-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Weber, Johann Auer, Michael F. O'Rourke, Erich Kvas, Elisabeth Lassnig, Gudrun Lamm, Nina Stark, Martin Rammer, Bernd Eber, Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions, European Heart Journal, Volume 26, Issue 24, December 2005, Pages 2657–2663, https://doi.org/10.1093/eurheartj/ehi504
Close - Share Icon Share
Abstract
Aims Increased arterial wave reflections are associated with the presence and extent of coronary atherosclerosis and with cardiovascular mortality in selected populations. We prospectively evaluated their prognostic value in the short- and long-term following percutaneous coronary interventions (PCIs).
Methods and results We non-invasively quantified wave reflections [expressed as augmentation index corrected for heart rate of 75 b.p.m. (AIx@75)] using applanation tonometry of the radial artery and a validated transfer function to obtain the corresponding aortic values in 262 patients undergoing PCI. During 2-year follow-up, 61 patients reached the primary endpoint [death, myocardial infarction (MI), and restenosis]. Increasing tertiles of Alx@75 were related to the rate of patients reaching the primary endpoint [15.2, 20 and 35.3%, respectively (P=0.001)], as well as the secondary endpoints total mortality, myocardial infarction and death plus myocardial infarction (RR for the third vs. the first tertile 4.33, 3.25 and 3.46, respectively, P<0.05). In a multivariable Cox-regression model, AIx@75 added prognostic value above and beyond clinical risk factors, angiographic variables, and medications (RR 1.8, 95%CI 1.18–2.76 per increasing AIx@75-tertile, P<0.01).
Conclusion Increased arterial wave reflections are independently associated with an increased risk for severe short- and long-term cardiovascular events in patients undergoing PCI.
See page 2609 for the editorial comment on this article (doi:10.1093/eurheartj/ehi607)
Introduction
Unfavourable alterations of the mechanical characteristics of the arterial system in general and the reflective properties in particular are increasingly recognized to play an important role in cardiovascular diseases. Brachial artery pulse pressure (PP), a crude estimate of arterial stiffness, has been identified as an independent predictor of cardiac (primarily coronary) events.1 Moreover, brachial artery PP has been independently associated with total mortality in the Balloon Angioplasty Revascularization Trial.2 In addition, pulsatility of the ascending aorta3 and earlier return of the reflected wave4, both determined invasively, appear to be powerful predictors of restenosis in patients undergoing conventional balloon angioplasty.
Arterial stiffness, determined by pulse wave velocity, has been linked to primary coronary events and cardiovascular mortality in end-stage renal failure patients5 and in hypertensives.6 An increased effect of arterial wave reflections on central arteries predicted impaired survival in dialysis patients.7 Recently, we observed that premature/increased arterial wave reflections, measured non-invasively and quantified as augmentation index (AIx), are associated with the presence and extent of coronary artery disease (CAD).8
We tested the hypothesis that non-invasive assessment of arterial wave reflections provides prognostic value in CAD patients undergoing percutaneous coronary interventions (PCI) and is capable of identifying patients who are prone to experience adverse cardiac events.
Methods
This study was conducted in a referral cardiology department in a 1050-bed tertiary care hospital in Austria. We prospectively screened 404 unselected patients undergoing PCIs, balloon angioplasty and/or stent implantation, for stable angina or acute coronary syndromes (ACSs) for study inclusion. Patients with more than mild valvular heart disease (n=6), left ventricular ejection fraction <35% (n=8), or atrial fibrillation (n=13) were excluded. Moreover, in 84 patients, pulse waveform analysis (PWA) data were not available due to their short hospital stay, and in 30 patients, PWA tracings were of poor quality. One patient was lost to follow-up. The latter 115 patients were slightly younger than the remaining 262 patients, who comprised our study population (mean age was 61.8 vs. 65.6 years), all other baseline and angiographic characteristics as well as medications and event rates during follow-up were similar between the two groups (data not shown). All patients were studied while on regular medications (discontinuation of drug therapy was not warranted in our patients, because most of them suffered from ACS) and gave written informed consent. The study was approved by the local Ethics Committee.
Hypertension was present with repeated measurements ≥140 mmHg systolic blood pressure (BP) and/or ≥90 mmHg diastolic BP or permanent antihypertensive drug treatment. Diabetes mellitus was defined as a fasting blood glucose concentration ≥126 mg/dl or antihyperglycaemic drug treatment. Current smoking was defined as having smoked the last cigarette less than 1 week before coronary angiography. Creatinine clearance was estimated using the Cockcroft–Gault formula.9
Coronary angiography and PCI
Coronary angiography and PCI were performed using standard techniques10 on a digitized coronary angiography equipment (Cathcor, Siemens, Germany). All clinical and procedural decisions were left to the discretion of the operator. No drug-eluting stents were used during the study period. The extent of CAD was defined as one-, two-, or three-vessel disease. Lesion complexity was characterized by standard angiographic criteria.11 Quantitative coronary angiography was performed offline, using the CMS software (Medis, Nuenen, The Netherlands), by one experienced interventionalist (J.A.), who was blinded to the patient's clinical course. Analysis was performed in each patient. (The lesion with the highest diameter stenosis at baseline was selected in patients with multivessel PCI.) Unsuccessful PCI was defined as a failure to achieve a minimum stenosis diameter reduction to <50%.10 A procedure-related myocardial infarction (MI) was defined as the occurrence of signs and/or symptoms suggestive of MI following the procedure, accompanied by angiographic evidence of abrupt vessel closure, important side branch occlusion, or new and persistent slow coronary flow, as well as elevation of necrosis markers troponin I or T or creatinine kinase of more than three times the upper limit.10 All patients were scheduled to receive weight-adjusted unfractionated heparin during the procedure, clopidogrel 75 mg/day for at least 4 weeks after stent implantation, and aspirin 100 mg/day indefinitely.
Pulse waveform analysis
Assessment of arterial stiffness was performed non-invasively with the commercially available SphygmoCor system (AtCor Medical, Sydney, Australia), as previously described.8 In brief, peripheral pressure waveforms were recorded from the radial artery at the wrist, using applanation tonometry with a high fidelity micromanometer. After 20 sequential waveforms had been acquired, a validated,12 generalized transfer function was used to generate the corresponding central aortic pressure waveform. AIx was derived from this with the technique of PWA.13 As AIx is influenced by heart rate, an index normalized for heart rate of 75 b.p.m. (AIx@75) was used in accordance to Wilkinson et al.14 Only high quality recordings, defined as an in-device quality index ≥80% and acceptable curves on visual inspection by the investigator (T.W.), were included into the analysis. All PWA-measurements were taken in the sitting position in a quiet, temperature-controlled room (22±1°C) after a brief period (at least 5 min) of rest, most often on the day following PCI, by nurses not involved in performance or interpretation of the angiograms. Repeatability of PWA is good, as previously reported.8
BP measurements
BP measurements were performed with a validated,15 automated wrist BP monitor (Omron R3, Omron Healthcare, Tokyo, Japan) with the radial artery kept at heart level during measurement.
Study endpoints
Primary endpoint was the combination of death, MI, and clinical restenosis observed within 24 months following PCI. Non-periprocedural MI was defined in accordance to previously published guidelines16 either as increased cardiac troponin (either T or I) exceeding the 99th percentile of a reference control group with acceptable imprecision, accompanied by ischaemic symptoms or typical ECG changes, or as typical findings on autopsy. Clinical restenosis was defined as re-occurrence of symptoms consistent with myocardial ischaemia in the presence of angiographically documented diameter stenosis ≥50% at the previously treated vessel site.
Follow-up
First data were collected at hospital discharge. Information after 3, 12, and 24 months was obtained primarily by a written questionnaire, which was returned by almost two-thirds of patients. The remaining patients were followed by a telephone interview or, if not achievable, by information obtained from their general practitioners. Overall, complete information of 262 patients on the 24-month status was available. If a patient reached an endpoint (death, MI, and clinically apparent restenosis), the hospital record or, if the patient was not hospitalized, information from the general practitioner was obtained. All endpoints were adjudicated by the two investigators (T.W. and E.L.) independently.
Statistical analysis
Patients were divided into tertiles, on the basis of their AIx@75 levels. All parametric values were expressed as mean±1 standard deviation. Baseline characteristics were analysed with the use of Kruskal–Wallis analysis of variance or χ2 test for continuous or categorical variables, as appropriate. To account for multiple testing, Bonferroni's correction was applied. All tests were two-sided. Numerical correlations were established by Spearman-correlation. AIx@75 levels at baseline were compared according to outcome, using Mann–Whitney U test. Unadjusted event rates in AIx@75-tertiles were compared by Pearson–Mantel–Haenszel test. To visualize the relation between AIx@75 and two-year event-free survival, Kaplan–Meier plots were generated, and the log-rank test was used for comparison of the resulting survival curves. Cox-proportional hazards modelling was used for the determination of multivariable predictors of the composite primary endpoint. AIx@75 was entered into the statistical model as tertiles or as a continuous variable. Other variables included in the model were known clinical predictors of cardiovascular events and all univariate predictors of the primary endpoint with a P-value of ≤0.20. Therefore, gender, age, smoking, prior MI, prior stroke, diabetes, peripheral arterial occlusive disease, extent of CAD as one-, two-, and three-vessel disease, clinical presentation, medications, triglyceride levels, creatinine clearance, body mass index, brachial systolic BP or brachial PP, all angiographic variables as presented in Table 2, and AIx@75 as a continuous variable or as tertiles were entered into the full multivariable model for prediction of the primary endpoint (no automatic variable selection algorithms were used). To examine whether the assumption of proportional hazards was met, we checked each covariate by plotting the survival curves on a log-minus-log scale and found no violation to this assumption. The linearity assumption was assessed by comparing the estimates of AIx@75 in models including the continuous variable as such with that in models in which the percentile dummies of AIx@75 were included. The linearity assumption was satisfied. A P-value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were performed using the Statistica 6.0 (StatSoft Inc., Tulsa, OK, USA), BiAS for Windows 7.05 (Hanns Ackermann, Frankfurt, Main, Germany), and SPSS 13.0 (SPSS GmbH software, Munich, Germany) software packages.
Results
Baseline characteristics
Demographic, angiographic, and procedural parameters of our patients, subdivided according to tertiles of AIx@75, are summarized in Tables 1 and 2. Increasing tertiles of AIx@75 were associated with female gender, older age, lower body mass index, lower triglycerides, lower creatinine clearance, higher inflammatory activity as assessed by hs-C-reactive protein, a higher proportion of patients with peripheral arterial occlusive disease, and higher systolic BP and PP, both brachial and estimated aortic. Angiographic variables of the culprit lesion did not differ between the tertiles.
Clinical determinant of prognosis
By the end of the follow-up, 12 patients died, 22 suffered from MI (five fatal and 17 non-fatal), and 40 were diagnosed with clinical restenosis (32 with worsening angina and eight with MI). In nine, these events occurred in hospital, including seven non-fatal and two fatal MIs, and in others after discharge from hospital in stable condition. Overall, 61 patients reached the primary endpoint (event group).
They did not differ from patients with an uneventful course of disease with the exception of diabetes, prior stroke, and three vessel CAD, which were more frequently observed in event-group patients.
Arterial wave reflections and clinical events
Mean baseline AIx@75 was significantly higher in patients reaching the primary endpoint as well as individual endpoints when compared with patients with an uneventful course (Table 3). There was an increased risk for the primary endpoint with increasing tertiles of AIx@75. At 2 years, 15.2, 20, and 35.3% of the patients reached the primary endpoint [unadjusted RRs 2.32 (95%CI 1.36–3.96, P=0.003) and 1.76 (95%CI 1.07–2.91, P=0.03) for the first (T1) and second (T2) tertiles of AIx@75 when compared with the third (T3) tertile, respectively].
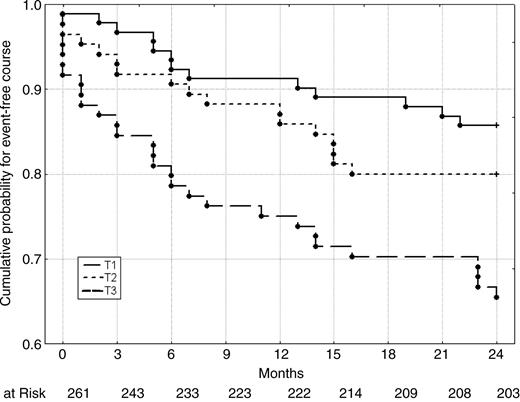
Figure 1 shows the cumulative proportion of patients remaining event-free according to tertiles of AIx@75. Event-free survival was significantly associated with AIx@75-tertiles, with outcome being worst in those patients with highest AIx@75-levels (P=0.001 and 0.03 for difference between T3 and T1 and between T3 and T2, respectively, log-rank test).
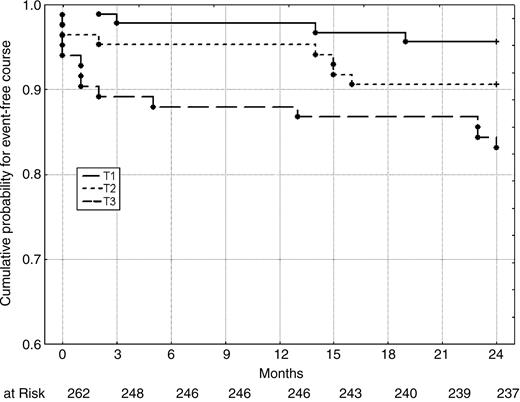
When considering the composite endpoint of death and MI, we again found a worse outcome in patients with increased wave reflections (P=0.006 for difference between AIx@75-T3 and -T1, log-rank test; Figure 2).
Moreover, in univariate analysis, a higher AIx@75 was associated with an increased risk for all-cause mortality (RR 4.33 for T3 vs. T1, 95%CI 1.08–17.25, P=0.04), MI (RR 3.25 for T3 vs. T1, 95%CI 1.17–9.02, P=0.02), and the combination of all-cause mortality plus MI (RR 3.46 for T3 vs. T1, 95%CI 1.43–8.41, P=0.006).
With respect to the primary endpoint in various clinical subgroups, we observed consistent and largely similar results in patients up to 65 years of age and in older patients (RRs 2.28 for AIx@75-T3 vs. -T1, 95%CI 1.02–5.10, and 2.32, 95%CI 1.09–4.96, respectively), in men and women (RRs 2.08 for AIx@75-T3 vs. -T1, 95%CI 1.09–3.95, and 4.13, 95%CI 0.87–19.63, respectively), in patients with successful PCI (RR 2.10 for AIx@75-T3 vs. -T1, 95%CI 1.22–3.59*), in patients with native vessel PCI (RR 2.44 for AIx@75-T3 vs. -T1, 95%CI 1.41–4.23*), and in patients with stent implantation (RR 2.54 for AIx@75-T3 vs. -T1, 95%CI 1.37–4.69*). The results in the subgroups marked with an asterisk were significant on a Bonferroni-corrected significance level (α=0.007).
Multivariable analysis of risk factors for the primary endpoint
In a multivariable Cox-regression analysis adjusting for a large number of covariables (Statistical Analysis), increasing tertiles of AIx@75 still independently contributed to the prediction of the primary endpoint (Table 4). The multiple adjusted RR for the primary endpoint was 1.8 per increasing AIx@75-tertile (95%CI 1.18–2.76, P=0.006). When we entered AIx@75 as a continuous variable into the model, the multiple adjusted RR for the primary endpoint was 1.04 per unit (95% CI 1.01–1.07, P=0.009). In addition, in both models, only a lower creatinine clearance, a lower incidence of beta-blocker use, more complex lesion type (B2 or C), and the use of GP IIb/IIIa inhibitors were independently associated with a worse outcome. The latter might largely reflect an unfavourable peri-interventional course, because GP IIb/IIIa inhibitors were not randomly distributed among our patients, but mainly used in ‘bail-out’ situations at that time in our institution.
When we replaced brachial systolic BP by brachial PP in our multivariable model, results were virtually unchanged: the multiple adjusted RR for the primary endpoint was 1.72 per increasing AIx@75-tertile (P=0.01), whereas brachial PP did not independently contribute to the prediction of the primary endpoint.
Discussion
The main novel finding of the present study is that increased arterial wave reflections, measured non-invasively through PWA, predicted the occurrence of severe cardiovascular events in CAD patients undergoing PCI, beyond the prediction provided by classic or angiographic risk factors. To the best of our knowledge, there has been only one prior study, relating wave reflections to survival, but only in a highly selected, small patient group (dialysis patients).7 In a recent study, aortic pressure augmentation, calculated from pressure tracings obtained during cardiac catheterization, predicted adverse cardiovascular events in patients with established CAD.17 However, it is obvious that a non-invasive approach has clear advantages for the determination of arterial wave reflections in larger patient groups and their changes in response to medical therapies. Therefore, both studies are complementary and, together, strongly suggest that increased arterial wave reflections could have detrimental consequences in the large group of CAD patients.
According to our multivariable model, it might be hypothesized that prognosis in patients undergoing PCI is determined by local factors (lesion complexity and GP IIb/IIIa use reflecting an unfavourable periprocedural course as in our study), systemic factors (renal function and arterial wave reflections), and medications (beta-blockers).
Several mechanisms may explain the contribution of arterial mechanics: increased stiffness of the central elastic arteries is the primary cause of increased systolic BP and PP with advancing age and in patients with cardiovascular disease and is due to degeneration and hyperplasia of the arterial wall. As central arterial stiffness increases, transmission velocity of both forward and backward (or reflected) pressure waves increases, which causes the reflected wave to arrive earlier in the central aorta, augments pressure in late systole, and reduces diastolic pressure. The former causes an increase in left ventricular after-load and myocardial oxygen consumption and the latter a decrease in coronary perfusion pressure.18 The reduction in coronary perfusion is especially pronounced in patients with advanced CAD with diminished coronary reserve, when aortic diastolic BP becomes the overriding haemodynamic factor, which controls coronary blood flow.1 The combined effect of higher systolic and lower diastolic BP is an unfavourable change in the myocardial oxygen demand/supply ratio, leading to increased coronary risk.1 Consequently, large artery stiffness has been shown to predict ischaemic threshold in patients with CAD.19
In contrast, in addition to structural alterations of large arteries, affecting the timing of wave reflections, functional changes including smooth muscle tone of small arteries and arterioles may exert an important influence on the intensity of wave reflections. The effects of therapeutic doses of nitrates on wave reflections and AIx have been described in detail20 and are consistent with our findings (Table 1). Moreover, AIx and its changes with pharmacological interventions have been proposed as an alternative way of measuring endothelial function.21 Given the well-known prognostic relevance of endothelial dysfunction,22 our results on the predictive value of AIx@75 might point to the same direction. The weak albeit statistically significant relationship between plasma levels of hs-C-reactive protein, a pro-inflammatory biomarker associated with endothelial dysfunction,22 and AIx@75 (Spearman-R 0.18, P=0.003; Table 1) seems to support this point of view.
Of note, in our study, AIx@75 was a much better measure for risk prediction than brachial artery PP. This confirms our previous findings8 and might be explained by several mechanisms: first, AIx@75 is derived from the (calculated) aortic pressure waveform and, therefore, not influenced by vascular amplification of the pressure wave in the upper limb, and second, as the pressure curve is obtained by high-fidelity tonometry, AIx@75 is not dependent on any inaccuracy in using the cuff sphygmomanometer.
What would be the clinical implications of our findings? The therapeutic benefit of antihypertensive drugs on arteries consists of two major effects: the effect due to BP lowering and the direct effect of the drug on the vessel wall. Drug therapy that favourably influences blood vessel function may directly improve the mechanical properties of the arterial vasculature, independent of changes in the BP. Indeed, it has been shown that for the same amount of diastolic BP reduction, the combination of a low-dose diuretic and an ACE-inhibitor decreased systolic BP, PP, and wave reflections to a significantly larger extent than a beta-blocker.23 A therapeutic trial in a population at high cardiovascular risk has recently shown that the pharmacological treatment of hypertension is associated with a longer survival, when the reduction in BP is associated with a significant improvement in large artery function in terms of pulse wave velocity.24 Thus, pharmacological agents favourably altering large artery function and reducing wave reflections25 might be needed to improve prognosis. This has been confirmed in a large-scale trial26 in high-risk patients using an ACE-inhibitor. Although arterial stiffness and/or wave reflections were not measured in that study, a comparison of the active drug (ramipril) against atenolol did show substantial reduction in wave reflection with ramipril but not with atenolol.27
In contrast, pure beta-blockers have little or no effect on wave reflections, but they enhance AIx (but not AIx@75; Table 1) by reduction in heart rate. Therefore, and consistent with our findings, their beneficial effects in CAD patients might relate to their myocardial protective actions,28 independent of their impact on the vasculature.
A possible limitation of our study is the relatively small number of patients. Thus, further studies in larger populations, ideally in a multicentre design, may add additional information. Moreover, because of the well-known effects of severe systolic dysfunction on AIx (i.e. reduction of AIx due to the incapability of the failing ventricle to generate a pressure boost to fully compensate the reflected pressure wave29), we excluded patients with an ejection fraction <35%. Therefore, the prognostic value of AIx@75 in these patients remains unknown. This is also true for patients with valvular heart disease and for those with atrial fibrillation.
In conclusion, the results presented herein indicate that increased arterial wave reflections are strong and independent predictors of severe cardiovascular events including death, MI, and clinical restenosis in CAD patients undergoing PCI. Considering our data together with previous observational studies, a therapeutic trial in these patients aiming to reduce wave reflections and, thus, improve prognosis seems to be warranted.
Acknowledgements
We are indebted to the cardiologists performing coronary interventions (Christian Punzengruber, MD, Edwin Maurer, MD, Herbert Mayr, MD, and Friederike Pichler, MD).
Conflict of interest: M.F.O. is a founding director of Atcor Medical and manufacturer of systems for pulse wave analysis.
Figure 1 Kaplan–Meier estimates of the rates of the primary endpoint (death, MI, and clinical restenosis), according to AIx@75-tertiles at baseline. Differences between Tertiles 1 and 2 vs. 3 were statistically significant (P=0.001 and 0.03, respectively, log-rank test).
Figure 2 Kaplan–Meier estimates of the secondary endpoint (death and MI) at 2 years, according to AIx@75-tertiles at baseline. Differences between Tertiles 1 and 3 were statistically significant (P=0.006, log-rank test).
Clinical characteristics of the patients, according to AIx@75-tertiles
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| AIx@75 | −19–18 | 19–25 | 26–53 | |
| Male, n (%) | 81(88.0) | 59 (69.4) | 45 (52.9) | <0.0001a |
| Age (years) | 62.3±9.8 | 66.8±8.6 | 67.9±11.3 | <0.001a |
| Prior MCI, n (%) | 11 (11.9) | 16 (18.8) | 17 (20) | 0.30 |
| Peripheral arterial disease, n (%) | 3 (3.3) | 11 (12.9) | 11 (12.9) | 0.04 |
| Hypertension, n (%) | 67 (72.8) | 61 (71.8) | 60 (70.6) | 0.95 |
| Diabetes, n (%) | 17 (18.5) | 23 (27.1) | 20 (23.5) | 0.39 |
| Smoking, n (%) | 21 (22.8) | 19 (22.4) | 16 (18.8) | 0.78 |
| Body mass index (kg/m2) | 28.6±4.1 | 27.1±3.5 | 26.9±3.3 | 0.005 |
| Total cholesterol (mg/dl) | 200.8±44.5 | 200.8±39.0 | 209.6±43.2 | 0.20 |
| LDL cholesterol (mg/dl) | 119.3±41.9 | 127.8±36.7 | 129.3±39.3 | 0.19 |
| HDL cholesterol (mg/dl) | 43.2±11.6 | 45.3±11.4 | 45.9±13.9 | 0.61 |
| Triglycerides (mg/dl) | 184.3±98.7 | 149.7±82.0 | 144.0±68.5 | 0.007 |
| Creatinine-clearance (ml/min) | 78.5±22.6 | 66.2±20.0 | 66.9±22.2 | <0.0001a |
| Hs-C-reactive protein (mg/dl) | 8.7±25.6 | 5.9±10.4 | 13.6±33.0 | 0.01 |
| Heart rate (b.p.m.) | 67.9±12.6 | 65.7±12.0 | 64.0±10.4 | 0.14 |
| Brachial SBP (mmHg) | 127±23 | 135±20 | 133±23 | 0.005 |
| Brachial DBP (mmHg) | 75±14 | 79±15 | 78±14 | 0.45 |
| Brachial PP (mmHg) | 51±19 | 57±17 | 55±18 | 0.02 |
| Estimated aortic systolic BP (mmHg) | 112±20 | 123±18 | 125±22 | <0.0001a |
| Estimated aortic diastolic BP (mmHg) | 76±14 | 79±15 | 79±14 | 0.43 |
| Estimated aortic PP (mmHg) | 36±15 | 44±13 | 46±17 | <0.0001a |
| Stable angina/ACS, n (%) | 29 (31.5)/63 (68.5) | 30 (35.3)/55 (64.7) | 22 (25.9)/63 (74.1) | 0.41 |
| EF normal, n(%) | 66 (71.7) | 57 (67.1) | 55 (64.7) | 0.59 |
| Medications at discharge | ||||
| Aspirin, n (%) | 91 (98.9) | 94 (98.8) | 84 (98.8) | 0.99 |
| Clopidogrel, n (%) | 76 (82.6) | 80 (94.1) | 70 (82.4) | 0.04 |
| Beta-blocker, n (%) | 67 (72.8) | 62 (72.9) | 63 (74.1) | 0.98 |
| ACE-inhibitor/ARB, n (%) | 51 (55.4) | 43 (50.6) | 35 (41.2) | 0.16 |
| CCB, n (%) | 14 (15.2) | 6 (7.1) | 6 (7.1) | 0.11 |
| Nitrates, n (%) | 32 (34.8) | 30 (35.3) | 16 (18.8) | 0.03 |
| Statin, n (%) | 71 (77.2) | 62 (72.9) | 61 (71.8) | 0.69 |
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| AIx@75 | −19–18 | 19–25 | 26–53 | |
| Male, n (%) | 81(88.0) | 59 (69.4) | 45 (52.9) | <0.0001a |
| Age (years) | 62.3±9.8 | 66.8±8.6 | 67.9±11.3 | <0.001a |
| Prior MCI, n (%) | 11 (11.9) | 16 (18.8) | 17 (20) | 0.30 |
| Peripheral arterial disease, n (%) | 3 (3.3) | 11 (12.9) | 11 (12.9) | 0.04 |
| Hypertension, n (%) | 67 (72.8) | 61 (71.8) | 60 (70.6) | 0.95 |
| Diabetes, n (%) | 17 (18.5) | 23 (27.1) | 20 (23.5) | 0.39 |
| Smoking, n (%) | 21 (22.8) | 19 (22.4) | 16 (18.8) | 0.78 |
| Body mass index (kg/m2) | 28.6±4.1 | 27.1±3.5 | 26.9±3.3 | 0.005 |
| Total cholesterol (mg/dl) | 200.8±44.5 | 200.8±39.0 | 209.6±43.2 | 0.20 |
| LDL cholesterol (mg/dl) | 119.3±41.9 | 127.8±36.7 | 129.3±39.3 | 0.19 |
| HDL cholesterol (mg/dl) | 43.2±11.6 | 45.3±11.4 | 45.9±13.9 | 0.61 |
| Triglycerides (mg/dl) | 184.3±98.7 | 149.7±82.0 | 144.0±68.5 | 0.007 |
| Creatinine-clearance (ml/min) | 78.5±22.6 | 66.2±20.0 | 66.9±22.2 | <0.0001a |
| Hs-C-reactive protein (mg/dl) | 8.7±25.6 | 5.9±10.4 | 13.6±33.0 | 0.01 |
| Heart rate (b.p.m.) | 67.9±12.6 | 65.7±12.0 | 64.0±10.4 | 0.14 |
| Brachial SBP (mmHg) | 127±23 | 135±20 | 133±23 | 0.005 |
| Brachial DBP (mmHg) | 75±14 | 79±15 | 78±14 | 0.45 |
| Brachial PP (mmHg) | 51±19 | 57±17 | 55±18 | 0.02 |
| Estimated aortic systolic BP (mmHg) | 112±20 | 123±18 | 125±22 | <0.0001a |
| Estimated aortic diastolic BP (mmHg) | 76±14 | 79±15 | 79±14 | 0.43 |
| Estimated aortic PP (mmHg) | 36±15 | 44±13 | 46±17 | <0.0001a |
| Stable angina/ACS, n (%) | 29 (31.5)/63 (68.5) | 30 (35.3)/55 (64.7) | 22 (25.9)/63 (74.1) | 0.41 |
| EF normal, n(%) | 66 (71.7) | 57 (67.1) | 55 (64.7) | 0.59 |
| Medications at discharge | ||||
| Aspirin, n (%) | 91 (98.9) | 94 (98.8) | 84 (98.8) | 0.99 |
| Clopidogrel, n (%) | 76 (82.6) | 80 (94.1) | 70 (82.4) | 0.04 |
| Beta-blocker, n (%) | 67 (72.8) | 62 (72.9) | 63 (74.1) | 0.98 |
| ACE-inhibitor/ARB, n (%) | 51 (55.4) | 43 (50.6) | 35 (41.2) | 0.16 |
| CCB, n (%) | 14 (15.2) | 6 (7.1) | 6 (7.1) | 0.11 |
| Nitrates, n (%) | 32 (34.8) | 30 (35.3) | 16 (18.8) | 0.03 |
| Statin, n (%) | 71 (77.2) | 62 (72.9) | 61 (71.8) | 0.69 |
Note: ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
aOn the basis of Bonferroni's correction, a P-value less than 0.0017 indicates statistical significance.
Clinical characteristics of the patients, according to AIx@75-tertiles
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| AIx@75 | −19–18 | 19–25 | 26–53 | |
| Male, n (%) | 81(88.0) | 59 (69.4) | 45 (52.9) | <0.0001a |
| Age (years) | 62.3±9.8 | 66.8±8.6 | 67.9±11.3 | <0.001a |
| Prior MCI, n (%) | 11 (11.9) | 16 (18.8) | 17 (20) | 0.30 |
| Peripheral arterial disease, n (%) | 3 (3.3) | 11 (12.9) | 11 (12.9) | 0.04 |
| Hypertension, n (%) | 67 (72.8) | 61 (71.8) | 60 (70.6) | 0.95 |
| Diabetes, n (%) | 17 (18.5) | 23 (27.1) | 20 (23.5) | 0.39 |
| Smoking, n (%) | 21 (22.8) | 19 (22.4) | 16 (18.8) | 0.78 |
| Body mass index (kg/m2) | 28.6±4.1 | 27.1±3.5 | 26.9±3.3 | 0.005 |
| Total cholesterol (mg/dl) | 200.8±44.5 | 200.8±39.0 | 209.6±43.2 | 0.20 |
| LDL cholesterol (mg/dl) | 119.3±41.9 | 127.8±36.7 | 129.3±39.3 | 0.19 |
| HDL cholesterol (mg/dl) | 43.2±11.6 | 45.3±11.4 | 45.9±13.9 | 0.61 |
| Triglycerides (mg/dl) | 184.3±98.7 | 149.7±82.0 | 144.0±68.5 | 0.007 |
| Creatinine-clearance (ml/min) | 78.5±22.6 | 66.2±20.0 | 66.9±22.2 | <0.0001a |
| Hs-C-reactive protein (mg/dl) | 8.7±25.6 | 5.9±10.4 | 13.6±33.0 | 0.01 |
| Heart rate (b.p.m.) | 67.9±12.6 | 65.7±12.0 | 64.0±10.4 | 0.14 |
| Brachial SBP (mmHg) | 127±23 | 135±20 | 133±23 | 0.005 |
| Brachial DBP (mmHg) | 75±14 | 79±15 | 78±14 | 0.45 |
| Brachial PP (mmHg) | 51±19 | 57±17 | 55±18 | 0.02 |
| Estimated aortic systolic BP (mmHg) | 112±20 | 123±18 | 125±22 | <0.0001a |
| Estimated aortic diastolic BP (mmHg) | 76±14 | 79±15 | 79±14 | 0.43 |
| Estimated aortic PP (mmHg) | 36±15 | 44±13 | 46±17 | <0.0001a |
| Stable angina/ACS, n (%) | 29 (31.5)/63 (68.5) | 30 (35.3)/55 (64.7) | 22 (25.9)/63 (74.1) | 0.41 |
| EF normal, n(%) | 66 (71.7) | 57 (67.1) | 55 (64.7) | 0.59 |
| Medications at discharge | ||||
| Aspirin, n (%) | 91 (98.9) | 94 (98.8) | 84 (98.8) | 0.99 |
| Clopidogrel, n (%) | 76 (82.6) | 80 (94.1) | 70 (82.4) | 0.04 |
| Beta-blocker, n (%) | 67 (72.8) | 62 (72.9) | 63 (74.1) | 0.98 |
| ACE-inhibitor/ARB, n (%) | 51 (55.4) | 43 (50.6) | 35 (41.2) | 0.16 |
| CCB, n (%) | 14 (15.2) | 6 (7.1) | 6 (7.1) | 0.11 |
| Nitrates, n (%) | 32 (34.8) | 30 (35.3) | 16 (18.8) | 0.03 |
| Statin, n (%) | 71 (77.2) | 62 (72.9) | 61 (71.8) | 0.69 |
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| AIx@75 | −19–18 | 19–25 | 26–53 | |
| Male, n (%) | 81(88.0) | 59 (69.4) | 45 (52.9) | <0.0001a |
| Age (years) | 62.3±9.8 | 66.8±8.6 | 67.9±11.3 | <0.001a |
| Prior MCI, n (%) | 11 (11.9) | 16 (18.8) | 17 (20) | 0.30 |
| Peripheral arterial disease, n (%) | 3 (3.3) | 11 (12.9) | 11 (12.9) | 0.04 |
| Hypertension, n (%) | 67 (72.8) | 61 (71.8) | 60 (70.6) | 0.95 |
| Diabetes, n (%) | 17 (18.5) | 23 (27.1) | 20 (23.5) | 0.39 |
| Smoking, n (%) | 21 (22.8) | 19 (22.4) | 16 (18.8) | 0.78 |
| Body mass index (kg/m2) | 28.6±4.1 | 27.1±3.5 | 26.9±3.3 | 0.005 |
| Total cholesterol (mg/dl) | 200.8±44.5 | 200.8±39.0 | 209.6±43.2 | 0.20 |
| LDL cholesterol (mg/dl) | 119.3±41.9 | 127.8±36.7 | 129.3±39.3 | 0.19 |
| HDL cholesterol (mg/dl) | 43.2±11.6 | 45.3±11.4 | 45.9±13.9 | 0.61 |
| Triglycerides (mg/dl) | 184.3±98.7 | 149.7±82.0 | 144.0±68.5 | 0.007 |
| Creatinine-clearance (ml/min) | 78.5±22.6 | 66.2±20.0 | 66.9±22.2 | <0.0001a |
| Hs-C-reactive protein (mg/dl) | 8.7±25.6 | 5.9±10.4 | 13.6±33.0 | 0.01 |
| Heart rate (b.p.m.) | 67.9±12.6 | 65.7±12.0 | 64.0±10.4 | 0.14 |
| Brachial SBP (mmHg) | 127±23 | 135±20 | 133±23 | 0.005 |
| Brachial DBP (mmHg) | 75±14 | 79±15 | 78±14 | 0.45 |
| Brachial PP (mmHg) | 51±19 | 57±17 | 55±18 | 0.02 |
| Estimated aortic systolic BP (mmHg) | 112±20 | 123±18 | 125±22 | <0.0001a |
| Estimated aortic diastolic BP (mmHg) | 76±14 | 79±15 | 79±14 | 0.43 |
| Estimated aortic PP (mmHg) | 36±15 | 44±13 | 46±17 | <0.0001a |
| Stable angina/ACS, n (%) | 29 (31.5)/63 (68.5) | 30 (35.3)/55 (64.7) | 22 (25.9)/63 (74.1) | 0.41 |
| EF normal, n(%) | 66 (71.7) | 57 (67.1) | 55 (64.7) | 0.59 |
| Medications at discharge | ||||
| Aspirin, n (%) | 91 (98.9) | 94 (98.8) | 84 (98.8) | 0.99 |
| Clopidogrel, n (%) | 76 (82.6) | 80 (94.1) | 70 (82.4) | 0.04 |
| Beta-blocker, n (%) | 67 (72.8) | 62 (72.9) | 63 (74.1) | 0.98 |
| ACE-inhibitor/ARB, n (%) | 51 (55.4) | 43 (50.6) | 35 (41.2) | 0.16 |
| CCB, n (%) | 14 (15.2) | 6 (7.1) | 6 (7.1) | 0.11 |
| Nitrates, n (%) | 32 (34.8) | 30 (35.3) | 16 (18.8) | 0.03 |
| Statin, n (%) | 71 (77.2) | 62 (72.9) | 61 (71.8) | 0.69 |
Note: ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
aOn the basis of Bonferroni's correction, a P-value less than 0.0017 indicates statistical significance.
Angiographic characteristics of the patients, according to AIx@75-tertiles
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| Three vessel diasease, n (%) | 12 (13.0) | 14 (16.5) | 13 (15.3) | 0.81 |
| PTCA, n (%) | 72 (78.3) | 66 (77.6) | 72 (84.7) | 0.44 |
| PTCA only, n (%) | 21 (22.8) | 10 (11.8) | 15 (17.6) | 0.16 |
| Stent, n (%) | 71 (77.2) | 75 (88.2) | 70 (82.4) | 0.16 |
| Direct stenting, n (%) | 20 (21.7) | 19 (22.4) | 20 (23.5) | 0.96 |
| Brachytherapy, n (%) | 2 (2.2) | 2 (2.4) | 1 (1.2) | 0.83 |
| More than one lesion treated, n (%) | 13 (14.1) | 11 (12.9) | 12 (14.1) | 0.97 |
| More than one vessel treated, n (%) | 7 (7.6) | 8 (9.4) | 10 (11.8) | 0.64 |
| Restenosis treated, n (%) | 9 (9.8) | 5 (5.9) | 13 (15.3) | 0.13 |
| VBP treated, n (%) | 2(2.2) | 3(3.5) | 0 (0) | 0.28 |
| Lesion B2 or C, n (%) | 45 (48.9) | 37 (43.5) | 45 (52.9) | 0.47 |
| Unsuccessful PCI, n (%) | 9 (9.8) | 3 (3.5) | 3 (3.5) | 0.12 |
| GP IIb/IIIa antagonist use n (%) | 9 (9.8) | 9 (10.6) | 14 (16.5) | 0.34 |
| MLD pre-procedure (mm) | 0.84±0.35 | 0.90±0.47 | 0.80±0.42 | 0.18 |
| Diameter stenosis pre-procedure (%) | 68.16±12.56 | 66.76±16.79 | 69.51±15.12 | 0.16 |
| Lesion length (mm) | 10.97±4.39 | 11.15±5.09 | 10.66±3.90 | 0.94 |
| MLD post-procedure (mm) | 2.41±0.82 | 2.43±0.68 | 2.46±0.64 | 0.66 |
| Reference diameter (mm) | 3.01±0.53 | 2.96±0.51 | 2.97±0.53 | 0.76 |
| Diameter stenosis post-procedure (%) | 21.44±22.82 | 18.50±17.78 | 17.57±14.45 | 0.88 |
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| Three vessel diasease, n (%) | 12 (13.0) | 14 (16.5) | 13 (15.3) | 0.81 |
| PTCA, n (%) | 72 (78.3) | 66 (77.6) | 72 (84.7) | 0.44 |
| PTCA only, n (%) | 21 (22.8) | 10 (11.8) | 15 (17.6) | 0.16 |
| Stent, n (%) | 71 (77.2) | 75 (88.2) | 70 (82.4) | 0.16 |
| Direct stenting, n (%) | 20 (21.7) | 19 (22.4) | 20 (23.5) | 0.96 |
| Brachytherapy, n (%) | 2 (2.2) | 2 (2.4) | 1 (1.2) | 0.83 |
| More than one lesion treated, n (%) | 13 (14.1) | 11 (12.9) | 12 (14.1) | 0.97 |
| More than one vessel treated, n (%) | 7 (7.6) | 8 (9.4) | 10 (11.8) | 0.64 |
| Restenosis treated, n (%) | 9 (9.8) | 5 (5.9) | 13 (15.3) | 0.13 |
| VBP treated, n (%) | 2(2.2) | 3(3.5) | 0 (0) | 0.28 |
| Lesion B2 or C, n (%) | 45 (48.9) | 37 (43.5) | 45 (52.9) | 0.47 |
| Unsuccessful PCI, n (%) | 9 (9.8) | 3 (3.5) | 3 (3.5) | 0.12 |
| GP IIb/IIIa antagonist use n (%) | 9 (9.8) | 9 (10.6) | 14 (16.5) | 0.34 |
| MLD pre-procedure (mm) | 0.84±0.35 | 0.90±0.47 | 0.80±0.42 | 0.18 |
| Diameter stenosis pre-procedure (%) | 68.16±12.56 | 66.76±16.79 | 69.51±15.12 | 0.16 |
| Lesion length (mm) | 10.97±4.39 | 11.15±5.09 | 10.66±3.90 | 0.94 |
| MLD post-procedure (mm) | 2.41±0.82 | 2.43±0.68 | 2.46±0.64 | 0.66 |
| Reference diameter (mm) | 3.01±0.53 | 2.96±0.51 | 2.97±0.53 | 0.76 |
| Diameter stenosis post-procedure (%) | 21.44±22.82 | 18.50±17.78 | 17.57±14.45 | 0.88 |
Note: PTCA, balloon angioplasty; VBP, venous bypass graft; MLD, minimum lumen diameter.
Angiographic characteristics of the patients, according to AIx@75-tertiles
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| Three vessel diasease, n (%) | 12 (13.0) | 14 (16.5) | 13 (15.3) | 0.81 |
| PTCA, n (%) | 72 (78.3) | 66 (77.6) | 72 (84.7) | 0.44 |
| PTCA only, n (%) | 21 (22.8) | 10 (11.8) | 15 (17.6) | 0.16 |
| Stent, n (%) | 71 (77.2) | 75 (88.2) | 70 (82.4) | 0.16 |
| Direct stenting, n (%) | 20 (21.7) | 19 (22.4) | 20 (23.5) | 0.96 |
| Brachytherapy, n (%) | 2 (2.2) | 2 (2.4) | 1 (1.2) | 0.83 |
| More than one lesion treated, n (%) | 13 (14.1) | 11 (12.9) | 12 (14.1) | 0.97 |
| More than one vessel treated, n (%) | 7 (7.6) | 8 (9.4) | 10 (11.8) | 0.64 |
| Restenosis treated, n (%) | 9 (9.8) | 5 (5.9) | 13 (15.3) | 0.13 |
| VBP treated, n (%) | 2(2.2) | 3(3.5) | 0 (0) | 0.28 |
| Lesion B2 or C, n (%) | 45 (48.9) | 37 (43.5) | 45 (52.9) | 0.47 |
| Unsuccessful PCI, n (%) | 9 (9.8) | 3 (3.5) | 3 (3.5) | 0.12 |
| GP IIb/IIIa antagonist use n (%) | 9 (9.8) | 9 (10.6) | 14 (16.5) | 0.34 |
| MLD pre-procedure (mm) | 0.84±0.35 | 0.90±0.47 | 0.80±0.42 | 0.18 |
| Diameter stenosis pre-procedure (%) | 68.16±12.56 | 66.76±16.79 | 69.51±15.12 | 0.16 |
| Lesion length (mm) | 10.97±4.39 | 11.15±5.09 | 10.66±3.90 | 0.94 |
| MLD post-procedure (mm) | 2.41±0.82 | 2.43±0.68 | 2.46±0.64 | 0.66 |
| Reference diameter (mm) | 3.01±0.53 | 2.96±0.51 | 2.97±0.53 | 0.76 |
| Diameter stenosis post-procedure (%) | 21.44±22.82 | 18.50±17.78 | 17.57±14.45 | 0.88 |
| . | Tertile 1 (n=92) . | Tertile 2 (n=85) . | Tertile 3 (n=85) . | P-values . |
|---|---|---|---|---|
| Three vessel diasease, n (%) | 12 (13.0) | 14 (16.5) | 13 (15.3) | 0.81 |
| PTCA, n (%) | 72 (78.3) | 66 (77.6) | 72 (84.7) | 0.44 |
| PTCA only, n (%) | 21 (22.8) | 10 (11.8) | 15 (17.6) | 0.16 |
| Stent, n (%) | 71 (77.2) | 75 (88.2) | 70 (82.4) | 0.16 |
| Direct stenting, n (%) | 20 (21.7) | 19 (22.4) | 20 (23.5) | 0.96 |
| Brachytherapy, n (%) | 2 (2.2) | 2 (2.4) | 1 (1.2) | 0.83 |
| More than one lesion treated, n (%) | 13 (14.1) | 11 (12.9) | 12 (14.1) | 0.97 |
| More than one vessel treated, n (%) | 7 (7.6) | 8 (9.4) | 10 (11.8) | 0.64 |
| Restenosis treated, n (%) | 9 (9.8) | 5 (5.9) | 13 (15.3) | 0.13 |
| VBP treated, n (%) | 2(2.2) | 3(3.5) | 0 (0) | 0.28 |
| Lesion B2 or C, n (%) | 45 (48.9) | 37 (43.5) | 45 (52.9) | 0.47 |
| Unsuccessful PCI, n (%) | 9 (9.8) | 3 (3.5) | 3 (3.5) | 0.12 |
| GP IIb/IIIa antagonist use n (%) | 9 (9.8) | 9 (10.6) | 14 (16.5) | 0.34 |
| MLD pre-procedure (mm) | 0.84±0.35 | 0.90±0.47 | 0.80±0.42 | 0.18 |
| Diameter stenosis pre-procedure (%) | 68.16±12.56 | 66.76±16.79 | 69.51±15.12 | 0.16 |
| Lesion length (mm) | 10.97±4.39 | 11.15±5.09 | 10.66±3.90 | 0.94 |
| MLD post-procedure (mm) | 2.41±0.82 | 2.43±0.68 | 2.46±0.64 | 0.66 |
| Reference diameter (mm) | 3.01±0.53 | 2.96±0.51 | 2.97±0.53 | 0.76 |
| Diameter stenosis post-procedure (%) | 21.44±22.82 | 18.50±17.78 | 17.57±14.45 | 0.88 |
Note: PTCA, balloon angioplasty; VBP, venous bypass graft; MLD, minimum lumen diameter.
Arterial wave reflections in patients reaching the primary endpoint (death, MI, and clinical restenosis), individual endpoints, and in patients with uneventful course
| . | Patients reaching endpoint . | Patients not reaching endpoint . | P-values . |
|---|---|---|---|
| Primary endpoint | |||
| n | 61 | 201 | |
| AIx@75 | 25.8±10.8 | 20.2±9.9 | <0.001 |
| Individual endpoints | |||
| Death | |||
| n | 12 | 250 | |
| AIx@75 | 27.3±12.4 | 21.2±10.2 | 0.047 |
| Myocardial infarction | |||
| n | 22 | 240 | |
| AIx@75 | 27.3±10.0 | 21.0±10.3 | 0.02 |
| Clinical restenosis | |||
| n | 40 | 222 | |
| AIx@75 | 25.4±11.2 | 20.8±10.1 | 0.04 |
| . | Patients reaching endpoint . | Patients not reaching endpoint . | P-values . |
|---|---|---|---|
| Primary endpoint | |||
| n | 61 | 201 | |
| AIx@75 | 25.8±10.8 | 20.2±9.9 | <0.001 |
| Individual endpoints | |||
| Death | |||
| n | 12 | 250 | |
| AIx@75 | 27.3±12.4 | 21.2±10.2 | 0.047 |
| Myocardial infarction | |||
| n | 22 | 240 | |
| AIx@75 | 27.3±10.0 | 21.0±10.3 | 0.02 |
| Clinical restenosis | |||
| n | 40 | 222 | |
| AIx@75 | 25.4±11.2 | 20.8±10.1 | 0.04 |
Arterial wave reflections in patients reaching the primary endpoint (death, MI, and clinical restenosis), individual endpoints, and in patients with uneventful course
| . | Patients reaching endpoint . | Patients not reaching endpoint . | P-values . |
|---|---|---|---|
| Primary endpoint | |||
| n | 61 | 201 | |
| AIx@75 | 25.8±10.8 | 20.2±9.9 | <0.001 |
| Individual endpoints | |||
| Death | |||
| n | 12 | 250 | |
| AIx@75 | 27.3±12.4 | 21.2±10.2 | 0.047 |
| Myocardial infarction | |||
| n | 22 | 240 | |
| AIx@75 | 27.3±10.0 | 21.0±10.3 | 0.02 |
| Clinical restenosis | |||
| n | 40 | 222 | |
| AIx@75 | 25.4±11.2 | 20.8±10.1 | 0.04 |
| . | Patients reaching endpoint . | Patients not reaching endpoint . | P-values . |
|---|---|---|---|
| Primary endpoint | |||
| n | 61 | 201 | |
| AIx@75 | 25.8±10.8 | 20.2±9.9 | <0.001 |
| Individual endpoints | |||
| Death | |||
| n | 12 | 250 | |
| AIx@75 | 27.3±12.4 | 21.2±10.2 | 0.047 |
| Myocardial infarction | |||
| n | 22 | 240 | |
| AIx@75 | 27.3±10.0 | 21.0±10.3 | 0.02 |
| Clinical restenosis | |||
| n | 40 | 222 | |
| AIx@75 | 25.4±11.2 | 20.8±10.1 | 0.04 |
Multivariable predictors of the primary endpoint (death, MI, and clinical restenosis)
| . | Risk ratio . | 95% CI . | P-values . |
|---|---|---|---|
| Creatinine clearance (ml/min) | 0.97 | 0.95–0.99 | 0.02 |
| Body mass index (kg/m2) | 1.13 | 1.02–1.25 | 0.02 |
| Prior stroke | 3.68 | 1.43–9.47 | 0.007 |
| Beta-blocker | 0.29 | 1.15–0.58 | < 0.001 |
| Lesion type B2 or C | 1.86 | 1.01–3.44 | 0.05 |
| GP IIb/IIIa | 3.37 | 1.52–7.45 | 0.003 |
| AIx@75 per tertile | 1.80 | 1.18–2.76 | 0.006 |
| . | Risk ratio . | 95% CI . | P-values . |
|---|---|---|---|
| Creatinine clearance (ml/min) | 0.97 | 0.95–0.99 | 0.02 |
| Body mass index (kg/m2) | 1.13 | 1.02–1.25 | 0.02 |
| Prior stroke | 3.68 | 1.43–9.47 | 0.007 |
| Beta-blocker | 0.29 | 1.15–0.58 | < 0.001 |
| Lesion type B2 or C | 1.86 | 1.01–3.44 | 0.05 |
| GP IIb/IIIa | 3.37 | 1.52–7.45 | 0.003 |
| AIx@75 per tertile | 1.80 | 1.18–2.76 | 0.006 |
Multivariable predictors of the primary endpoint (death, MI, and clinical restenosis)
| . | Risk ratio . | 95% CI . | P-values . |
|---|---|---|---|
| Creatinine clearance (ml/min) | 0.97 | 0.95–0.99 | 0.02 |
| Body mass index (kg/m2) | 1.13 | 1.02–1.25 | 0.02 |
| Prior stroke | 3.68 | 1.43–9.47 | 0.007 |
| Beta-blocker | 0.29 | 1.15–0.58 | < 0.001 |
| Lesion type B2 or C | 1.86 | 1.01–3.44 | 0.05 |
| GP IIb/IIIa | 3.37 | 1.52–7.45 | 0.003 |
| AIx@75 per tertile | 1.80 | 1.18–2.76 | 0.006 |
| . | Risk ratio . | 95% CI . | P-values . |
|---|---|---|---|
| Creatinine clearance (ml/min) | 0.97 | 0.95–0.99 | 0.02 |
| Body mass index (kg/m2) | 1.13 | 1.02–1.25 | 0.02 |
| Prior stroke | 3.68 | 1.43–9.47 | 0.007 |
| Beta-blocker | 0.29 | 1.15–0.58 | < 0.001 |
| Lesion type B2 or C | 1.86 | 1.01–3.44 | 0.05 |
| GP IIb/IIIa | 3.37 | 1.52–7.45 | 0.003 |
| AIx@75 per tertile | 1.80 | 1.18–2.76 | 0.006 |
References
Safar ME, Smulyan H. Coronary ischemic disease, arterial stiffness, and pulse pressure.
Domanski MJ, Sutton-Tyrrell K, Mitchell GF, Faxon DP, Pitt B, Sopko G. Determinants and prognostic information provided by pulse pressure in patients with coronary artery disease undergoing revascularization. The Balloon Angioplasty Revascularization Trial (BARI).
Nakayama Y, Tsumura K, Yamashita N, Yoshimaru K, Hayashi T. Pulsatility of the ascending aortic pressure waveform is a powerful predictor of restenosis after percutaneous transluminal coronary angioplasty.
Ueda H, Nakayama Y, Tsumura K, Yoshimaru K, Hayashi T, Yoshikawa J. Inflection point of ascending aortic waveform is a powerful predictor of restenosis after percutaneous transluminal coronary angioplasty.
Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease.
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study.
London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure.
Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Aortic stiffness, wave reflections, and the risk of coronary artery disease.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine.
Smith SC Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, Kuntz RE, Popma JJ, Schaff HV, Williams DO, Gibbons RJ, Alpert JP, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO. ACC/AHA guidelines for percutaneous coronary intervention.
Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease: implications for patient selection.
Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans.
Eckert S, Gleichmann U, Zagorski O, Klapp A. Validation of the Omron R3 blood pressure self-measuring device through simultaneous comparative invasive measurements according to protocol 58130 of the German Institute of Validation.
The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction.
Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease.
Nichols WW, O'Rourke MF. Wave reflections. In: Nichols WW, O'Rourke MF, eds.
Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease.
Jiang XJ, O'Rourke MF, Jin WQ, Liu LS, Li CW, Tai PC, Zhang XC, Liu SZ. Quantification of glyceryl trinitrate effect through analysis of the synthesized ascending aortic pressure waveform.
Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, Shu YE, MacKay LS, Webb DJ, Cockcroft JR. Pulse wave analysis. Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function.
Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease.
Asmar RG, London GM, O'Rourke MF, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patients. A comparison with atenolol.
Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure.
London GM, Asmar RG, O'Rourke MF, Safar ME. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol.
The Heart Outcomes Prevention Evaluation Study Investigators. Effects of angiotensin-converting-enzyme inhibitor ramipril on cardiovascular events in high-risk patients.
Hirata K, Vlachopoulos C, Adji A, O'Rourke MF. Benefits ‘beyond blood pressure lowering’-beyond blood pressure or beyond the brachial artery.
Gersh BJ, Braunwald E, Bonow. Chronic coronary artery disease. In: Braunwald E, Zipes DP, Libby P, eds.