LDL = low-density lipoprotein.
9.3 Older people
The proportion of older people (defined herein as those aged >65 years) in society is increasing and, as a consequence, >80% of individuals who die from CVD are >65 years of age. The proportion of patients with MI >85 years of age has increased several-fold.393
A meta-analysis of observational studies has shown that higher TC is associated with increased CAD mortality at all ages.62,394 However, since the absolute risk of CAD is higher in older persons, the associated absolute increase in risk for a given increment in TC is larger with increasing age.217
9.3.1 Effects of statins in primary and secondary prevention
The use of statin therapy declines with increasing age, reflecting differences in both prescription and compliance.395,396 This trend is even more prominent among older patients who do not have evidence of occlusive vascular disease.396 One explanation for this pattern may be uncertainty about the effects of statins in older people due to the relatively small number of people aged >75 years who have been included in statin trials.233,397,398 The CTT collaboration recently provided a comprehensive assessment of the randomized evidence on the effects of statin therapy at different ages.217 Among 186 854 participants in 28 trials, 14 483 (8%) were aged >75 years at randomization. Overall, statin therapy produced a 21% relative reduction in major vascular events (relative risk 0.79, 95% CI 0.77–0.81) per 1.0 mmol/L reduction in LDL-C, and there was direct evidence of benefit among those aged >75 years. The relative reduction in major vascular events was similar, irrespective of age, among patients with pre-existing vascular disease, but appeared smaller among older individuals not known to have vascular disease. Therefore, the available evidence from trials indicates that statin therapy produces significant reductions in major vascular events irrespective of age. However, there is less direct evidence of benefit among patients aged >75 years who do not already have evidence of occlusive vascular disease, and this limitation is currently being addressed by the STAtin therapy for Reducing Events in the Elderly (STAREE) trial in Australia.
9.3.2 Adverse effects, interactions, and adherence
The safety and adverse effects of statins are a matter of special concern in older adults because they often have comorbidities, take multiple medications, and have altered pharmacokinetics and pharmacodynamics. Statin–drug interactions are a concern, primarily because of their potential to increase muscle-related statin-associated adverse effects such as myalgia without CK elevation, myopathy with CK elevation, and the rare but serious rhabdomyolysis. It is recommended that a statin is started at a low dose if there is significant renal impairment and/or the potential for drug interactions, and then titrated upwards to achieve LDL-C treatment goals.
The recommendations for the treatment of dyslipidaemias in older people are shown below.
Recommendations for the treatment of dyslipidaemias in older people (aged >65 years)
 |
 |
ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol.
Class of recommendation.
Level of evidence.
Recommendations for the treatment of dyslipidaemias in older people (aged >65 years)
 |
 |
ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol.
Class of recommendation.
Level of evidence.
9.4 Diabetes and metabolic syndrome
The number of people with DM will increase from ∼415 million today up to 550 million by 2030, but the situation may get even worse.399 Despite significant advantages in the management strategies that lessen atherosclerotic CVD risk factors, CVD has remained the leading cause of morbidity and mortality in patients with T2DM. The good news is that fatal CVD outcomes have declined significantly in both T1DM and T2DM between 1998 and 2014.400 DM itself is an independent risk factor for CVD and is associated with a higher risk of CVD, even more so in women. The difference in CVD risk between individuals with and without DM has narrowed substantially over the last few decades,401 and there are strong associations between DM and vascular outcomes.402,403 Recent data indicate that DM per se increases CVD risk about two-fold on average, but the risk is subject to wide variation depending on the population and current aggressive prophylactic therapy.401,404 Importantly, those with DM and CAD are at substantially higher CVD risk for future events. In T2DM, the risk of ASCVD is strongly determined by the presence of target organ damage—including nephropathy (microalbuminuria), neuropathy, or retinopathy—with the risks increasing in relation to the number of conditions present.405 Hypertension, dyslipidaemia, abdominal obesity, and non-alcoholic fatty liver disease (NAFLD) commonly coexist with T2DM and further aggravate the risk, which is highest in people with T2DM and multiple cardiometabolic risk factors.406–408 Importantly, DM confers excess mortality risk following ACS despite modern therapies, highlighting the poor prognosis of coronary patients with T2DM and the need for intensive therapy.409
How to capture the extra risk beyond the traditional risk factors in clinical practice is a debated issue. A practical approach is that if one component is identified, a systematic search should be made for the others.410
9.4.1 Specific features of dyslipidaemia in insulin resistance and type 2 diabetes mellitus
Diabetic dyslipidaemia is a cluster of plasma lipid and lipoprotein abnormalities that are metabolically interrelated. The increase in large VLDL particles in T2DM initiates a sequence of events that generates atherogenic remnants, small dense LDL, and small TG-rich dense HDL particles.411 These components are not isolated abnormalities but are closely linked to each other. Both LDL and HDL particles show variable compositional changes that are reflected in their functions. Notably, ApoC-III levels are increased in people with T2DM.412 High ApoC-III concentrations prevent the clearance of both TRLs and remnants, resulting in prolonged residence times of these particles in the circulation.413,414 In fact, the defective catabolism of TRLs seems to be a more important contributor to the elevation of plasma TGs than the increased production rate leading to an excess of remnant particles. Together, TRL remnants, small dense LDL, and small dense HDL comprise the atherogenic lipid profile, which is also characterized by an increase in ApoB concentration due to an increased number of ApoB-containing particles. Importantly, TRLs—including chylomicrons, VLDL, and their remnants—carry a single ApoB molecule, also like LDL particles. Therefore, the malignant nature of diabetic dyslipidaemia is not always revealed by the lipid measures used in clinical practice, as LDL-C levels may remain within the normal range. It may be better revealed by non-HDL-C levels.415 Elevation of TGs or low HDL-C levels in the fasting or post-prandial state is seen in about one-half of all people with T2DM,416,417 and is also often present in people with abdominal adiposity, insulin resistance or impaired glucose tolerance.413
Box 7 summarizes dyslipidaemia in MetS and T2DM.
Summary of dyslipidaemia in metabolic syndrome and type 2 diabetes mellitus
| Dyslipidaemia represents a cluster of lipid and lipoprotein abnormalities, including elevation of both fasting and post-prandial TG, ApoB, and small dense LDL, and low HDL-C and ApoA1 levels. |
| Non-HDL-C or ApoB are good markers of TRLs and remnants, and are a secondary objective of therapy. Non-HDL-C <2.6 mmol/L (<100 mg/dL) and ApoB <80 mg/dL are desirable in those at high-risk, and non-HDL-C <2.2 mmol/L (<85 mg/dL) and ApoB <65 mg/dL in those at very high-risk. For those at very high-risk with recurrent ASCVD events, a goal of non-HDL-C <1.8 mmol/L (<70 mg/dL) and ApoB <55 mg/dL may be considered. |
| Atherogenic dyslipidaemia is one of the major risk factors for CVD in people with type 2 diabetes, and in people with abdominal obesity and insulin resistance or impaired glucose tolerance. |
| Dyslipidaemia represents a cluster of lipid and lipoprotein abnormalities, including elevation of both fasting and post-prandial TG, ApoB, and small dense LDL, and low HDL-C and ApoA1 levels. |
| Non-HDL-C or ApoB are good markers of TRLs and remnants, and are a secondary objective of therapy. Non-HDL-C <2.6 mmol/L (<100 mg/dL) and ApoB <80 mg/dL are desirable in those at high-risk, and non-HDL-C <2.2 mmol/L (<85 mg/dL) and ApoB <65 mg/dL in those at very high-risk. For those at very high-risk with recurrent ASCVD events, a goal of non-HDL-C <1.8 mmol/L (<70 mg/dL) and ApoB <55 mg/dL may be considered. |
| Atherogenic dyslipidaemia is one of the major risk factors for CVD in people with type 2 diabetes, and in people with abdominal obesity and insulin resistance or impaired glucose tolerance. |
Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG = triglyceride; TRLs = triglyceride-rich lipoproteins.
Summary of dyslipidaemia in metabolic syndrome and type 2 diabetes mellitus
| Dyslipidaemia represents a cluster of lipid and lipoprotein abnormalities, including elevation of both fasting and post-prandial TG, ApoB, and small dense LDL, and low HDL-C and ApoA1 levels. |
| Non-HDL-C or ApoB are good markers of TRLs and remnants, and are a secondary objective of therapy. Non-HDL-C <2.6 mmol/L (<100 mg/dL) and ApoB <80 mg/dL are desirable in those at high-risk, and non-HDL-C <2.2 mmol/L (<85 mg/dL) and ApoB <65 mg/dL in those at very high-risk. For those at very high-risk with recurrent ASCVD events, a goal of non-HDL-C <1.8 mmol/L (<70 mg/dL) and ApoB <55 mg/dL may be considered. |
| Atherogenic dyslipidaemia is one of the major risk factors for CVD in people with type 2 diabetes, and in people with abdominal obesity and insulin resistance or impaired glucose tolerance. |
| Dyslipidaemia represents a cluster of lipid and lipoprotein abnormalities, including elevation of both fasting and post-prandial TG, ApoB, and small dense LDL, and low HDL-C and ApoA1 levels. |
| Non-HDL-C or ApoB are good markers of TRLs and remnants, and are a secondary objective of therapy. Non-HDL-C <2.6 mmol/L (<100 mg/dL) and ApoB <80 mg/dL are desirable in those at high-risk, and non-HDL-C <2.2 mmol/L (<85 mg/dL) and ApoB <65 mg/dL in those at very high-risk. For those at very high-risk with recurrent ASCVD events, a goal of non-HDL-C <1.8 mmol/L (<70 mg/dL) and ApoB <55 mg/dL may be considered. |
| Atherogenic dyslipidaemia is one of the major risk factors for CVD in people with type 2 diabetes, and in people with abdominal obesity and insulin resistance or impaired glucose tolerance. |
Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG = triglyceride; TRLs = triglyceride-rich lipoproteins.
9.4.2 Evidence for lipid-lowering therapy
9.4.2.1 Low-density lipoprotein cholesterol.
LDL-C is the primary target of lipid-lowering therapy in patients with DM. Trials specifically performed in people with T2DM, as well as subsets of individuals with DM in major statin trials, have consistently demonstrated significant benefits of statin therapy on CVD events in people with T2DM.418 Statin therapy reduces the 5 year incidence of major CVD events by 23% per 1 mmol/L reduction in LDL-C, regardless of the initial LDL-C level or other baseline characteristics based on meta-analysis.418 The CTT meta-analysis further indicates that people with T2DM will have a relative risk reduction that is comparable to that seen in non-diabetic patients; however, being at higher absolute risk, the absolute benefit will be greater, resulting in a lower number needed to treat (NNT). Thus, statin therapy is the first-line treatment for LDL-C lowering and for the reduction of CVD burden.419
Ezetimibe lowers LDL-C by ∼24% and, when added to statin therapy, decreases the risk of major vascular events.33 The relative risk reduction in major vascular events is proportional to the absolute degree of LDL-C lowering and consistent with the relationship seen for statins. The subset of patients with DM in IMPROVE-IT had, as expected, a higher rate of major vascular events than patients without DM (46 vs. 31% 7 year Kaplan–Meier rate in the placebo arm). Ezetimibe appeared particularly efficacious in patients with DM, with a relative risk reduction of 15% (95% CI 6–22%) and an absolute risk reduction of 5.5%.299
The mAb PCSK9 inhibitors evolocumab and alirocumab lower LDL-C levels by ∼60% and, when added to statin therapy, decrease the risk of major vascular events.119 In the FOURIER study, the relative risk reduction for major vascular events was similar in patients with and without DM; however, given the higher baseline risk in patients with DM, the absolute risk reductions tended to be greater in patients with DM (2.7% absolute decrease in major vascular events over 3 years).297 Of note, the achieved LDL-C in the evolocumab arm was 0.8 mmol/L. The same benefits were also recently demonstrated for diabetic patients after ACS in the ODYSSEY trial.420
Recent studies have suggested an increased incidence of DM in patients treated with statins.247 These observations have been seen in Mendelian randomization studies and in clinical trials, although the effect appears greatest in patients already at high risk for DM (e.g. those with pre-diabetes). These observations should not lessen our attention to the treatment of patients, as the overall benefits in CV event reduction remain and greatly outweigh the increased incidence of DM. In RCTs, neither ezetimibe nor the PCSK9 inhibitors have been reported to increase the risk of DM.297
9.4.2.2 Triglycerides and high-density lipoprotein cholesterol.
Lifestyle modification provides the first option to improve atherogenic dyslipidaemia due to its multifaceted effects. Weight loss is, in most cases, the most effective measure since it is associated with very pronounced effects on plasma TG and HDL levels, together with a modest decrease in TC and LDL-C levels. Moderate-to-heavy aerobic exercise is also associated with improvement of the plasma lipid profile by reducing TG levels and increasing HDL-C concentrations. In relation to diet composition, besides the need to eliminate trans fat, the available evidence supports the reduction of saturated fat intake and its substitution with unsaturated fat, as well as the replacement of a major proportion of refined starchy foods and simple sugars with fibre-rich foods like fruits, vegetables, and wholegrains.179
The clinical benefits achieved by the treatment of high TG and low HDL-C levels (frequently seen with DM) are still a matter of debate, as the effects of fenofibrate therapy on the major outcome (MACE) remained negative in both the FIELD and the ACCORD studies performed in T2DM cohorts.306,307 In a post hoc analysis of the FIELD study, fenofibrate reduced CVD events by 27% in those with elevated TGs [∼2.3 mmol/L (200 mg/dL)] and increased HDL-C levels (NNT = 23).416 The ACCORD trial confirmed the following: patients who had both TG levels in the higher third [∼2.3 mmol/L (200 mg/dL)] and an HDL-C level in the lower third [≤0.4 mmol/L (≤34 mg/dL)], representing 17% of all participants, appeared to benefit from the addition of fenofibrate to simvastatin.306
Recently, post-trial follow-up of the ACCORD lipid trial participants reported the beneficial effect of fenofibrate in people with HTG and low HDL-C levels at baseline.421 Consistent with these findings, a meta-analysis of fibrates in the prevention of CVD in 11 590 people with T2DM showed that fibrates significantly reduced the risk of non-fatal MI by 21%, but had no effect on the risk of overall mortality or coronary mortality.422 In CV outcome trials of fibrates, the risk reduction has appeared to simply be proportional to the degree of non-HDL-C lowering.50
Overall, available data indicate that diabetic patients with atherogenic dyslipidaemia may derive clinical benefits from TG-lowering therapy as an add-on to statin treatment.354 The ongoing PROMINENT trial is exploring the efficacy of pemafibrate, a new selective PPAR-α modulator, in reducing CVD outcomes in ∼10 000 diabetic patients with atherogenic dyslipidaemia on a statin.317,423
There are limited data on the impacts on CVD of adding omega-3 fatty acids to statin therapy in patients with high plasma TG levels who are treated with statins. The REDUCE-IT trial examined the effects of icosapent ethyl 2 g b.i.d. on CV events in 8179 high-risk patients with HTG who were taking a statin. Over a median of 4.9 years, there was a significant (P < 0.001) 25% reduction in the composite primary outcome of CV death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina, corresponding with an absolute reduction of 4.8%, which was offset by a 1% increased absolute risk of hospitalization for atrial fibrillation or flutter.194 The STRENGTH trial is investigating the effect of omega-3 fatty acids, in addition to a statin, in individuals with HTG and low HDL-C levels who are at high-risk for CVD. The ASCEND trial was a randomized 2×2 factorial design study of aspirin and omega-3 fatty acid supplements for the primary prevention of CV events in people with DM, but not specifically with HTG. Among 15 480 people randomized to omega-3 fatty acid supplements vs. placebo over a mean follow-up of 7.4 years, there was no significant effect (HR 0.97, 95% CI 0.87–1.08) on serious vascular events [non-fatal MI, non-fatal stroke, transient ischaemic attack (TIA), or vascular death].329,424–426
9.4.3 Type 1 diabetes mellitus
T1DM is associated with high CVD risk, in particular in patients with microalbuminuria and renal disease.427 Conclusive evidence supports the proposition that hyperglycaemia accelerates atherosclerosis. Emerging evidence highlights the frequent coexistence of MetS with T1DM, resulting in the so-called double diabetes increasing CVD risk.428
The lipid profile in T1DM patients with good glycaemic control is ‘supernormal’, and is characterized by subnormal TG and LDL-C levels, whereas HDL-C levels are usually within the upper normal range or slightly elevated. This is explained by subcutaneous administration of insulin that increases LPL activity in adipose tissue and skeletal muscle, and consequently the turnover rate of VLDL particles.429 However, there are potentially atherogenic changes in the compositions of both HDL and LDL particles.
Consistent data have demonstrated the efficacy of statins in preventing CV events and reducing CV mortality in patients with DM, with no evidence of sex differences.430,431 A meta-analysis including 18 686 patients with DM demonstrated that a statin-induced reduction of LDL-C yielded a 9% reduction in all-cause mortality and a 21% reduction in the incidence of major CV events per 1.0 mmol/L (40mg/dL) lower LDL cholesterol.418 Similar benefits were seen in patients with T1DM and T2DM. In diabetic patients with ACS, intensive statin treatment led to a reduction in all-cause mortality and CV death, and contributed to a reduction in atheroma progression.432
9.4.4 Management of dyslipidaemia for pregnant women with diabetes
In both T1DM and young-onset T2DM patients, there is a paucity of evidence to indicate the age at which statin therapy should be initiated. To guide an approach, statins are not indicated in pregnancy,433 and should be avoided in both T1DM and T2DM individuals who are planning pregnancy. If diabetic individuals aged ≤30 years have no evidence of vascular damage and, in particular, microalbuminuria, it seems reasonable to delay statin therapy in asymptomatic patients until the age of 30. Below this age, statin therapy should be managed on a case-by-case basis, taking into account the presence of microalbuminuria, end organ damage, and ambient LDL-C levels.
Recommendations for the treatment of dyslipidaemias in DM are shown in the table below.
Recommendations for the treatment of dyslipidaemias in diabetes mellitus
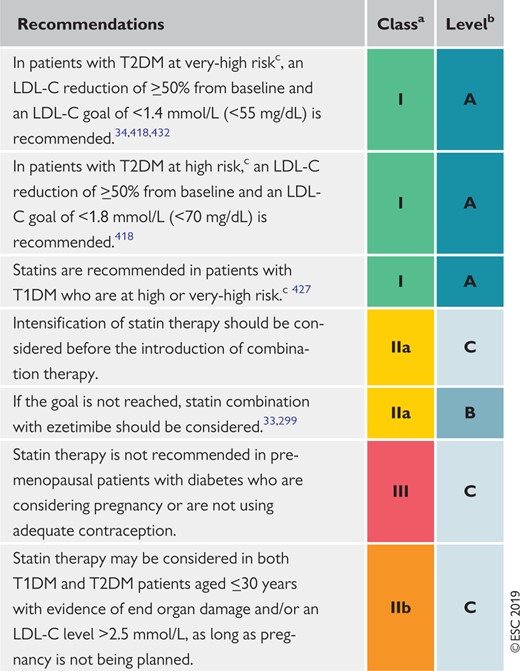 |
 |
LDL-C = low-density lipoprotein cholesterol; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 6.
Recommendations for the treatment of dyslipidaemias in diabetes mellitus
 |
 |
LDL-C = low-density lipoprotein cholesterol; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 6.
9.5 Patients with acute coronary syndromes and patients undergoing percutaneous coronary intervention
Patients who present with ACS are at increased risk of experiencing recurrent CV events. For these patients, lipid management should be undertaken in the context of a comprehensive global risk reduction strategy including lifestyle adaptations, risk factor management, and the implementation of cardioprotective drug strategies. Ideally, patients should be signed up to cardiac rehabilitation programmes to enhance the control of lipid levels434 and improve overall survival following ACS.435 Despite the acknowledged clinical benefits of lowering LDL-C in patients with ACS,436 attainment of LDL-C target values remains suboptimal in this very high-risk setting.437
9.5.1 Lipid-lowering therapy in patients with acute coronary syndromes
LDL-C levels tend to decrease during the first days of ACS and therefore a lipid profile should be obtained as soon as possible after admission for ACS. Patients do not have to be fasting as this has little impact on LDL-C levels.100 Lipid-lowering treatment should be initiated as early as possible to increase patient adherence after discharge. Lipid levels should be re-evaluated 4–6 weeks after ACS to determine whether treatment goals have been achieved and to check for any safety issues; the therapeutic regimen can then be adapted accordingly.
9.5.1.1 Statins.
Data from RCTs and meta-analyses indicate that routine early use of high-intensity statin therapy is associated with rapid and sustained clinical benefits.438–442 We recommend the initiation of high-intensity statin therapy in all statin-naïve ACS patients with no contraindication, regardless of initial LDL-C values; the treatment goal is to reach a 50% LDL-C reduction from baseline and an LDL-C goal of <1.4 mmol/L (<55 mg/dL). In those with recurrent events within 2 years while taking maximally tolerated statin therapy, a goal of <1.0 mmol/L (<40 mg/dL) for LDL-C should be considered. The intensity of statin therapy should be increased in those patients receiving low- or moderate-intensity statin treatment at presentation, unless there is a definite history of intolerance to high-intensity statin therapy. The use of lower-intensity statin therapy should be considered in patients at increased risk of adverse effects with high-intensity statin therapy, such as in the elderly, patients diagnosed with hepatic or renal impairment, or in the case of a potential risk of drug–drug interactions with other essential concomitant therapies.
Regarding the timing of statin treatment initiation, the Statins Evaluation in Coronary Procedures and Revascularization (SECURE-PCI) randomized, placebo-controlled trial recently assessed the impact of peri-procedural loading with atorvastatin [two loading doses of 80 mg, before and 24 h after the planned percutaneous coronary intervention (PCI)] on MACE at 30 days in 4191 patients with ACS and planned invasive management.443 All patients received atorvastatin 40 mg per day starting 24 h after the second loading dose. The authors found no significant treatment benefit in the overall study population. In a pre-specified analysis, a significant 28% relative risk reduction in MACE was observed among patients who underwent PCI (65% of all patients). The benefit was even more pronounced (46% relative risk reduction) in a post hoc analysis including 865 ST-elevation MI (STEMI) patients undergoing reperfusion by primary PCI.443 Based on current evidence, we recommend the initiation of high-intensity statin therapy during the first 1–4 days of hospitalization for the index ACS.438–442 Moreover, pre-treatment (or loading dose for patients already on a statin) with a high-intensity statin should be considered in ACS patients with planned invasive management.443
9.5.1.2 Ezetimibe.
In the IMPROVE-IT trial, the addition of ezetimibe to simvastatin therapy provided an additional benefit (6.4% relative risk reduction in the composite clinical endpoint) to post-ACS patients.33 The clinical benefit of adding ezetimibe was consistent across patient subgroups299,444 and also led to a reduction of total CV events,445 stroke,446 and rehospitalizations.447 Patients at higher atherothrombotic risk [as assessed by the TIMI (Thrombolysis In Myocardial Infarction) risk score for secondary prevention] benefitted the most from the addition of ezetimibe.448 In another randomized, open-label trial including 1734 patients with ACS, the addition of ezetimibe to moderate-intensity statin (pitavastatin 2 mg) therapy failed to improve outcomes overall, but did reduce the composite primary endpoint (death, MI, stroke, unstable angina, and ischaemia-driven revascularization) during 3.9-year follow-up in patients with increased intestinal absorption of cholesterol (as assessed by elevated levels of sitosterol)449; however, this finding requires further confirmation.
9.5.1.3 Proprotein convertase subtilisin/kexin type 9 inhibitors.
In the FOURIER trial, which included 27 564 patients with atherosclerotic CV disease, the addition of evolocumab to statin therapy (69% high-intensity therapy) resulted in a 15% relative risk reduction of the composite primary endpoint throughout a 2.2 year follow-up. Results were consistent in the subgroup of patients with a history of MI (81% of all patients).119,450 A subanalysis of the FOURIER trial showed that patients who achieved the lowest LDL-C values under PCSK9 treatment also had the lowest risk of future MACE.451 In the ODYSSEY Outcomes trial, which included 18 924 patients with recent ACS (1–12 months prior to enrolment, median 2.6 months), alirocumab added to statin therapy (89% high-intensity therapy) also resulted in a 15% relative risk reduction in the primary composite endpoint and was associated with a 15% relative reduction in all-cause mortality throughout a 2.8 year follow-up.120 No serious side effects or safety concerns were reported in these two large trials. The optimal timing of initiating PCSK9 inhibition after ACS and its impact on clinical outcomes remain to be determined. Regarding the timing of PCSK9 inhibitor treatment initiation, post hoc analyses from the FOURIER trial have shown that the closer to the event this is done, the better. Treatment initiation with PCSK9 inhibitors during the acute phase of ACS is under investigation in the EVOlocumab for Early Reduction of LDL-cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS) trial.452 Based on current evidence, we recommend the initiation of treatment with PCSK9 inhibitors in patients with ACS who do not reach their respective LDL-C goals (as outlined in Table 7) after 4–6 weeks of maximum tolerated statin and ezetimibe therapy. In patients who present with an ACS and whose LDL-C levels are not at goal, despite already taking a maximally tolerated statin dose and ezetimibe prior to the event, the addition of a PCSK9 inhibitor early after the event (during the hospitalization for the ACS event if possible) should be considered.
9.5.1.4 n-3 polyunsaturated fatty acids.
Oral supplementation with highly purified n-3 PUFAs reduced mortality in survivors of MI in one study [Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Prevenzione (GISSI-P)] but failed to affect clinical outcomes in subsequent trials using contemporary secondary prevention therapies. A recent meta-analysis of available RCTs showed no reduction in mortality, MI, or major vascular events associated with n-3 PUFAs, including the subgroup of patients with known CAD.453 Therefore, routine treatment with n-3 PUFAs cannot be recommended.
9.5.1.5 Cholesteryl ester transfer protein inhibitors.
In 2007, a large prospective study using the CETP inhibitor torcetrapib failed to show any clinical benefit in more than 15 000 high-risk patients, and was potentially harmful.336 The CETP inhibitors dalcetrapib (in >30 000 patients with recent ACS65) and evacetrapib (in >12 000 high-risk patients63) were investigated in 2012 and 2017, respectively. Neither clinical study was able to show any clinical benefit associated with CETP inhibitors.65 More recently, the REVEAL study investigated anacetrapib in >30 000 patients with atherosclerotic vascular disease and resulted in a lower incidence of MACE compared with placebo after 4 years, with no safety concerns.64 However, this compound was not filed for marketing authorization.
9.5.2 Lipid-lowering therapy in patients undergoing percutaneous coronary intervention
In a meta-analysis of 13 randomized studies including 3341 patients who were planned to undergo PCI, pre-treatment with a high-dose statin (statin-naïve patients, 11 studies) or a high-dose statin loading dose reduced the risk of MACE (death, MI, or target vessel revascularization) by 44% both for peri-procedural MI and MACE at 30 days.454 In all but one study, PCI was performed in the setting of stable angina or in a non-emergency setting in non-ST elevation ACS (NSTE-ACS). One of the studies that was included in the meta-analysis showed an improvement in coronary flow when primary PCI was used for the treatment of STEMI.455 A routine strategy of either short pre-treatment or loading (on the background of pre-existing therapy) with a high-dose statin before PCI should be considered in elective PCI or NSTE-ACS.454,456,457
In addition, pre-treatment with a statin has also been shown to reduce the risk of contrast-induced acute kidney injury after coronary angiography or intervention.458
Recommendations for lipid-lowering therapy in patients with ACS and patients undergoing PCI are summarized below.
Recommendations for lipid-lowering therapy in very-high-risk patients with acute coronary syndromes
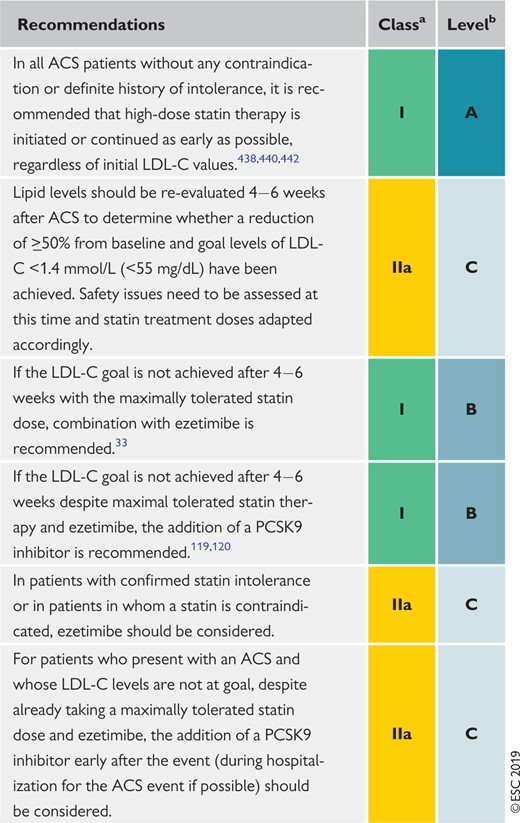 |
 |
ACS = acute coronary syndrome; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
Recommendations for lipid-lowering therapy in very-high-risk patients with acute coronary syndromes
 |
 |
ACS = acute coronary syndrome; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
9.6 Stroke
Stroke has a heterogeneous aetiology, including cardiac thromboembolism [often associated with atrial fibrillation, but also of uncertain source (embolic stroke of undetermined source)], carotid artery and proximal aortic atherosclerosis and thromboembolism, small-vessel CVD, and intracranial haemorrhage (including intracerebral and subarachnoid haemorrhage). Dyslipidaemia may play a variable role in the pathogenesis of stroke according to the particular aetiology. The relationship between dyslipidaemia and atherothrombotic events, including ischaemic stroke and TIA, is well recognized, while the association of dyslipidaemia with other types of stroke is uncertain. Notwithstanding, concomitant control of other aetiological factors, such as hypertension, is of paramount importance.
Following ischaemic stroke or TIA, patients are at risk not only of recurrent cerebrovascular events, but also of other major CV events, including MI. Secondary prevention therapy with statins reduces the risk of recurrent stroke (by 12% per mmol/L reduction in LDL cholesterol), MI, and vascular death.459,460 Statin pre-treatment at TIA onset was associated with reduced recurrent early stroke risk in patients with carotid stenosis in a pooled data analysis, supporting as-early-as-possible initiation of statins after stroke.460–462 Statin therapy may yield a small increase in the risk of haemorrhagic stroke, but the evidence regarding this risk is uncertain.34,36,251,252
Recommendations for lipid-lowering therapy in very-high-risk patients undergoing percutaneous coronary intervention
 |
 |
ACS = acute coronary syndrome; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Recommendations for lipid-lowering therapy in very-high-risk patients undergoing percutaneous coronary intervention
 |
 |
ACS = acute coronary syndrome; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Recommendations for lipid-lowering therapy for the prevention of atherosclerotic cardiovascular disease events in patients with prior ischaemic stroke
 |
 |
ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol; TIA = transient ischaemic attack.
Class of recommendation.
Level of evidence.
Recommendations for lipid-lowering therapy for the prevention of atherosclerotic cardiovascular disease events in patients with prior ischaemic stroke
 |
 |
ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol; TIA = transient ischaemic attack.
Class of recommendation.
Level of evidence.
9.7 Heart failure and valvular diseases
9.7.1 Prevention of incident heart failure in coronary artery disease patients
Cholesterol lowering with statins reduces the incidence of HF in patients with CAD (stable CAD or a history of ACS) without previous HF; this has been shown consistently in RCTs that have compared statin vs. no statin treatment463,464 as well as more-intensive vs. less-intensive statin therapy.465–468 A large-scale meta-analysis of primary and secondary prevention RCTs with statins showed a modest (10%) reduction in first non-fatal HF hospitalizations with statin treatment, with no effect on HF death within the limited RCT period.469 There is no evidence that statins can prevent HF of non-ischaemic origin.
9.7.2 Chronic heart failure
Two large RCTs466,470 (mainly including patients with systolic HF), as well as a meta-analysis of 24 RCTs, have shown no benefit of statin treatment on CV mortality or stroke;471 a reduction in HF hospitalizations,218,471 as well as a small reduction in MI, was observed in a pooled analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and GISSI-HF trials.472 Based on current evidence, routine administration of statins in patients with HF without other indications for their use (e.g. CAD) is not recommended. Because there is no evidence of harm in patients on statin treatment after the occurrence of HF, there is no need for statin discontinuation for patients already on treatment.
There is no evidence regarding the effect of PCSK9 inhibition in patients with chronic HF. In the recent PCSK9 clinical outcomes trials, FOURIER119 and ODYSSEY Outcomes,120 PCSK9 inhibition in patients with atherosclerotic CVD or after an ACS did not reduce the risk of HF hospitalization. In the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT-CHF) study of 2174 patients with worsening HF, multivariable analysis revealed a positive linear association between PCSK9 levels and the risk of mortality, and the composite of mortality and unplanned HF hospitalization.473 Similarly, there was a negative association between LDLR levels and mortality, indicating a potential relationship between the PCSK9–LDLR axis and outcomes among patients with HF that requires further investigation.473,474
Treatment with n-3 PUFAs 1 g o.d. may confer a small benefit in patients with chronic HF, as shown by a significant 9% relative risk reduction for mortality in the GISSI-HF RCT.475
9.7.3 Valvular heart diseases
Aortic stenosis increases the risk of CV events and mortality, and frequently coexists with atherosclerotic CVD. Life-long high levels of LDL-C476 and Lp(a)477 have been associated with incident aortic valve stenosis and aortic valve calcification in genetic Mendelian randomization studies. Observational studies have suggested possible beneficial effects of intensive lipid lowering in slowing the progression of native valve aortic stenosis.478 However, this has not been confirmed in RCTs,266,479–481 or in meta-analyses of observational and randomized trials.482,483 Three modestly sized trials479–481 and one large randomized trial (SEAS, which included 1873 patients treated with simvastatin 40 mg plus ezetimibe 10 mg or placebo)266 failed to show a reduction in the clinical progression of aortic stenosis in patients with mild-to-moderate native valve aortic stenosis. In a post hoc analysis of the SEAS trial, the efficacy of lipid-lowering therapy in impeding the progression of aortic stenosis increased with higher pre-treatment LDL-C levels and lower peak aortic jet velocity (i.e. milder stenosis at baseline).484 Similarly, a post hoc analysis of three RCTs, including patients without known aortic valve stenosis at baseline [Treating to New Targets (TNT), Incremental Decrease In End-points Through Aggressive Lipid-lowering (IDEAL), and Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL)] showed no impact of high-dose vs. usual-dose statin therapy on the incidence of aortic valve stenosis.485 In patients who underwent transcatheter aortic valve replacement, statin therapy was associated with improved outcomes in a small observational study.486
Aortic valve sclerosis (calcification of the aortic leaflets without significant transvalvular pressure gradient) is associated with an increased risk of CAD even in the absence of increased risk profiles. Whether or not statins may be useful both for aortic valve disease and CAD progression in such patients warrants further investigation.487
Recommendations for lipid-lowering therapy in patients with HF and valvular diseases are shown below.
Recommendations for the treatment of dyslipidaemias in chronic heart failure or valvular heart diseases
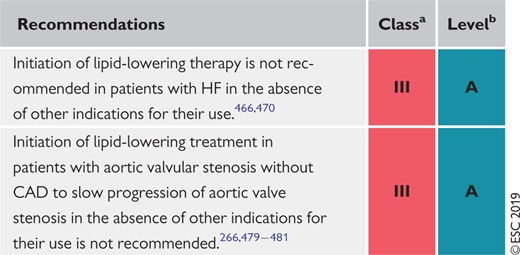 |
 |
CAD = coronary artery disease; HF = heart failure.
Class of recommendation.
Level of evidence.
Recommendations for the treatment of dyslipidaemias in chronic heart failure or valvular heart diseases
 |
 |
CAD = coronary artery disease; HF = heart failure.
Class of recommendation.
Level of evidence.
9.8 Chronic kidney disease
CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health. CKD is classified on the basis of the cause, GFR category, and category of albuminuria.488 In the adult population, decreasing GFR is associated with increased CVD risk, independent of other CV risk factors.489–492 There is an increased risk of both atherosclerotic vascular disease and structural heart disease.492 Patients with CKD and established CVD have a much higher mortality rate compared with patients with CVD and normal renal function.493 Therefore, patients with CKD are considered to be at high (stage 3 CKD) or very-high risk (stage 4–5 CKD or on dialysis) of CVD, and there is no need to use risk estimation models in these patients.
9.8.1 Lipoprotein profile in chronic kidney disease
In the initial stages of CKD, TG levels are specifically elevated and HDL-C levels are lowered. LDL subclasses display a shift to an excess of small dense LDL particles. Studies suggest that the kidney has a role in Lp(a) catabolism and that Lp(a) levels are increased in association with kidney disease. Such acquired abnormalities can be reversed by kidney transplantation or remission of nephrosis.
9.8.2 Evidence for risk reduction through statin-based therapy in patients with chronic kidney disease
In the Die Deutsche Diabetes Dialyse Studie (4D) trial, which involved 1200 patients with diabetes on haemodialysis, atorvastatin had no significant effect on risk of CVD.220 Similar results were obtained in the AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events) trial, which involved 2776 patients on haemodialysis.221
In the SHARP study,222 simvastatin and ezetimibe combination therapy reduced the risk for major atherosclerotic events (coronary death, MI, non-haemorrhagic stroke, or any revascularization) compared with placebo in persons with CKD stage 3A–5. The trial did not have sufficient power to separately assess the effects on the primary outcome in dialysis and non-dialysis patients. Although statin-based therapy is clearly effective in mild-to-moderate CKD, a major controversy that remained after the publication of the 4D, AURORA, and SHARP studies was whether statin therapy is effective in more advanced CKD, particularly dialysis patients. By combining data from the three CKD trials with other trials in the existing database, the CTT investigators found that, even after adjusting for the smaller LDL-C reductions achieved among patients with more advanced CKD and for differences in outcome definitions between dialysis trials, there was a trend towards smaller relative reductions per mmol/L reduction in LDL-C in major atherosclerotic events as estimated GFR (eGFR) declines (with little evidence of benefit among dialysis patients).214 This diminution in relative risk reduction as GFR declines implies that, at least in non-dialysis patients, more intensive LDL-lowering regimens are required to achieve the same benefit.
9.8.3 Safety of lipid management in patients with chronic kidney disease
Safety issues and dose adjustments are important in advanced stages of CKD (stages 3–5), as adverse events are commonly dose-related and due to increased blood concentrations of compounds. Although it has been suggested that preference should be given to regimens and doses that have been shown to be beneficial in RCTs conducted specifically in such patients,494 the CTT meta-analysis makes clear that the goal—as in patients without CKD—should be to achieve the largest possible absolute reduction in LDL-C safely. Although there were no specific safety concerns raised by the 4D, AURORA, or SHARP trials, statins metabolized via CYP3A4 may result in adverse effects due to drug–drug interactions and caution is required.
Based on the evidence for lipid management in patients with CKD, the Kidney Disease: Improving Global Outcomes (KDIGO) organization has developed an updated clinical practice guideline for lipid management in CKD.494 In line with this, but with a focus on those patients at high or very-high risk for developing CVD, recommendations are summarized below.
Recommendations for lipid management in patients with moderate-to-severe (Kidney Disease Outcomes Quality Initiative stages 3–5) chronic kidney disease
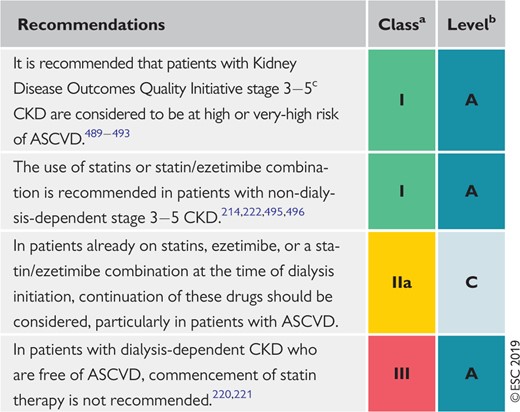 |
 |
ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Class of recommendation.
Level of evidence.
Defined as eGFR < 60ml/min/1.73m2 on two measurements more than 3 months apart.
Recommendations for lipid management in patients with moderate-to-severe (Kidney Disease Outcomes Quality Initiative stages 3–5) chronic kidney disease
 |
 |
ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Class of recommendation.
Level of evidence.
Defined as eGFR < 60ml/min/1.73m2 on two measurements more than 3 months apart.
9.9 Transplantation
Dyslipidaemias are very common in patients who have undergone heart, lung, liver, kidney, or allogenic haematopoietic stem cell transplantation, and predispose such patients to an increased risk of developing ASCVD and transplant arterial vasculopathy.497–501 In patients with CKD undergoing renal transplantation, the risk of ASCVD may be determined, at least in part, by the increased risk resulting from CKD itself.
Immunosuppressive drug regimens may have adverse effects on lipid metabolism leading to increases in TC, VLDL, and TGs, and in the size and density of LDL particles. These effects vary with different immunosuppressive drugs.497,498,502–506
The management of dyslipidaemias in transplant recipients is comparable to what is recommended for patients at high or very high ASCVD risk, although more attention is needed regarding the causes of the lipid disturbances and possible side effects due to drug–drug interactions (see Recommendations for low-density lipoprotein in solid organ transplant patients below).
The clinical effectiveness of statins in renal transplant patients is uncertain owing to a lack of randomized trials in this population. A systematic review of the benefits and harms of statins in patients with a functioning kidney transplant included 3465 patients, free of CHD, from 22 studies. Although the authors concluded that statins may reduce CV events, they also suggested a need for additional studies.253 However, in patients with a functioning renal transplant at increased risk of CVD, it may be appropriate to extrapolate from the clear evidence of benefit from statin therapy, without safety concerns, in people with moderate reductions in GFR.214
Several potential drug interactions must also be considered, especially with ciclosporin, which is metabolized through CYP3A4, and may increase systemic statin exposure and the risk of myopathy. Ciclosporin increases the blood levels of all statins.
Fluvastatin, pravastatin, pitavastatin, and rosuvastatin are metabolized through different CYP enzymes than the others and have less potential for interaction.507
Tacrolimus is also metabolized by CYP3A4, but appears to have less potential for harmful interaction with statins than ciclosporin. Other drugs that influence CYP3A4 activity should be avoided if possible, and used with extreme caution in patients receiving both calcineurin inhibitors and statins.
For transplant patients with dyslipidaemia, ezetimibe could be considered as an alternative for patients unable to take a statin or added to the highest tolerated statin dose.507–509 No outcome data are available for this drug, which should generally be reserved for second-line use. Ciclosporin can induce a 2–12-fold increase in the ezetimibe level.
Care is required with the use of fibrates, as they can decrease ciclosporin levels and have the potential to cause myopathy. Extreme caution is required if fibrate therapy is planned in combination with a statin. Cholestyramine is not effective as monotherapy in heart transplant patients and has the potential to reduce absorption of immunosuppressants; this potential is minimized by separate administration.
Recommendations for low-density lipoprotein lowering in solid organ transplant patients
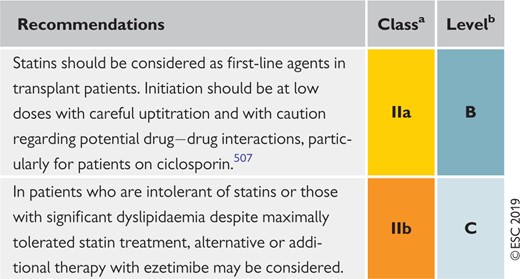 |
 |
Class of recommendation.
Level of evidence.
Recommendations for low-density lipoprotein lowering in solid organ transplant patients
 |
 |
Class of recommendation.
Level of evidence.
9.10 Peripheral arterial disease
PAD encompasses all vascular sites, including carotid, vertebral, upper extremity, mesenteric, renal, and lower extremity arteries. The aorta is often included in the term.510 PAD is a common manifestation of atherosclerosis and such patients are at elevated risk of coronary events, with PAD representing an independent risk factor for MI and CV death.510,511 Patients with PAD are at very-high risk and should be managed according to the recommendations in Table 7. Elevated CV risk has led to the inclusion of PAD among the list of ‘risk equivalent’ conditions, and therapeutic strategies of secondary prevention should be implemented [see Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease) below]. Yet, despite the high CV morbidity and mortality risk, PAD patients are usually inadequately managed compared with CAD patients.511
9.10.1 Lower extremity arterial disease
A low ABI (0.90) is diagnostic for lower extremity arterial disease (LEAD). Either a low (0.90) or high (1.40, related to stiffened arteries) ABI is predictive of CV morbidity and mortality. Lowering LDL-C levels reduces the risk of ischaemic CV events and worsening of claudication, while it also improves walking performance. As for cardiac events, a systematic review of 18 trials including 10 000 patients, with cholesterol levels ranging from normal to elevated, reported that lipid-lowering therapy in people affected by atherosclerosis of the lower limbs was associated with a 20% reduction in total CV events, together with a non-significant 14% reduction of all-cause mortality.512 In the Heart Protection Study, the need for non-coronary revascularization was reduced by 16% with statin therapy.513
In addition to statins, PSCK9 inhibitors have also been shown to reduce CV events in PAD patients. In a pre-specified subgroup analysis of the FOURIER trial, evolocumab significantly reduced the primary endpoint in patients with PAD.514 PAD had larger absolute risk reductions for the primary endpoint (3.5% with PAD and 1.6% without PAD). Evolocumab also reduced the risk of major adverse limb events by 42% in patients, with consistent effects in those with and without known PAD. In the FIELD trial, fenofibrate reduced the risk of amputations, particularly minor amputations without known large-vessel disease, probably through non-lipid mechanisms.515
9.10.2 Carotid artery disease
While there are currently no randomized studies that have assessed whether lipid-lowering treatments reduce the incidence of CV events in patients enrolled on the basis of carotid atherosclerotic disease and without previous CV events, lipid-lowering therapy had reduced stroke in numerous studies. In a meta-analysis of RCTs enrolling >90 000 patients, statin therapy did lead to a 21% reduction in the incidence of all strokes in different populations; this effect was mainly driven by the extent of LDL-C reduction.460
9.10.3 Retinal vascular disease
Atherosclerotic changes of retinal arteries correlate with TC, LDL-C, TG, and apoB levels and also with CAD.516 Fenofibrate reduces the progression of diabetic retinopathy.517,518
9.10.4 Secondary prevention in patients with abdominal aortic aneurysm
The presence of an abdominal aortic aneurysm represents a risk-equivalent condition for CAD and is associated with age, male gender, personal history of atherosclerotic CVD, smoking, hypertension, and dyslipidaemia;519 in contrast, diabetic patients are at decreased risk.
There are currently no available clinical trials on the reduction of CV risk with lipid-lowering therapy in patients affected by this condition. Systematic reviews,520 mostly based on retrospective non-randomized studies, have reported that there is still inconclusive evidence that statin therapy reduces peri-operative CV morbidity and mortality. In an RCT comparing atorvastatin 20 mg with placebo, the composite endpoint of cardiac death, MI, stroke, and unstable angina was significantly reduced in 100 patients undergoing vascular non-cardiac surgery, including abdominal aortic aneurysm repair.521 In another double-blind, placebo-controlled trial in 497 patients undergoing vascular surgery, peri-operative fluvastatin therapy (80 mg/day) was associated with an improvement in post-operative cardiac outcome.522
9.10.5 Renovascular atherosclerosis
Lipid-lowering therapy has never been tested in an RCT in patients affected by renovascular atherosclerosis; however, a recent non-randomized population-based study showed that in patients older than 65 years of age with atherosclerotic renovascular disease raised the hypothesis that such treatment may yield cardiorenal benefits; the risk of a major cardiorenal composite endpoint (MI, stroke, HF, acute renal failure, dialysis, and death) was significantly lower in statin users than in non-users.523
Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease)
 |
 |
ASCVD = atherosclerotic cardiovascular disease; PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease)
 |
 |
ASCVD = atherosclerotic cardiovascular disease; PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Class of recommendation.
Level of evidence.
9.11 Other special populations at risk of atherosclerotic cardiovascular disease
In general, the effects of lowering LDL-C are determined by the absolute risk of ASCVD and the achieved reduction in LDL-C, so it is important to identify and treat all those at increased risk of ASCVD. There are a few specific groups of patients in whom an underlying disease confers such increased risk, and in addition in whom the standard treatments may themselves cause dyslipidaemia that may contribute to the risk of ASCVD. These include: (i) chronic immune-mediated inflammatory disease, (ii) patients with human immunodeficiency virus (HIV), and (iii) patients with severe mental illness. The management principles are the same in these patient groups, but their management may need to address specific issues related to individual dyslipidaemias and drug safety. Details are provided in the Supplementary Data document.
10 Inflammation
Recent advances in basic science have established a fundamental role for low-degree chronic inflammation in mediating all stages of atherosclerosis, from initiation through progression and, ultimately, to the rupture of plaque and ensuing thrombotic complications of atherosclerosis. The cellular and molecular interactions involved during atherogenesis are fundamentally not different from those in chronic inflammatory–fibroproliferative diseases, such as rheumatoid arthritis (RA), glomerulosclerosis, or pulmonary fibrosis.525 Almost all cell types of the immuno-inflammatory system, such as macrophages, and T- and B-cells, as well as many pro- and anti-inflammatory cytokines and chemokines, have been identified during the process of atherosclerosis.526
Interestingly, cholesterol accumulation in cells triggers the inflammasome response and results in the production of inflammatory mediators such as interleukin (IL)-1β. Numerous animal studies, using the knockout model, have demonstrated that inflammation and the immune system both play crucial roles during atherogenesis.527
During inflammatory processes, large numbers of acute-phase proteins have been identified, and several clinical studies have reported C-reactive protein528 to be the most useful serum marker of inflammation, even though it has poor specificity for any particular inflammation process, including atherosclerosis. The high-sensitivity C-reactive protein diagnostic test was developed to detect very low levels of C-reactive protein, and thereby enable a more accurate and precise measure of chronic inflammation compared with standard C-reactive protein.529 This diagnostic tool differs only in the range of C-reactive protein levels that it can detect. Several studies have found that elevated levels of high-sensitivity C-reactive protein in the blood are associated with an increased risk of CV events and could be used to predict clinical outcomes independently of cholesterol levels.530,531 Other studies have not been able to show any relationship between low-grade chronic inflammation, as indicated by high-sensitivity C-reactive protein levels, and increased risk of CV.532–535 Finally, genetic studies of large population cohorts have not demonstrated that chronic elevated high-sensitivity C-reactive protein increases the risk of atherosclerotic events.536 Nevertheless, in some guidelines, high-sensitivity C-reactive protein has been added to traditional risk factors for prognostic information, especially for patients at intermediate risk.537,538
Statins have been shown to reduce C-reactive protein secretion by hepatocytes,539 and a series of clinical trials and post hoc analyses have found that beneficial outcomes after statin therapy relate both to a reduction in cholesterol levels and reduced inflammation.540–544 The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial542 demonstrated that in primary prevention for individuals with chronically elevated C-reactive protein (>2 mg/L), statin treatment markedly reduced CV events.545 It is of note that other lipid-lowering agents, such as ezetimibe and more recently the anti-PCSK9 mAbs, do not influence high-sensitivity C-reactive protein levels,546,547 but lead to further significant reductions in CV events when added to statin therapy.
Specific anti-inflammatory treatment was tested in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial.548 In patients with previous MI and chronic elevated high-sensitivity C-reactive protein levels, all on optimal medical treatment, including statins, the anti-IL-1β mAb canakinumab dose-dependently reduced high-sensitivity C-reactive protein and significantly lowered the rate of recurrent CV events compared with placebo, independently of the level of lipid lowering. Not surprisingly, there was a slight increase in the risk of severe and fatal infections associated with canakinumab. This study was the first to highlight the positive correlation between high-sensitivity C-reactive protein and CV events, where lower achieved high-sensitivity C-reactive protein values were directly correlated with a lower risk of future CV events.548 Nevertheless, the FDA declined to approve canakinumab for CV risk reduction on the strength of data from the CANTOS study. As canakinumab treatment has not been tested against anti-PCSK9 mAb and/or ezetimibe added to statin therapy, the question of residual risk remains for patients with elevated high-sensitivity C-reactive protein despite achieving very low (below goal) LDL-C values, and whether patients with very low LDL-C would benefit from anti-IL-1β treatment or other anti-inflammatory agents. In addition, all currently recommended lipid-lowering drugs, including anti-PCSK9 mAbs, have demonstrated beneficial effects on atherosclerotic plaque composition as well as plaque volume regression; such results are still missing for anti-inflammatory treatment. Another anti-inflammatory approach using methotrexate was tested in the Cardiovascular Inflammation Reduction Trial (CIRT).549 Very low-dose methotrexate (10 mg weekly), a proven anti-inflammatory regimen that reduces tumour necrosis factor (TNF), IL-6, and C-reactive protein levels and is widely used in the treatment of RA, was allocated vs. placebo to 7000 stable CAD patients. This study was stopped prematurely due to futility. Interestingly, this regimen of methotrexate had no effect on either IL-6 or high-sensitivity C-reactive protein blood levels in this population, which could explain the neutral results of this trial.550 Based on the current level of evidence, no further recommendations on the use of anti-inflammatory agents can be made.551
11 Monitoring of lipids and enzymes in patients on lipid-lowering therapy
Evidence concerning which tests should be carried out to monitor lipids in patients on treatment is limited. Similar limited evidence applies to tests of possible toxicity, such as ALT and CK. Recommendations stem from consensus rather than evidence-based medicine.
Response to therapy can be assessed at 6–8 weeks from initiation of therapy, but response to lifestyle may take longer. Standard practice for subsequent follow-up monitoring is 6–12 months, but such monitoring intervals are arbitrary. As a minimum, LDL-C should be assessed whenever available, but better management decisions will probably occur if a full lipid profile is performed, including HDL-C and TGs. Non-HDL-C or ApoB should also be analysed, and used as a secondary treatment target. A separate issue is the impact of regular lipid monitoring in promoting patient adherence to lifestyle changes or drug regimens that impact positively on their health, as found in a range of studies. It is unclear whether only the process of monitoring is critical in achieving this or whether a combination of education, regular contact, and adherence assessment is required.
Where pharmacological lipid-lowering therapy is implemented, safety blood tests are advised, including ALT and CK at baseline, to identify the limited number of patients where treatment is contraindicated. CK should be checked in patients at high-risk for myopathy, such as the very elderly with comorbidities, patients with antecedents of muscle symptoms, or patients receiving interacting drugs. A mild and typically transient elevation of ALT is seen in about 2% of patients and normalization is seen with continuing therapy.240,244,552 Recent reviews are encouraging regarding the safety of long-term statin therapy and statin-induced liver injury is reported to be very uncommon.243,244,553–555 ALT is recommended before the start of statin therapy; routine control of ALT during treatment is not recommended but should be performed, if indicated, based on clinical observations. During fibrate therapy, regular ALT control is still recommended. In patients whose liver function tests increase to above three times the ULN, explanations such as alcohol ingestion or NAFLD should be sought, and the levels monitored. If levels remain elevated then lipid-lowering therapy should be stopped, but may be cautiously reintroduced under monitoring after levels have returned to normal.
There is no predictive value of routine repeat CK testing for rhabdomyolysis since the level can increase for many reasons, including muscle injury or excess muscular exercise. However, CK must be assessed immediately in patients who present with muscle pain and weakness, and especially in the elderly, and treatment stopped if CK rises to >10 times the ULN. Strategies to handle CK elevations are given in Table 13.
Due to the increased frequency of DM during statin treatment,247,249,556,557 regular checks of HbA1c should be considered in patients at high-risk of developing DM and under high-dose statin treatment. Groups to be considered for glucose control are the elderly or those with MetS, obesity, or signs of insulin resistance.
Summary of recommendations for monitoring lipids and enzymes in patients, before and on lipid-lowering therapy
| Testing lipids |
How often should lipids be tested?
|
How often should a patient’s lipids be tested after starting lipid-lowering treatment?
|
How often should lipids be tested once a patient has achieved the target or optimal lipid level?
|
| Monitoring liver and muscle enzymes |
How often should liver enzymes (ALT) be routinely measured in patients on lipid-lowering drugs?
|
What if liver enzymes become elevated in a person taking lipid-lowering drugs? If ALT <3× ULN:
|
How often should CK be measured in patients taking lipid-lowering drugs?
|
| Be alert regarding myopathy and CK elevation in patients at risk, such as: elderly patients, those on concomitant interfering therapy, multiple medications, liver or renal disease, or athletes. |
What if CK becomes elevated in a person taking lipid-lowering drugs? Re-evaluate indication for statin treatment. If ≥4× ULN:
|
| Testing lipids |
How often should lipids be tested?
|
How often should a patient’s lipids be tested after starting lipid-lowering treatment?
|
How often should lipids be tested once a patient has achieved the target or optimal lipid level?
|
| Monitoring liver and muscle enzymes |
How often should liver enzymes (ALT) be routinely measured in patients on lipid-lowering drugs?
|
What if liver enzymes become elevated in a person taking lipid-lowering drugs? If ALT <3× ULN:
|
How often should CK be measured in patients taking lipid-lowering drugs?
|
| Be alert regarding myopathy and CK elevation in patients at risk, such as: elderly patients, those on concomitant interfering therapy, multiple medications, liver or renal disease, or athletes. |
What if CK becomes elevated in a person taking lipid-lowering drugs? Re-evaluate indication for statin treatment. If ≥4× ULN:
|
ACS = acute coronary syndrome; ALT = alanine aminotransferase; CK = creatine kinase; ULN = upper limit of normal.
Summary of recommendations for monitoring lipids and enzymes in patients, before and on lipid-lowering therapy
| Testing lipids |
How often should lipids be tested?
|
How often should a patient’s lipids be tested after starting lipid-lowering treatment?
|
How often should lipids be tested once a patient has achieved the target or optimal lipid level?
|
| Monitoring liver and muscle enzymes |
How often should liver enzymes (ALT) be routinely measured in patients on lipid-lowering drugs?
|
What if liver enzymes become elevated in a person taking lipid-lowering drugs? If ALT <3× ULN:
|
How often should CK be measured in patients taking lipid-lowering drugs?
|
| Be alert regarding myopathy and CK elevation in patients at risk, such as: elderly patients, those on concomitant interfering therapy, multiple medications, liver or renal disease, or athletes. |
What if CK becomes elevated in a person taking lipid-lowering drugs? Re-evaluate indication for statin treatment. If ≥4× ULN:
|
| Testing lipids |
How often should lipids be tested?
|
How often should a patient’s lipids be tested after starting lipid-lowering treatment?
|
How often should lipids be tested once a patient has achieved the target or optimal lipid level?
|
| Monitoring liver and muscle enzymes |
How often should liver enzymes (ALT) be routinely measured in patients on lipid-lowering drugs?
|
What if liver enzymes become elevated in a person taking lipid-lowering drugs? If ALT <3× ULN:
|
How often should CK be measured in patients taking lipid-lowering drugs?
|
| Be alert regarding myopathy and CK elevation in patients at risk, such as: elderly patients, those on concomitant interfering therapy, multiple medications, liver or renal disease, or athletes. |
What if CK becomes elevated in a person taking lipid-lowering drugs? Re-evaluate indication for statin treatment. If ≥4× ULN:
|
ACS = acute coronary syndrome; ALT = alanine aminotransferase; CK = creatine kinase; ULN = upper limit of normal.
12 Cost-effectiveness of cardiovascular disease prevention by lipid modification
In 2015, there were >85 million people in Europe living with CVD.558 Aging populations,559 unhealthy diets, smoking, sedentary lifestyles, increasing obesity, and diabetes560–563 are the main contributors. CVD cost the European Union about €210 billion in 2015, one-half of which was in healthcare costs (∼8% of total healthcare expenditure), and the other half in productivity losses and informal care.558
In these Guidelines, the Joint Task Force recommends a range of actions to reduce CVD by targeting plasma lipids, ranging from population-wide initiatives to promote healthy lifestyles to individual-level interventions to reduce CVD risk factors, such as unhealthy diets and high lipid levels. Cost-effectiveness analysis can help target resources for interventions where the net health gain is greatest in relation to the net resources, and is increasingly required across Europe.564 However, cost-effectiveness depends on available resources, the costs of services, and disease risk in the population, and results obtained in one country might not be valid in another.565 In addition, to fully capture the long-term effects of interventions, cost-effectiveness studies combining evidence from RCTs with modelling and limitations in both could affect the reliability of findings. Here, the evidence for the cost-effectiveness of ASCVD preventive interventions with respect to lipid modification is summarized; further scrutiny in view of local circumstances is recommended.
The health impact pyramid summarizes the evidence on the relative effort in relation to health impact (Figure 5), with interventions with the broadest impact on populations at the base and interventions requiring considerable individual effort at the top.566 There is consensus that all the levels of the pyramid should be targeted but that emphasis should be placed on the lower levels. This would address the persistent socio-economic divide in CV health despite major improvements in ASCVD treatment.558
More than one-half of the reduction in CV mortality over the last three decades has been attributed to population-level changes in CV risk factors, primarily reductions in plasma cholesterol, BP levels, and smoking.560–563,567 Lifestyle changes at the population level may be more cost-effective than lifestyle and drug interventions at the individual level, particularly when targeted to populations at increased risk. Awareness and knowledge of how lifestyle risk factors lead to CVD has increased in recent decades. Moreover, legislation promoting a healthy lifestyle, such as reduced salt intake and smoking bans, has been reported to be cost-effective in preventing CVD,568–573 and initiatives to improve infrastructure and promote physical activity have shown promise.574,575 A number of structural strategies at international, national, and regional levels combined can substantially reduce CVD morbidity and mortality.576,577 Individual-level interventions to improve diet,578,579 increase physical activity,580 and stop smoking581 could also be cost-effective.582 However, suboptimal adherence limits benefits,583,584 and interventions to improve adherence, such as electronic device reminders to reinforce favourable health behaviours, are increasingly being investigated.585
All statin regimens and ezetimibe are now generically available across Europe. There is strong evidence that lowering blood cholesterol levels using low-cost statins is widely cost-effective586–590 in many categories of patients. For secondary prevention of CVD, the evidence suggests that statin treatments are highly cost-effective,586,590,591 and adding low-cost ezetimibe to high-intensity statin therapy further reduces LDL-C and CVD risk cost-effectively.592 In primary ASCVD prevention, the evidence indicates that generic statin-based treatments are cost-effective for people at least down to 1% annual total CVD risk and could be cost-effective at even lower risk,589 with the highest tolerated statin dose likely the most cost-effective.591,593,594 Importantly, many patients on statin treatment fail to take their medications adequately and/or to reach their therapeutic goals,595 with clinical and economic consequences.596,597 Reinforcing measures aimed at improving adherence to treatment is cost-effective.598–600
Studies have shown that at mid-2018 prices PCSK9 inhibitors were largely not cost-effective.601–604 Their cost-effectiveness is improved in selected high-risk patients, such as those with clinical CVD or FH, other comorbidities, and high LDL-C levels.605,606 However, at lower prices, PCSK9 inhibitors would become cost-effective in a wider range of high-risk patients; recent price reductions may therefore lead to increased use.607 Cost-effectiveness evidence for other lipid-modifying therapies is lacking.
Effective interventions to prevent ASCVD, including statins, typically exhibit similar relative risk reductions across categories of patients, including by ASCVD risk; therefore, health benefits and cost-effectiveness are greater among people at higher ASCVD risk (Figure 6).36,233 Consequently, increased efforts and higher-intensity interventions should be aimed at individuals and populations at higher ASCVD risk.
Absolute reductions in major vascular events with statin therapy.233 LDL = low-density lipoprotein. Reproduced from The Lancet, 388/10059, Collins et al., ‘Interpretation of the evidence for the efficacy and safety of statin therapy’, 2532-2561, 2016, with permission from Elsevier.
Box 8 lists the key messages regarding the cost-effectiveness of CVD prevention by lipid modification, and Box 9 highlights gaps in the evidence.
Key messages
| Prevention of CVD by lifestyle changes, medication, or both is cost-effective in many scenarios, including population-based approaches and actions directed at individuals at increased CVD risk. |
| Cost-effectiveness depends on several factors, including baseline CVD risk and LDL levels, cost of treatment, and uptake of preventive strategies. |
| Interventions to prevent CVD are more cost-effective among individuals and populations at higher CVD risk. |
| Cost-effectiveness analyses are importantly informed by assumptions about long-term disease prognosis and treatment effects. Strengthening of the evidence to inform these assumptions is encouraged. |
| Prevention of CVD by lifestyle changes, medication, or both is cost-effective in many scenarios, including population-based approaches and actions directed at individuals at increased CVD risk. |
| Cost-effectiveness depends on several factors, including baseline CVD risk and LDL levels, cost of treatment, and uptake of preventive strategies. |
| Interventions to prevent CVD are more cost-effective among individuals and populations at higher CVD risk. |
| Cost-effectiveness analyses are importantly informed by assumptions about long-term disease prognosis and treatment effects. Strengthening of the evidence to inform these assumptions is encouraged. |
CVD = cardiovascular disease; LDL = low-density lipoprotein.
Key messages
| Prevention of CVD by lifestyle changes, medication, or both is cost-effective in many scenarios, including population-based approaches and actions directed at individuals at increased CVD risk. |
| Cost-effectiveness depends on several factors, including baseline CVD risk and LDL levels, cost of treatment, and uptake of preventive strategies. |
| Interventions to prevent CVD are more cost-effective among individuals and populations at higher CVD risk. |
| Cost-effectiveness analyses are importantly informed by assumptions about long-term disease prognosis and treatment effects. Strengthening of the evidence to inform these assumptions is encouraged. |
| Prevention of CVD by lifestyle changes, medication, or both is cost-effective in many scenarios, including population-based approaches and actions directed at individuals at increased CVD risk. |
| Cost-effectiveness depends on several factors, including baseline CVD risk and LDL levels, cost of treatment, and uptake of preventive strategies. |
| Interventions to prevent CVD are more cost-effective among individuals and populations at higher CVD risk. |
| Cost-effectiveness analyses are importantly informed by assumptions about long-term disease prognosis and treatment effects. Strengthening of the evidence to inform these assumptions is encouraged. |
CVD = cardiovascular disease; LDL = low-density lipoprotein.
Gaps in the evidence
| Cost-effectiveness requires evidence for effects of interventions on health and healthcare over a long time period; modelling techniques fill gaps. More data are needed from RCTs and observational studies. |
| Direct evidence of effects of lipid-modifying treatments on overall mortality, particularly among people at low-to-moderate CVD risk, older people, and for newer interventions, is lacking. Long-term post-trial follow-up in RCTs should be encouraged. |
| The cost-effectiveness of using lifetime CVD risk and more precise CVD risk scores to target interventions needs further investigation. |
| Cost-effectiveness requires evidence for effects of interventions on health and healthcare over a long time period; modelling techniques fill gaps. More data are needed from RCTs and observational studies. |
| Direct evidence of effects of lipid-modifying treatments on overall mortality, particularly among people at low-to-moderate CVD risk, older people, and for newer interventions, is lacking. Long-term post-trial follow-up in RCTs should be encouraged. |
| The cost-effectiveness of using lifetime CVD risk and more precise CVD risk scores to target interventions needs further investigation. |
CVD = cardiovascular disease; RCT = randomized controlled trial.
Gaps in the evidence
| Cost-effectiveness requires evidence for effects of interventions on health and healthcare over a long time period; modelling techniques fill gaps. More data are needed from RCTs and observational studies. |
| Direct evidence of effects of lipid-modifying treatments on overall mortality, particularly among people at low-to-moderate CVD risk, older people, and for newer interventions, is lacking. Long-term post-trial follow-up in RCTs should be encouraged. |
| The cost-effectiveness of using lifetime CVD risk and more precise CVD risk scores to target interventions needs further investigation. |
| Cost-effectiveness requires evidence for effects of interventions on health and healthcare over a long time period; modelling techniques fill gaps. More data are needed from RCTs and observational studies. |
| Direct evidence of effects of lipid-modifying treatments on overall mortality, particularly among people at low-to-moderate CVD risk, older people, and for newer interventions, is lacking. Long-term post-trial follow-up in RCTs should be encouraged. |
| The cost-effectiveness of using lifetime CVD risk and more precise CVD risk scores to target interventions needs further investigation. |
CVD = cardiovascular disease; RCT = randomized controlled trial.
13 Strategies to encourage adoption of healthy lifestyle changes and adherence to lipid-modifying therapies
Helping patients to change to healthier lifestyle habits is most effectively achieved through formal programmes of preventive care, possibly because of the intensive follow-up and multidisciplinary expertise they provide.608 However, in everyday care, adherence to both healthy lifestyle changes and medication regimens is a challenge to patients and professionals.
A comprehensive patient- and family-centred approach located in one healthcare setting is recommended rather than addressing single risk factors with more than one intervention in different locations. Box 10 includes some useful techniques when counselling patients for behavioural change.
A comprehensive approach to improving adherence to medication is described in the Supplementary Data document.
Methods for enhancing adherence to lifestyle changes
| 1. Explore motivation and identify ambivalence. Weigh pros and cons for change, assess and build self-efficacy and confidence, and avoid circular discussion. |
| 2. Offer support, and establish an alliance with the patient and his/her family. |
| 3. Involve the partner, other household members, or caregiver who may be influential in the lifestyle of the patient. |
| 4. Use the OARS method (Open-ended questions, Affirmation, Reflective listening, Summarising when discussing behaviour changes (www.smartrecovery.org/wp-content/uploads/2017/03/UsingMIinSR.pdf). |
| 5. Tailor advice to an individual patient’s culture, habits, and situation. |
| 6. Use SMART goal setting (negotiate goals for change that are Specific, Measurable, Achievable, Realistic, and Timely). Follow-up on goals and record progress on a shared record. |
| 1. Explore motivation and identify ambivalence. Weigh pros and cons for change, assess and build self-efficacy and confidence, and avoid circular discussion. |
| 2. Offer support, and establish an alliance with the patient and his/her family. |
| 3. Involve the partner, other household members, or caregiver who may be influential in the lifestyle of the patient. |
| 4. Use the OARS method (Open-ended questions, Affirmation, Reflective listening, Summarising when discussing behaviour changes (www.smartrecovery.org/wp-content/uploads/2017/03/UsingMIinSR.pdf). |
| 5. Tailor advice to an individual patient’s culture, habits, and situation. |
| 6. Use SMART goal setting (negotiate goals for change that are Specific, Measurable, Achievable, Realistic, and Timely). Follow-up on goals and record progress on a shared record. |
Methods for enhancing adherence to lifestyle changes
| 1. Explore motivation and identify ambivalence. Weigh pros and cons for change, assess and build self-efficacy and confidence, and avoid circular discussion. |
| 2. Offer support, and establish an alliance with the patient and his/her family. |
| 3. Involve the partner, other household members, or caregiver who may be influential in the lifestyle of the patient. |
| 4. Use the OARS method (Open-ended questions, Affirmation, Reflective listening, Summarising when discussing behaviour changes (www.smartrecovery.org/wp-content/uploads/2017/03/UsingMIinSR.pdf). |
| 5. Tailor advice to an individual patient’s culture, habits, and situation. |
| 6. Use SMART goal setting (negotiate goals for change that are Specific, Measurable, Achievable, Realistic, and Timely). Follow-up on goals and record progress on a shared record. |
| 1. Explore motivation and identify ambivalence. Weigh pros and cons for change, assess and build self-efficacy and confidence, and avoid circular discussion. |
| 2. Offer support, and establish an alliance with the patient and his/her family. |
| 3. Involve the partner, other household members, or caregiver who may be influential in the lifestyle of the patient. |
| 4. Use the OARS method (Open-ended questions, Affirmation, Reflective listening, Summarising when discussing behaviour changes (www.smartrecovery.org/wp-content/uploads/2017/03/UsingMIinSR.pdf). |
| 5. Tailor advice to an individual patient’s culture, habits, and situation. |
| 6. Use SMART goal setting (negotiate goals for change that are Specific, Measurable, Achievable, Realistic, and Timely). Follow-up on goals and record progress on a shared record. |
14 Key messages
Cholesterol and risk. Prospective studies, randomized trials, and Mendelian randomization studies have all shown that raised LDL-C is a cause of ASCVD. Throughout the range of LDL-C levels, ‘lower is better’ with no lower threshold, at least down to ∼1 mmol/L. Lowering LDL-C may yield worthwhile benefits in patients with average or below average LDL-C who are already receiving LDL-C-lowering treatment. The proportional reduction in ASCVD risk achieved by lowering LDL-C (e.g. with a statin, ezetimibe, or PCSK9-inhibitor) depends on the absolute reduction in LDL-C, with each 1 mmol/L reduction corresponding to a reduction of about one-fifth in ASCVD.
PCSK-9 inhibitors. Large trials have shown that PCSK9 inhibitors further reduce ASCVD risk when given on top of statin-based therapy and their use may need to be restricted to those at the highest risk for ASCVD.
Use of cardiac imaging for risk stratification. CAC score assessment with CT may be helpful in reaching decisions about treatment in people who are at moderate risk of ASCVD. Obtaining such a score may assist in discussions about treatment strategies in patients where the LDL-C goal is not achieved with lifestyle intervention alone and there is a question of whether to institute LDL-C-lowering treatment. Assessment of arterial (carotid or femoral) plaque burden on ultrasonography may also be informative in these circumstances.
Use of ApoB in risk stratification. ApoB may be a better measure of an individual’s exposure to pro atherogenic lipoproteins, and hence its use may be particularly helpful for risk assessment in people where measurement of LDL-C underestimates this burden, such as those with high TG, DM, obesity, or very low LDL-C.
Use of Lp(a) in risk stratification. A one-off measurement of Lp(a) may help to identify people with very high inherited Lp(a) levels who may have a substantial lifetime risk of ASCVD. A high Lp(a) plasma level may also be helpful in further risk stratification of patients at high risk of ASCVD, in patients with a family history of premature CVD, and to determine treatment strategies in people whose estimated risk is on the border of risk categories.
Intensification of treatment goals. It is important to ensure that treatment of the highest-risk patients achieves the largest LDL-C reduction possible. These Guidelines aim to support this by setting both a minimum percentage LDL-C reduction (50%) and an absolute LDL-C treatment goal of <1.4 mmol/L (<55 mg/dL) for very-high-risk patients, and <1.8 mmol/L (<70 mg/dL) for high-risk patients. It is recommended that FH patients with ASCVD or who have another major risk factor are treated as very-high-risk, and those with no prior ASCVD or other risk factors as high-risk.
Treatment of patients with recent ACS. New randomized trials support a strategy of intensification of LDL-C-lowering therapy in very-high-risk patients with ACS (MI or unstable angina). If the specified LDL-C treatment goal is not achieved after 4–6 weeks with the highest tolerated statin dose and ezetimibe, it is appropriate to add a PCSK9 inhibitor.
Safety of low LDL cholesterol concentrations. To date there are no known adverse effects of very low LDL-C concentrations [e.g. <1 mmol/L (40 mg/dL)].
Management of statin ‘intolerance’. While statins rarely cause serious muscle damage (myopathy, or rhabdomyolysis in the most severe cases), there is much public concern that statins may commonly cause less serious muscle symptoms. Such statin ‘intolerance’ is frequently encountered by practitioners and may be difficult to manage. However, placebo-controlled randomized trials have shown very clearly that true statin intolerance is rare, and that it is generally possible to institute some form of statin therapy (e.g. by changing the statin or reducing the dose) in the overwhelming majority of patients at risk of ASCVD.
Statin treatment for older people. A meta-analysis of randomized trials has shown that the effects of statin therapy are determined by the absolute reduction in LDL-C as well as the baseline ASCVD risk, and are independent of all known risk factors, including age. Statin therapy in older people should therefore be considered according to the estimated level of risk and baseline LDL-C, albeit with due regard to an individual’s underlying health status and the risk of drug interactions. There is less certainty about the effects of statins in individuals aged >75 years, particularly in primary prevention. Statin therapy should be started at a low dose if there is significant renal impairment and/or the potential for drug interactions, and then titrated upwards to achieve LDL-C treatment goals.
15 Gaps in the evidence
|
|
|
|
16 ‘What to do’ and ‘what not to do’ messages from the Guidelines
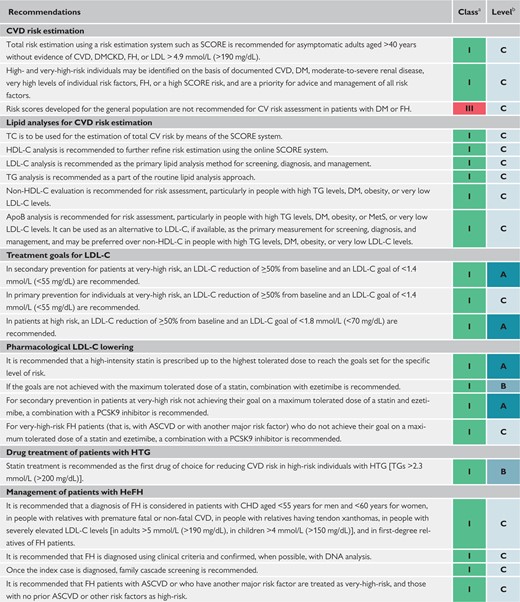 |
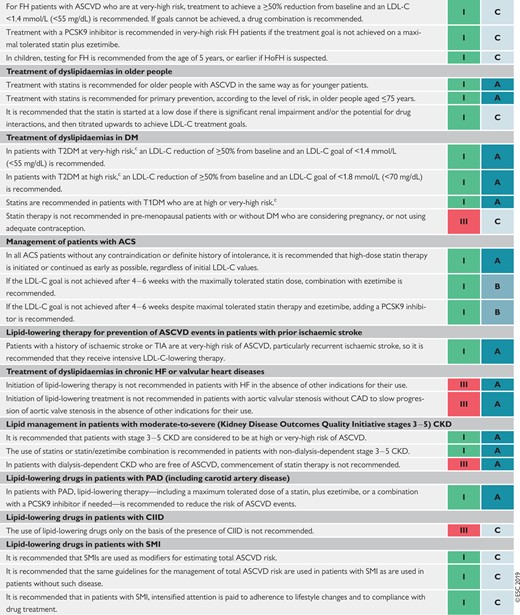 |
 |
 |
ACS = acute coronary syndrome(s); Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CAD = coronary artery disease; CHD = coronary heart disease; CIID = chronic immune-mediated inflammatory diseases; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; HDL-C = high-density lipoprotein cholesterol; HeFH = heterozygous FH; HF = heart failure; HoFH = homozygous FH; HTG = hypertriglyceridaemia; LDL-C = low-density lipoprotein cholesterol; MetS = metabolic syndrome; PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9; SCORE = Systematic Coronary Risk Estimation; SMI = severe mental illness; TC = total cholesterol; TG = triglycerides; TIA = transient ischaemic event; T1DM = type 1 DM; T2DM = type 2 DM.
Class of recommendation.
Level of evidence.
 |
 |
 |
 |
ACS = acute coronary syndrome(s); Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CAD = coronary artery disease; CHD = coronary heart disease; CIID = chronic immune-mediated inflammatory diseases; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; FH = familial hypercholesterolaemia; HDL-C = high-density lipoprotein cholesterol; HeFH = heterozygous FH; HF = heart failure; HoFH = homozygous FH; HTG = hypertriglyceridaemia; LDL-C = low-density lipoprotein cholesterol; MetS = metabolic syndrome; PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9; SCORE = Systematic Coronary Risk Estimation; SMI = severe mental illness; TC = total cholesterol; TG = triglycerides; TIA = transient ischaemic event; T1DM = type 1 DM; T2DM = type 2 DM.
Class of recommendation.
Level of evidence.
17 Supplementary data
Supplementary Data with additional Supplementary Tables, Boxes, and text complementing the full text—as well as sections on other features of a healthy diet contributing to cardiovascular disease prevention, chronic immune-mediated inflammatory diseases, HIV patients, severe mental illness, and adhering to medications along with the related references—are available on the European Heart Journal website and via the ESC website at www.escardio.org/guidelines.
18 Appendix
Author/Task Force Member Affiliations:
Konstantinos C. Koskinas, Cardiology, Bern University Hospital (Inselspital), Bern, Switzerland; Manuela Casula, Epidemiology and Preventive Pharmacology Service (SEFAP), Department of Pharmacological and Biomolecular Sciences (DiSFeB), University of Milan, Milan, Italy and IRCCS MultiMedica, Sesto S. Giovanni (Milan), Italy; Lina Badimon, Cardiovascular Program-ICCC and CiberCV, IR-Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; M. John Chapman, National Institute for Health and Medical Research (INSERM), Paris, France; Sorbonne University, Paris, France; and Division of Endocrinology- Metabolism, Pitie-Salpetriere University Hospital, Paris, France; Guy G. De Backer, Public Health and Primary Care, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium; Victoria Delgado, Cardiology, Leiden University Medical Center, Leiden, Netherlands; Brian A. Ference, Centre for Naturally Randomized Trials, Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom; Ian M. Graham, Cardiology, Trinity College, Dublin, Ireland; Alison Halliday, Nuffield Department of Surgery, University of Oxford, Oxford, United Kingdom and NIHR Oxford Biomedical Research Centre, Oxford, United Kingdom; Ulf Landmesser, Department of Cardiology, Charite Universitätsmedizin Berlin, Berlin, Germany; Berlin Institute of Health (BIH), Berlin, Germany; and German Center of Cardiovascular Research (DZHK), Berlin, Germany; Borislava Mihaylova, Nuffield Department of Population Health and NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, United Kingdom and Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom; Terje R. Pedersen, Preventive Cardiology, Oslo University Hospital, Aker, Oslo, Norway; Gabriele Riccardi, Clinical Medicine and Surgery, Federico II University, Naples, Italy; Dimitrios J. Richter, Cardiac Department, Euroclinic, Athens, Greece; Marc S. Sabatine, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States of America; Marja-Riitta Taskinen, Research Program for Clinical and Molecular Metabolism, University of Helsinki, Helsinki, Finland; Lale Tokgozoglu, Cardiology, Hacettepe University, Ankara, Turkey; Olov Wiklund, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden.
ESC Committee for Practice Guidelines (CPG): Stephan Windecker (Chairperson) (Switzerland), Victor Aboyans (France), Colin Baigent (United Kingdom), Jean-Philippe Collet (France), Veronica Dean (France), Victoria Delgado (Netherlands), Donna Fitzsimons (United Kingdom), Chris P. Gale (United Kingdom), Diederick E. Grobbee (Netherlands), Sigrun Halvorsen (Norway), Gerhard Hindricks (Germany), Bernard Iung (France), Peter Jüni (Canada), Hugo A. Katus (Germany), Ulf Landmesser (Germany), Christophe Leclercq (France), Maddalena Lettino (Italy), Basil S. Lewis (Israel), Bela Merkely (Hungary), Christian Mueller (Switzerland), Steffen Petersen (United Kingdom), Anna Sonia Petronio (Italy), Dimitrios J. Richter (Greece), Marco Roffi (Switzerland), Evgeny Shlyakhto (Russian Federation), Iain A. Simpson (United Kingdom), Miguel Sousa-Uva (Portugal), Rhian M. Touyz (United Kingdom).
ESC National Cardiac Societies actively involved in the review process of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk.
Algeria: Algerian Society of Cardiology, Djamaleddine Nibouche; Armenia: Armenian Cardiologists Association, Parounak H. Zelveian; Austria: Austrian Society of Cardiology, Peter Siostrzonek; Azerbaijan: Azerbaijan Society of Cardiology, Ruslan Najafov; Belgium: Belgian Society of Cardiology, Philippe van de Borne; Bosnia and Herzegovina: Association of Cardiologists of Bosnia and Herzegovina, Belma Pojskic; Bulgaria: Bulgarian Society of Cardiology, Arman Postadzhiyan; Cyprus: Cyprus Society of Cardiology, Lambros Kypris; Czech Republic: Czech Society of Cardiology, Jindřich Špinar; Denmark: Danish Society of Cardiology, Mogens Lytken Larsen; Egypt: Egyptian Society of Cardiology, Hesham Salah Eldin; Estonia: Estonian Society of Cardiology, Margus Viigimaa; Finland: Finnish Cardiac Society, Timo E. Strandberg; France: French Society of Cardiology, Jean Ferrières; Georgia: Georgian Society of Cardiology, Rusudan Agladze; Germany: German Cardiac Society, Ulrich Laufs; Greece: Hellenic Society of Cardiology, Loukianos Rallidis; Hungary: Hungarian Society of Cardiology, László Bajnok; Iceland: Icelandic Society of Cardiology, Thorbjörn Gudjónsson; Ireland: Irish Cardiac Society, Vincent Maher; Israel: Israel Heart Society, Yaakov Henkin; Italy: Italian Federation of Cardiology, Michele Massimo Gulizia; Kazakhstan: Association of Cardiologists of Kazakhstan, Aisulu Mussagaliyeva; Kosovo (Republic of): Kosovo Society of Cardiology, Gani Bajraktari; Kyrgyzstan: Kyrgyz Society of Cardiology, Alina Kerimkulova; Latvia: Latvian Society of Cardiology, Gustavs Latkovskis; Lebanon: Lebanese Society of Cardiology, Omar Hamoui; Lithuania: Lithuanian Society of Cardiology, Rimvydas Slapikas; Luxembourg: Luxembourg Society of Cardiology, Laurent Visser; Malta: Maltese Cardiac Society, Philip Dingli; Moldova (Republic of): Moldavian Society of Cardiology, Victoria Ivanov; Montenegro: Montenegro Society of Cardiology, Aneta Boskovic; Morocco: Moroccan Society of Cardiology, Mbarek Nazzi; Netherlands: Netherlands Society of Cardiology, Frank Visseren; North Macedonia: North Macedonian Society of Cardiology, Irena Mitevska; Norway: Norwegian Society of Cardiology, Kjetil Retterstøl; Poland: Polish Cardiac Society, Piotr Jankowski; Portugal: Portuguese Society of Cardiology, Ricardo Fontes-Carvalho; Romania: Romanian Society of Cardiology, Dan Gaita; Russian Federation: Russian Society of Cardiology, Marat Ezhov; San Marino: San Marino Society of Cardiology, Marina Foscoli; Serbia: Cardiology Society of Serbia, Vojislav Giga; Slovakia: Slovak Society of Cardiology, Daniel Pella; Slovenia: Slovenian Society of Cardiology, Zlatko Fras; Spain: Spanish Society of Cardiology, Leopoldo Perez de Isla; Sweden: Swedish Society of Cardiology, Emil Hagström; Switzerland: Swiss Society of Cardiology, Roger Lehmann; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Leila Abid; Turkey: Turkish Society of Cardiology, Oner Ozdogan; Ukraine: Ukrainian Association of Cardiology, Olena Mitchenko; United Kingdom of Great Britain and Northern Ireland: British Cardiovascular Society, Riyaz S. Patel.
The disclosure forms of all experts involved in the development of these Guidelines are available on the ESC website www.escardio.org/guidelines
ESC Committee for Practice Guidelines (CPG), National Cardiac Societies document reviewers and Author/Task Force Member affiliations: listed in the Appendix.
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing & Allied Professions (ACNAP), European Association of Cardiovascular Imaging (EACVI), European Association of Preventive Cardiology (EAPC), European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Councils: Council for Cardiology Practice, Council on Hypertension, Council on Stroke.
Working Groups: Aorta and Peripheral Vascular Diseases, Atherosclerosis and Vascular Biology, Cardiovascular Pharmacotherapy, e-Cardiology, Thrombosis.
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC (journals.permissions@oxfordjournals.org).
Disclaimer. The ESC/EAS Guidelines represent the views of the ESC and EAS, and were produced after careful consideration of the scientific and medical knowledge, and the evidence available at the time of their publication. The ESC and EAS is not responsible in the event of any contradiction, discrepancy, and/or ambiguity between the ESC/EAS Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC/EAS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic, or therapeutic medical strategies; however, the ESC/EAS Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. Nor do the ESC/EAS Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
19 References
World Health Organization.
Cholesterol Treatment Trialists Collaboration,
Cholesterol Treatment Trialists Collaboration,
Cholesterol Treatment Trialists Collaboration,
Emerging Risk Factors Collaboration,
Triglyceride Coronary Disease Genetics Consortium, Emerging Risk Factors Collaboration,
Prospective Studies Collaboration,
HPS/TIMI/REVEAL Collaborative Group,
Aim-High Investigators,
Emerging Risk Factors Collaboration,
National Clinical Guideline Centre (UK).
Joint British Societies Board.
European Association for Cardiovascular Prevention & Rehabilitation,
Look Ahead Research Group,
Global Burden of Disease 2016 Alcohol Collaborators.
Cholesterol Treatment Trialists Collaboration,
Cholesterol Treatment Trialists C.
Cholesterol Treatment Trialists Collaboration,
Sharp Collaborative Group.
Myocardial Infarction Genetics Consortium Investigators,
The Lipid Research Clinics Program.
The Lipid Research Clinics Program.
The Lipid Research Clinics Program.
Accord Study Group,
Lipids and lipoproteins in symptomatic coronary heart disease. Distribution, intercorrelations, and significance for risk classification in 6,700 men and 1,500 women.
ASCEND Study Collaborative Group,
Global Lipids Genetics Consortium,
Risk of fatal coronary heart disease in familial hypercholesterolaemia.
World Health Organization. Human Genetics Programme. Familial hypercholesterolemia: Report of a second WHO consultation. WHO/HGN/FH/Cons/99.2. Geneva: World Health Organization;
Second Joint Task Force of European and other Societies.
Emerging Risk Factors Collaboration,
Cholesterol Treatment Trialists Collaboration,
American Diabetes Association.
ASCEND Study Collaborative Group,
The Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group.
Chronic Kidney Disease Prognosis Consortium
European Stroke Organisation
Accord Study Group, Accord Eye Study Group,
Emerging Risk Factors Collaboration,
Atlas Writing Group
Heart Protection Study C,
Author notes
François Mach, Colin Baigent and Alberico L. Catapano chairpersons contributed equally to the document.
Alberico L. Catapano, Manuela Casula, Gabriele Riccardi, Marja-Riitta Taskinen, Lale Tokgozoglu, Olov Wiklund, Alberto Corsini, Meral Kayikcioglu, Philippe Moulin, Xavier Pintó, Kausik K. Ray, Željko Reiner, Erik Stroes, Alexandros D. Tselepis, Margus Viigimaa and Michal Vrablik: representing the EAS.