-
PDF
- Split View
-
Views
-
Cite
Cite
Holger Thiele, Alexander Jobs, Dagmar M Ouweneel, Jose P S Henriques, Melchior Seyfarth, Steffen Desch, Ingo Eitel, Janine Pöss, Georg Fuernau, Suzanne de Waha, Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials, European Heart Journal, Volume 38, Issue 47, 14 December 2017, Pages 3523–3531, https://doi.org/10.1093/eurheartj/ehx363
Close - Share Icon Share
Abstract
Evidence on the impact on clinical outcome of active mechanical circulatory support (MCS) devices in cardiogenic shock (CS) is scarce. This collaborative meta-analysis of randomized trials thus aims to investigate the efficacy and safety of percutanzeous active MCS vs. control in CS.
Randomized trials comparing percutaneous active MCS to control in patients with CS were identified through searches of medical literature databases. Risk ratios (RR) and 95% confidence intervals (95% CI) were calculated to analyse the primary endpoint of 30-day mortality and device-related complications including bleeding and leg ischaemia. Mean differences (MD) were calculated for mean arterial pressure (MAP), cardiac index (CI), pulmonary capillary wedge pressure (PCWP), and arterial lactate. Four trials randomizing 148 patients to either TandemHeart™ or Impella® MCS (n = 77) vs. control (n = 71) were identified. In all four trials intra-aortic balloon pumping (IABP) served as control. There was no difference in 30-day mortality (RR 1.01, 95% CI 0.70 to 1.44, P = 0.98, I 2 = 0%) for active MCS compared with control. Active MCS significantly increased MAP (MD 11.85 mmHg, 95% CI 3.39 to 20.31, P = 0.02, I 2 = 32.7%) and decreased arterial lactate (MD − 1.36 mmol/L, 95% CI − 2.52 to − 0.19, I 2 = 0%, P = 0.02) at comparable CI (MD 0.32, 95% CI − 0.24 to 0.87, P = 0.14, I 2 = 44.1%) and PCWP (MD − 5.59, 95% −15.59 to 4.40, P = 0.14, I 2 = 81.1%). No significant difference was observed in the incidence of leg ischaemia (RR 2.64, 95% CI 0.83 to 8.39, P = 0.10, I 2 = 0%), whereas the rate of bleeding was significantly increased in MCS compared to IABP (RR 2.50, 95% CI 1.55 to 4.04, P < 0.001, I 2 = 0%).
Results of this collaborative meta-analysis do not support the unselected use of active MCS in patients with CS complicating AMI.
Introduction
Cardiogenic shock (CS) is defined as a state of critical end-organ hypoperfusion due to reduced cardiac output. Advances in treatment led to mortality reduction over the last decades, mainly driven by early revascularization in patients with infarct-related CS. Nevertheless, CS mortality rates are still approaching 40–50% according to recent registries and randomized trials.1–4
The use of active mechanical circulatory support (MCS) appears to be a promising therapeutic concept to improve cardiac output while avoiding the possible cardiotoxicity of catecholamines. Passive intra-aortic balloon pumping (IABP) has been the most widely used MCS device for the last decades.5 Based on neutral results of the IABP-SHOCK II trial,3 , 6 European guidelines downgraded routine IABP use in CS to a class III B recommendation.7–9 The lack of efficacy of IABP led to an increased use of more potent active MCS devices.5 , 10 Among the currently available percutaneous devices left atrial-to-femoral artery MCS such as the TandemHeart™ (TandemHeart, Cardiac Assist, Pittsburgh, PA, USA), axial flow MCS from the Impella® family (Impella 2.5 and Impella CP, Abiomed Europe, Aachen, Germany) and extracorporeal membrane oxygenation (ECMO) are predominantly used for short-term support.4 , 10
Several controlled trials comparing the efficacy and safety of active percutaneous MCS vs. control in CS complicating acute myocardial infarction (AMI) have been performed.11–14 The individual trials were underpowered to adequately evaluate a potential mortality benefit. Consequently, clinical evidence on the impact of MCS use on outcome is scarce.15
We thus performed a collaborative meta-analysis to investigate the effects of MCS vs. control with respect to mortality, haemodynamic variables as well as major device-related complications.
Methods
Studies eligible for inclusion had to compare active percutaneous MCS vs. control (defined as either no support or IABP) in patients with CS predominantly complicated by AMI reporting at least short-term all-cause mortality assessed at 30 days. Medical literature databases including Pubmed/Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE as well as abstracts and presentations from major cardiovascular meetings were searched using the following keywords ‘ventricular assist device’ OR ‘intra-aortic balloon pump’ OR ‘intra-aortic balloon pumping’ OR ‘VAD’ OR ‘LVAD’ OR ‘IABP’ AND ‘cardiogenic shock’. Reference lists from review articles and eligible studies were further checked to identify additional citations. The reference lists of retrieved publications as well as clinical trials registration websites were scrutinized to identify additional trials as well as ongoing studies. The search was last updated on 17 January 2017. No language, publication date, or publication status restrictions were imposed. The most updated and inclusive data for each study were chosen. Two investigators (H.T. and A.J.) independently reviewed the titles, abstracts and studies to determine whether they met the inclusion criteria. Conflict between reviewers was resolved by consensus. Internal validity of randomized controlled trials was assessed by evaluating concealment of allocation, blind adjudication of events, and inclusion of all randomized patients in the analysis. Owing to the nature of the compared interventions, blinding of patients or physicians was not feasible. Risk of bias was assessed according the Cochrane Collaboration’s tool independently by two investigators (H.T. and A.J.).16 The present meta-analysis was performed according to PRISMA statement (PMID: 19622552, PRISMA checklist see Supplementary material online, Table S1).
Data acquisition, endpoints, and definitions
Patient and outcome data were independently extracted by two investigators (H.T. and A.J.) from the original publications. Furthermore, the corresponding authors were contacted to provide additional data if necessary. Except for one trial where only the individual mortality data were available and the original database was not retrievable anymore12 all other trials could confirm data from the original database. The primary endpoint of the present meta-analysis was all-cause short-term mortality assessed at 30 days after randomization for the intention-to-treat population. Secondary endpoints were haemodynamic parameters including mean arterial pressure (MAP), pulmonary capillary wedge pressure (PCWP), and cardiac index (CI) as well as arterial lactate pre vs. post (within 2 h) MCS implantation. In addition, typical device associated complications such as bleeding and leg ischaemia were analysed. The endpoint definitions as applied in each trial were used (Supplement Table 4). Evaluation of publication bias was not conducted due to the small number of the studies included.
Statistical analysis
Baseline characteristics including demographics, medical history, haemodynamic parameters, and angiography parameters were tabulated by treatment group for each study. Continuous variables were summarized as mean and standard deviation (SD). Frequencies and percentages were used to summarize categorical variables. Random effects meta-analyses of clinical outcomes were performed by calculating risk ratios (RR) with 95% confidence intervals (95% CI) for MCS vs. control of each individual study and consecutive pooling by means of the Mantel–Haenszel method. Mean differences (MD) with 95% CI were calculated for random effects meta-analyses of continuous outcomes (i.e. haemodynamic parameters and lactate) and pooled using the inverse variance method. Between-study variances τ 2 were calculated according DerSimonian and Laird. Cochran’s Q statistic and Higgins and Thompsons I 2 were calculated to assess heterogeneity.2 In case of heterogeneity (i.e. I 2 > 0%) Hartung-Knapp adjustment to our random effects meta-analyses was applied. A P-value <0.05 and <0.10 were considered statistically significant for clinical outcomes and heterogeneity, respectively. Clinical outcome measures are presented by means of forest plots. In addition, 30-day cumulative mortality rate was estimated with the Kaplan–Meier method based on individual patient data. All analyses were performed with R software version 3.1.0 (The R Project for Statistical Computing, Vienna, Austria) and its meta package version 4.7-0 (cran.r-project.org/web/packages/meta/).
Results
In total four randomized trials comparing active percutaneous MCS published between 2005 and 2016 were identified and included in the collaborative meta-analysis (Figure 1).11–14 All four trials randomly assigned patients to treatment with percutaneous active MCS vs. IABP serving as control. Two trials used the TandemHeart™ device 11 , 12 and two trials used the Impella® device (Impella 2.5 in 1 trial and Impella CP in the other trial).13 , 14 All trials reported adequate sequence generation and methods for allocation concealment (Table 1). The Cochrane Risk of Bias tool is displayed in Supplementary material online, Table S2. Complete 30-day follow-up was available in all trials.
Characteristics of the individual randomized trials in cardiogenic shock
| . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . |
|---|---|---|---|---|
| Number of patients | 41 | 33 | 26 | 48 |
| MCS | TandemHeart™ | TandemHeart™ | Impella® 2.5 | Impella® CP |
| Control | IABP | IABP | IABP | IABP |
| Setting | Single centre | Multicentre | Multicentre | Multicentre |
| Inclusion period | 2000–2003 | 2002–2004 | 2004–2007 | 2012–2015 |
| Sequence generation | Drawing envelopes | Drawing envelopes | Drawing envelopes | Internet-based program |
| Allocation concealment | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Internet-based program |
| Blinding | Not possible | Not possible | Not possible | Not possible |
| Primary endpoint | Cardiac power index | Haemodynamic profile improvementa | Cardiac index | 30-day mortality |
| Haemodynamic measurements | Pulmonary artery catheter | Pulmonary artery catheter | Pulmonary artery catheter | Arterial line |
| Follow-up 30 days | Complete | Complete | Complete | Complete |
| . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . |
|---|---|---|---|---|
| Number of patients | 41 | 33 | 26 | 48 |
| MCS | TandemHeart™ | TandemHeart™ | Impella® 2.5 | Impella® CP |
| Control | IABP | IABP | IABP | IABP |
| Setting | Single centre | Multicentre | Multicentre | Multicentre |
| Inclusion period | 2000–2003 | 2002–2004 | 2004–2007 | 2012–2015 |
| Sequence generation | Drawing envelopes | Drawing envelopes | Drawing envelopes | Internet-based program |
| Allocation concealment | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Internet-based program |
| Blinding | Not possible | Not possible | Not possible | Not possible |
| Primary endpoint | Cardiac power index | Haemodynamic profile improvementa | Cardiac index | 30-day mortality |
| Haemodynamic measurements | Pulmonary artery catheter | Pulmonary artery catheter | Pulmonary artery catheter | Arterial line |
| Follow-up 30 days | Complete | Complete | Complete | Complete |
IABP, intra-aortic balloon pumping; MCS, mechanical circulatory support.
All the following four criteria needed to be met: (1) patient did not die during support or within 24 h of device removal, (2) cardiac index ≥2.2 L/min/m2, (3) pulmonary capillary wedge pressure ≤24 mmHg, and (4) mean arterial pressure ≥70 mmHg.
Characteristics of the individual randomized trials in cardiogenic shock
| . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . |
|---|---|---|---|---|
| Number of patients | 41 | 33 | 26 | 48 |
| MCS | TandemHeart™ | TandemHeart™ | Impella® 2.5 | Impella® CP |
| Control | IABP | IABP | IABP | IABP |
| Setting | Single centre | Multicentre | Multicentre | Multicentre |
| Inclusion period | 2000–2003 | 2002–2004 | 2004–2007 | 2012–2015 |
| Sequence generation | Drawing envelopes | Drawing envelopes | Drawing envelopes | Internet-based program |
| Allocation concealment | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Internet-based program |
| Blinding | Not possible | Not possible | Not possible | Not possible |
| Primary endpoint | Cardiac power index | Haemodynamic profile improvementa | Cardiac index | 30-day mortality |
| Haemodynamic measurements | Pulmonary artery catheter | Pulmonary artery catheter | Pulmonary artery catheter | Arterial line |
| Follow-up 30 days | Complete | Complete | Complete | Complete |
| . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . |
|---|---|---|---|---|
| Number of patients | 41 | 33 | 26 | 48 |
| MCS | TandemHeart™ | TandemHeart™ | Impella® 2.5 | Impella® CP |
| Control | IABP | IABP | IABP | IABP |
| Setting | Single centre | Multicentre | Multicentre | Multicentre |
| Inclusion period | 2000–2003 | 2002–2004 | 2004–2007 | 2012–2015 |
| Sequence generation | Drawing envelopes | Drawing envelopes | Drawing envelopes | Internet-based program |
| Allocation concealment | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Sealed opaque sequentially numbered envelopes | Internet-based program |
| Blinding | Not possible | Not possible | Not possible | Not possible |
| Primary endpoint | Cardiac power index | Haemodynamic profile improvementa | Cardiac index | 30-day mortality |
| Haemodynamic measurements | Pulmonary artery catheter | Pulmonary artery catheter | Pulmonary artery catheter | Arterial line |
| Follow-up 30 days | Complete | Complete | Complete | Complete |
IABP, intra-aortic balloon pumping; MCS, mechanical circulatory support.
All the following four criteria needed to be met: (1) patient did not die during support or within 24 h of device removal, (2) cardiac index ≥2.2 L/min/m2, (3) pulmonary capillary wedge pressure ≤24 mmHg, and (4) mean arterial pressure ≥70 mmHg.
Flow diagram of the study selection process. IABP, intra-aortic balloon pumping; MCS, active mechanical support device.
In- and exclusion criteria as well as study-specific definitions of e.g. CS did not differ significantly between the individual studies (see Supplementary material online, Tables S3 and S4). Characteristics of each study are depicted in Table 1. Three trials were multicentre studies and one trial was performed at a single centre. Altogether 148 patients were included with 77 (52%) randomized to active MCS and 71 (48%) to IABP.
Baseline characteristics of individual studies did not show major discrepancies (Table 2).
Baseline characteristics according to treatment strategy in individual studies
| Variable . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | MCS (n = 21) . | IABP (n = 20) . | MCS (n = 19) . | IABP (n = 14) . | MCS (n = 13) . | IABP (n = 13) . | MCS (n = 24) . | IABP (n = 24) . |
| Age (years) | 63 ± 10 | 66 ± 10 | 66 ± 14 | 60 ± 11 | 65 ± 10 | 67 ± 19 | 58 ± 9 | 59 ± 11 |
| Male gender, n (%) | 16 (76) | 15 (75) | 14 (74) | 9 (64) | 8 (62) | 11 (85) | 18 (75) | 20 (83) |
| Cardiovascular risk factors | ||||||||
| Arterial hypertension, n (%) | 19 (91) | 15 (75) | n.a. | n.a. | 7 (54) | 9 (69) | 4 (20) | 6 (29) |
| Hyperlipidaemia, n (%) | 11 (52) | 9 (45) | n.a. | n.a. | 8 (62) | 7 (54) | 4 (20) | 5 (24) |
| Diabetes mellitus, n (%) | 11 (52) | 11 (55) | n.a. | n.a. | 5 (39) | 3 (23) | 2 (9) | 3 (13) |
| Current smoking, n (%) | 9 (43) | 6 (30) | n.a. | n.a. | 8 (62) | 7 (54) | 11 (61) | 6 (32) |
| Baseline and catheterization laboratory parameters | ||||||||
| Multivessel CAD, n (%) | 13 (62) | 14 (70) | n.a. | n.a. | 9 (69) | 10 (77) | 15 (63) | 21 (88) |
| TIMI-flow 2 or 3 post-PCI, n (%) | 17 (81) | 19 (95) | n.a. | n.a. | 12 (92) | 12 (92) | 23 (96) | 24 (100) |
| Device insertion pre-PCI, n (%) | 9 (43) | 9 (45) | n.a. | n.a. | 0 | 0 | 5 (21) | 3 (13) |
| Acute myocardial infarction, n (%) | 21 (100) | 20 (100) | 11 (58) | 10 (71) | 13 (100) | 13 (100) | 24 (100) | 24 (100) |
| Anterior myocardial infarction, n (%) | 18 (86) | 13 (65) | n.a. | n.a. | 7 (54) | 8 (62) | 16 (67) | 15 (63) |
| Catecholamines at baseline, n (%) | 21 (100) | 20 (100) | 19 (100) | 14 (100) | 11 (84) | 12 (92) | 24 (100) | 24 (100) |
| Mechanical ventilation, n (%) | 20 (95) | 20 (100) | n.a. | n.a. | 12 (92) | 12 (92) | 24 (100) | 24 (100) |
| CPR, VT, or VF pre randomization, n (%) | 11 (55) | 11 (52) | n.a. | n.a. | 11 (85) | 9 (69) | 24 (100) | 20 (83) |
| Left ventricular ejection fraction (%) | 26 ± 9 | 27 ± 7 | 19 ± 14 | 22 ± 9 | 28 ± 14 | 31 ± 16 | 30 ± 16 | 28 ± 16 |
| Variable . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | MCS (n = 21) . | IABP (n = 20) . | MCS (n = 19) . | IABP (n = 14) . | MCS (n = 13) . | IABP (n = 13) . | MCS (n = 24) . | IABP (n = 24) . |
| Age (years) | 63 ± 10 | 66 ± 10 | 66 ± 14 | 60 ± 11 | 65 ± 10 | 67 ± 19 | 58 ± 9 | 59 ± 11 |
| Male gender, n (%) | 16 (76) | 15 (75) | 14 (74) | 9 (64) | 8 (62) | 11 (85) | 18 (75) | 20 (83) |
| Cardiovascular risk factors | ||||||||
| Arterial hypertension, n (%) | 19 (91) | 15 (75) | n.a. | n.a. | 7 (54) | 9 (69) | 4 (20) | 6 (29) |
| Hyperlipidaemia, n (%) | 11 (52) | 9 (45) | n.a. | n.a. | 8 (62) | 7 (54) | 4 (20) | 5 (24) |
| Diabetes mellitus, n (%) | 11 (52) | 11 (55) | n.a. | n.a. | 5 (39) | 3 (23) | 2 (9) | 3 (13) |
| Current smoking, n (%) | 9 (43) | 6 (30) | n.a. | n.a. | 8 (62) | 7 (54) | 11 (61) | 6 (32) |
| Baseline and catheterization laboratory parameters | ||||||||
| Multivessel CAD, n (%) | 13 (62) | 14 (70) | n.a. | n.a. | 9 (69) | 10 (77) | 15 (63) | 21 (88) |
| TIMI-flow 2 or 3 post-PCI, n (%) | 17 (81) | 19 (95) | n.a. | n.a. | 12 (92) | 12 (92) | 23 (96) | 24 (100) |
| Device insertion pre-PCI, n (%) | 9 (43) | 9 (45) | n.a. | n.a. | 0 | 0 | 5 (21) | 3 (13) |
| Acute myocardial infarction, n (%) | 21 (100) | 20 (100) | 11 (58) | 10 (71) | 13 (100) | 13 (100) | 24 (100) | 24 (100) |
| Anterior myocardial infarction, n (%) | 18 (86) | 13 (65) | n.a. | n.a. | 7 (54) | 8 (62) | 16 (67) | 15 (63) |
| Catecholamines at baseline, n (%) | 21 (100) | 20 (100) | 19 (100) | 14 (100) | 11 (84) | 12 (92) | 24 (100) | 24 (100) |
| Mechanical ventilation, n (%) | 20 (95) | 20 (100) | n.a. | n.a. | 12 (92) | 12 (92) | 24 (100) | 24 (100) |
| CPR, VT, or VF pre randomization, n (%) | 11 (55) | 11 (52) | n.a. | n.a. | 11 (85) | 9 (69) | 24 (100) | 20 (83) |
| Left ventricular ejection fraction (%) | 26 ± 9 | 27 ± 7 | 19 ± 14 | 22 ± 9 | 28 ± 14 | 31 ± 16 | 30 ± 16 | 28 ± 16 |
CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; n.a., not available; PCI, percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction; VT, ventricular tachycardia; VF, ventricular fibrillation.
Baseline characteristics according to treatment strategy in individual studies
| Variable . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | MCS (n = 21) . | IABP (n = 20) . | MCS (n = 19) . | IABP (n = 14) . | MCS (n = 13) . | IABP (n = 13) . | MCS (n = 24) . | IABP (n = 24) . |
| Age (years) | 63 ± 10 | 66 ± 10 | 66 ± 14 | 60 ± 11 | 65 ± 10 | 67 ± 19 | 58 ± 9 | 59 ± 11 |
| Male gender, n (%) | 16 (76) | 15 (75) | 14 (74) | 9 (64) | 8 (62) | 11 (85) | 18 (75) | 20 (83) |
| Cardiovascular risk factors | ||||||||
| Arterial hypertension, n (%) | 19 (91) | 15 (75) | n.a. | n.a. | 7 (54) | 9 (69) | 4 (20) | 6 (29) |
| Hyperlipidaemia, n (%) | 11 (52) | 9 (45) | n.a. | n.a. | 8 (62) | 7 (54) | 4 (20) | 5 (24) |
| Diabetes mellitus, n (%) | 11 (52) | 11 (55) | n.a. | n.a. | 5 (39) | 3 (23) | 2 (9) | 3 (13) |
| Current smoking, n (%) | 9 (43) | 6 (30) | n.a. | n.a. | 8 (62) | 7 (54) | 11 (61) | 6 (32) |
| Baseline and catheterization laboratory parameters | ||||||||
| Multivessel CAD, n (%) | 13 (62) | 14 (70) | n.a. | n.a. | 9 (69) | 10 (77) | 15 (63) | 21 (88) |
| TIMI-flow 2 or 3 post-PCI, n (%) | 17 (81) | 19 (95) | n.a. | n.a. | 12 (92) | 12 (92) | 23 (96) | 24 (100) |
| Device insertion pre-PCI, n (%) | 9 (43) | 9 (45) | n.a. | n.a. | 0 | 0 | 5 (21) | 3 (13) |
| Acute myocardial infarction, n (%) | 21 (100) | 20 (100) | 11 (58) | 10 (71) | 13 (100) | 13 (100) | 24 (100) | 24 (100) |
| Anterior myocardial infarction, n (%) | 18 (86) | 13 (65) | n.a. | n.a. | 7 (54) | 8 (62) | 16 (67) | 15 (63) |
| Catecholamines at baseline, n (%) | 21 (100) | 20 (100) | 19 (100) | 14 (100) | 11 (84) | 12 (92) | 24 (100) | 24 (100) |
| Mechanical ventilation, n (%) | 20 (95) | 20 (100) | n.a. | n.a. | 12 (92) | 12 (92) | 24 (100) | 24 (100) |
| CPR, VT, or VF pre randomization, n (%) | 11 (55) | 11 (52) | n.a. | n.a. | 11 (85) | 9 (69) | 24 (100) | 20 (83) |
| Left ventricular ejection fraction (%) | 26 ± 9 | 27 ± 7 | 19 ± 14 | 22 ± 9 | 28 ± 14 | 31 ± 16 | 30 ± 16 | 28 ± 16 |
| Variable . | Thiele et al. 11 . | Burkhoff et al. 12 . | ISAR-SHOCK13 . | IMPRESS in Severe Shock14 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | MCS (n = 21) . | IABP (n = 20) . | MCS (n = 19) . | IABP (n = 14) . | MCS (n = 13) . | IABP (n = 13) . | MCS (n = 24) . | IABP (n = 24) . |
| Age (years) | 63 ± 10 | 66 ± 10 | 66 ± 14 | 60 ± 11 | 65 ± 10 | 67 ± 19 | 58 ± 9 | 59 ± 11 |
| Male gender, n (%) | 16 (76) | 15 (75) | 14 (74) | 9 (64) | 8 (62) | 11 (85) | 18 (75) | 20 (83) |
| Cardiovascular risk factors | ||||||||
| Arterial hypertension, n (%) | 19 (91) | 15 (75) | n.a. | n.a. | 7 (54) | 9 (69) | 4 (20) | 6 (29) |
| Hyperlipidaemia, n (%) | 11 (52) | 9 (45) | n.a. | n.a. | 8 (62) | 7 (54) | 4 (20) | 5 (24) |
| Diabetes mellitus, n (%) | 11 (52) | 11 (55) | n.a. | n.a. | 5 (39) | 3 (23) | 2 (9) | 3 (13) |
| Current smoking, n (%) | 9 (43) | 6 (30) | n.a. | n.a. | 8 (62) | 7 (54) | 11 (61) | 6 (32) |
| Baseline and catheterization laboratory parameters | ||||||||
| Multivessel CAD, n (%) | 13 (62) | 14 (70) | n.a. | n.a. | 9 (69) | 10 (77) | 15 (63) | 21 (88) |
| TIMI-flow 2 or 3 post-PCI, n (%) | 17 (81) | 19 (95) | n.a. | n.a. | 12 (92) | 12 (92) | 23 (96) | 24 (100) |
| Device insertion pre-PCI, n (%) | 9 (43) | 9 (45) | n.a. | n.a. | 0 | 0 | 5 (21) | 3 (13) |
| Acute myocardial infarction, n (%) | 21 (100) | 20 (100) | 11 (58) | 10 (71) | 13 (100) | 13 (100) | 24 (100) | 24 (100) |
| Anterior myocardial infarction, n (%) | 18 (86) | 13 (65) | n.a. | n.a. | 7 (54) | 8 (62) | 16 (67) | 15 (63) |
| Catecholamines at baseline, n (%) | 21 (100) | 20 (100) | 19 (100) | 14 (100) | 11 (84) | 12 (92) | 24 (100) | 24 (100) |
| Mechanical ventilation, n (%) | 20 (95) | 20 (100) | n.a. | n.a. | 12 (92) | 12 (92) | 24 (100) | 24 (100) |
| CPR, VT, or VF pre randomization, n (%) | 11 (55) | 11 (52) | n.a. | n.a. | 11 (85) | 9 (69) | 24 (100) | 20 (83) |
| Left ventricular ejection fraction (%) | 26 ± 9 | 27 ± 7 | 19 ± 14 | 22 ± 9 | 28 ± 14 | 31 ± 16 | 30 ± 16 | 28 ± 16 |
CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; n.a., not available; PCI, percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction; VT, ventricular tachycardia; VF, ventricular fibrillation.
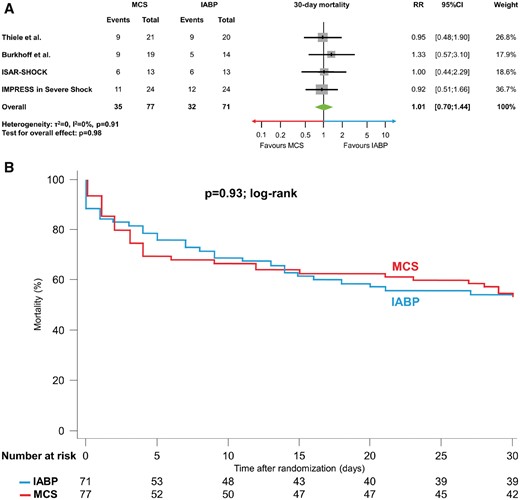
All-cause mortality
Short-term mortality was similar in patients treated with active MCS in comparison to those undergoing IABP (45.5% vs. 45.1%; RR 1.01, 95% CI 0.70 to 1.44, P = 0.98, I 2 = 0%, Figure 2A). Similarly, no difference in time-to-event analyses for mortality was detected (P = 0.93 by log-rank test) (Figure 2B).
(A, B) 30-day mortality. (A) Forest plot with results for 30-day mortality. (B) Kaplan–Meier curve for 30-day mortality using individual patient data. IABP, intra-aortic balloon pumping; MCS, active mechanical support device; RR, relative risk; 95% CI, 95% confidence interval.
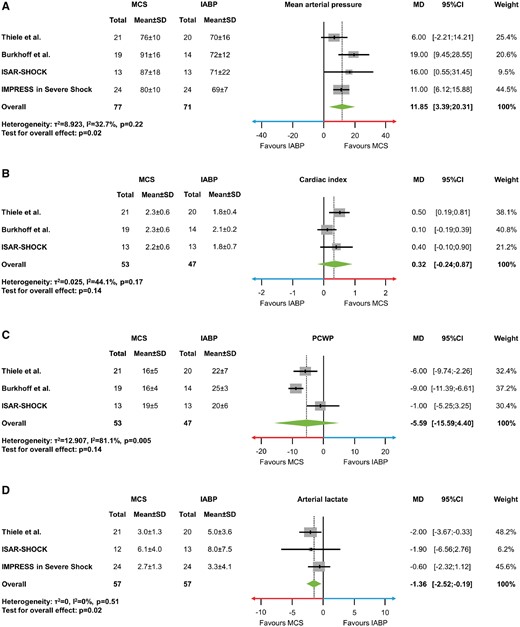
Haemodynamic and metabolic variables
Haemodynamic and metabolic variables were available for most of the trials. In IMPRESS in Severe Shock no pulmonary artery catheter monitoring was performed, thus no data on CI and PCWP were available. Arterial lactate was not assessed in the trial by Burkhoff et al. Active MCS significantly increased MAP (MD 11.85 mmHg, 95% CI 3.39 to 20.31, P = 0.02, I 2 = 32.7%; Figure 3A), whereas CI (MD 0.32, 95% CI −0.24 to 0.87, P = 0.14, I 2 = 44.1%; Figure 3B) and PCWP (MD −5.59, 95% −15.59 to 4.40, P = 0.14, I 2 = 81.1%; Figure 3C) were not significantly improved. Arterial lactate levels were lower in MCS patients as compared with control (MD −1.36 mmol/L, 95% CI −2.52 to −0.19, P = 0.02, I 2 = 0%; Figure 3D).
(A–D) Mean difference with 95% confidence intervals in haemodynamic and metabolic variables. (A) Mean arterial pressure (mmHg). (B) Cardiac index (L/min/m2). (C) Pulmonary capillary wedge pressure (mmHg). (D) Arterial lactate (mmol/L). IABP, intra-aortic balloon pumping; MCS, active mechanical support device; MD, mean difference; PCWP, pulmonary capillary wedge pressure; SD, standard deviation; 95% CI, 95% confidence interval.
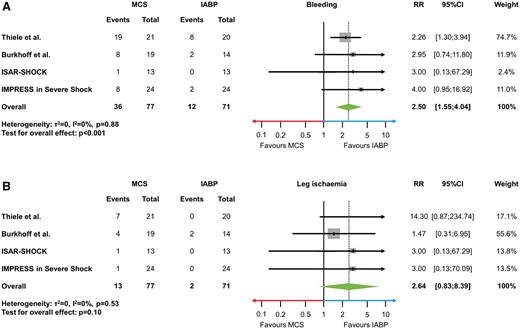
Complications
Bleeding and leg ischaemia were reported in all trials. In the ISAR-SHOCK trial bleeding events differed from the original publication and could be confirmed by individual data. Bleeding (RR 2.50, 95% CI 1.55 to 4.04, P < 0.001, I 2 = 0%; Figure 4A) occurred more frequently in MCS compared with control. The rate of leg ischaemia was numerically higher in patients undergoing MCS (RR 2.64, 95% CI 0.83 to 8.39, P = 0.10, I 2 = 0%; Figure 4B).
(A, B) Potential device-related complications. (A) Forest plot showing risk estimates of major bleeding. (B) Forest plot showing risk estimates of leg ischaemia. IABP, intra-aortic balloon pumping; MCS, active mechanical support device; RR, relative risk; 95% CI, 95% confidence interval.
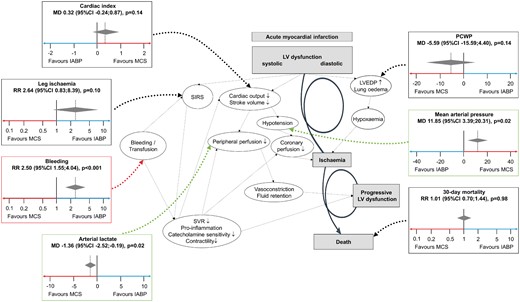
Potential beneficial and negative effects of mechanical support devices in the context of the downward spiral of cardiogenic shock. IABP, intra-aortic balloon pumping; MCS, active mechanical support device; MD, mean difference; PCWP, pulmonary capillary wedge pressure; 95% CI, 95% confidence interval; LV, left ventricular; LVEDP, left ventricular end-diastolic pressure; SIRS, systemic inflammatory response syndrome; SVR, systemic vascular resistance.
Discussion
This collaborative meta-analysis of four randomized trials investigating the efficacy and safety of percutaneous active MCS vs. control with IABP demonstrates similar short-term mortality despite initial beneficial effects on MAP and reduction of arterial lactate. There was a higher rate of bleeding and a numerically higher incidence of limb ischaemia following active percutaneous MCS.
Mortality of CS complicating AMI remains high despite modern treatment strategies including early revascularization and best available medical therapy. The latter mainly consists of volume management as well as administration of inotropic agents and vasopressors enhancing cardiac output and vascular tone. The haemodynamic benefits of inotropes and vasopressors appear to be counterbalanced by adverse effects such as increased myocardial oxygen demand, arrhythmogenicity, and compromize of tissue microcirculation which may translate into an increased mortality risk. MCS are an alternative to increase systemic blood flow while avoiding the possible cardiotoxicity and long-term morbidity of medical therapy.4 , 17
The current meta-analysis demonstrates an initial improvement of MAP and arterial lactate in patients treated by active MCS, whereas CI and PCWP did not differ significantly. The benefits of active MCS on MAP and arterial lactate must be weighed against the potential complications associated with the invasiveness of MCS with respect to the implantation procedure, leg ischaemia due to large arterial cannula size and bleeding. This meta-analysis confirmed a more than two-fold increase of bleeding in patients with MCS as compared with IABP control, which most likely affects clinical outcome due to the fact that bleeding is known to be a powerful predictor for mortality in acute coronary syndromes. Although the rate of leg ischaemia did not differ between the MCS and IABP groups, one might speculate that the occurrence of leg ischaemia would be even lower in a control arm using neither MCS nor IABP resulting in a significant decrease as compared with an active arm. The contact with artificial surfaces from MCS and secondary haemolysis might further promote systemic inflammatory response syndrome.4 In a previous meta-analysis there was a trend towards more fever and sepsis in MCS treated patients, which were not assessed in the current meta-analysis due to inconsistent reporting and definitions.18 These complications inherent to MCS appear to counterbalance the beneficial effects on MAP and tissue hypoxaemia as indicated by arterial lactate measurements resulting in neutral effects with respect to all-cause mortality (Summarizing Figure).6 , 19
Based on animal studies the beneficial effects of MCS are often believed to be more pronounced when started before revascularization. The time point of initiation of the MCS device (before PCI vs. after PCI) was at the discretion of the treating physician in all four included trials. In the ISAR-SHOCK study all patients underwent MCS insertion post PCI. Conversely, MCS support was initiated before PCI in 21% of patients enrolled in the IMPRESS in Severe Shock trial and in 43% in the trial performed by Thiele et al. 11–14 Data on timing of active MCS insertion in humans in CS are limited. In the USpella registry patients directly treated with Impella® prior to PCI in CS had an overall better survival at hospital discharge compared with those treated after PCI, even when adjusting for potential confounding variables.20 Concerning IABP, there are conflicting data with more evidence demonstrating harm rather than benefit by IABP insertion before PCI.21 , 22 This might be at least partly explained by further deferral of revascularization, which is the therapeutic cornerstone in CS complicating AMI. This is also supported by findings of a randomized trial investigating the impact of IABP insertion prior to PCI in high-risk anterior AMI patients on infarct size demonstrating neutral results.23
The current meta-analysis does not demonstrate a reduced mortality in unselected CS patients treated with active MCS used on a routine basis. Therefore, patient selection may play a crucial role. It is well known, that approximately 50–60% of CS patients survive without any MCS.3 Thus, a positive impact of MCS on outcome in this patient group appears to be unlikely. There may also be futile situations where MCS devices might not even theoretically be able to change clinical outcome such as patients with severe brain injury. MCS appears to stabilize the initial haemodynamic situation but will not be able to influence prognosis. In clinical practice MCS is often chosen on a subjective basis and readily available scores are currently not well established. The newly introduced IABP-SHOCK II score, based on 6 easily assessable parameters dividing patients into low, intermediate, and high-risk cohorts, may be helpful for MCS selection but this needs further evaluation in randomized trials.24 Evidently, timing and appropriate patient selection are influenced by the balance between efficacy of the device and its device-related complications. Devices with low complication rates may be chosen more liberally in the early stage of CS whereas more aggressive devices such as extracorporeal life support (ECLS) with higher flow rates may be reserved for more severe CS. The evidence for ECLS is, however, even more limited.25 Recent animal data suggest better haemodynamic support with the TandemHeart™ in comparison to the Impella® CP,26 however, based on the current meta-analysis no preference for any device can be made. According to current guidelines, MCS should be mainly considered in patients with refractory CS.8 , 27
IABP is in place for more than five decades and remains the most widely used device. All trials included in the current meta-analysis used IABP as comparator as the individual studies were performed or started before the downgrading of IABP use in current European guidelines.7–9 The recent class III recommendation for routine use of IABP in CS is based on the findings of the IABP-SHOCK II trial demonstrating similar 30-day and 12-month mortality in patients treated with or without IABP. Furthermore, IABP did not show any differences in secondary endpoints such as MAP, arterial lactate, renal function, catecholamine doses, or length of intensive care unit treatment.3 Moreover, a previous trial also showed no beneficial haemodynamic effects in IABP vs. control such as CI, cardiac power output, and systemic vascular resistance.28 Changes in MAP and arterial lactate observed in the current meta-analysis would have most likely also been observed in active MCS vs. no IABP.
The following limitations should be acknowledged. First, IMPRESS in Severe Shock contributed 32% of patients to the collaborative meta-analysis. Therefore, the statistical weight to the calculated models of mortality and the secondary as well as safety outcomes of IMPRESS in Severe Shock ranged between 11% and 46%. Second, the data on mortality need to be interpreted with caution due to the limited statistical power and analysis of two different MCS. However, the observed RR of 1.01 (95% CI 0.70 to 1.44) with a P-value of 0.98 between MCS and IABP makes a possible positive effect of an unselected use even in larger populations unlikely. This is also underlined by the consistency of the findings in the individual studies. Third, adjustment for baseline characteristics or subanalyses aiming to identify specific subgroups with potential benefit from MCS could not be performed, as these individual patient data were not retrievable anymore in one trial. Additional prospective randomized studies are warranted enrolling specific subgroups of patients (e.g. moderate vs. severe CS). Forth, effects on haemodynamic parameters and arterial lactate also must be cautiously interpreted based on the non-blinded evaluation in the four trials. Further, a minority of patients (n = 12) enrolled by Burkhoff et al. presented with CS due to decompensated heart failure in the absence of acute myocardial ischaemia. This refers to less than 10% of all patients and thus a significant impact on the main results of the current meta-analysis appears unlikely. Moreover, data on mechanical ventilation, neurological outcome, therapeutic hypothermia, organ dysfunction including need for renal replacement therapy or doses of inotropes and vasopressors were not available in all patients and could thus not be analysed. Finally, definitions of bleeding and leg ischaemia were not uniformly reported and occurrence of endpoints was only adjudicated by a clinical event committee in one trial. However, the primary endpoint of the current meta-analysis is 30-day all-cause mortality and thus the results are independent of adjudication.
In conclusion, despite an initial beneficial effect on MAP and arterial lactate active percutaneous MCS did not improve mortality in comparison to control in patients with CS complicating AMI, which may be partly explained by an excess of complications such as bleeding. The use of active percutaneous MCS may thus be restricted to selected patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: none declared.
References
Author notes
Holger Thiele, Alexander Jobs and Dagmar M. Ouweneel authors should be considered as first authors.
See page 3532 and 3535 for the editorial comments on this article (doi: 10.1093/eurheartj/ehx406 and 10.1093/eurheartj/ehx405)
- heart failure, acute
- myocardial infarction, acute
- ischemia
- intra-aortic balloon pumping
- hemorrhage
- cardiac support procedures
- cardiogenic shock
- lactates
- pulmonary wedge pressure
- safety
- leg
- mortality
- treatment outcome
- cardiac index
- mean arterial pressure
- medical devices
- medical literature
- bleeding rate