-
PDF
- Split View
-
Views
-
Cite
Cite
Kurt Huber, Raffaele De Caterina, Steen D. Kristensen, Freek W.A. Verheugt, Gilles Montalescot, Lina Badimon Maestro, Frans Van de Werf, for the Task Force on Pre-hospital Reperfusion Therapy of the Working Group on Thrombosis of the ESC, Pre-hospital reperfusion therapy: a strategy to improve therapeutic outcome in patients with ST-elevation myocardial infarction, European Heart Journal, Volume 26, Issue 19, October 2005, Pages 2063–2074, https://doi.org/10.1093/eurheartj/ehi413
Close - Share Icon Share
This paper was guest edited by Elliott M. Antman, Brigham and Women's Hospital, Cardiovascular Division, Boston, MA, USA
Introduction
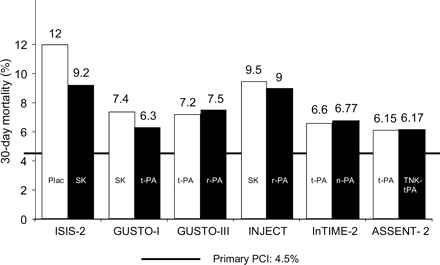
Therapy of acute ST-elevation myocardial infarction (STEMI) has undergone dramatic improvements during the past three decades, and in-hospital and 30-day mortality rates have tremendously decreased from >15–20% in the pre-thrombolytic area to 8–10% using fibrin-non-specific agents, to 6–8% using fibrin-specific thrombolytic agents, and down to 4.5% by the use of primary percutaneous coronary intervention (PPCI) in controlled trials by experienced centres under optimal trial conditions (Figure 1). However, translation of these results, obtained in the setting of controlled trials, into the real world, is different, as demonstrated by the results of registries. Registry data show mortality rates of >20% in patients who do not undergo reperfusion therapy within 12 h, of ∼10% in thrombolysed patients, and of up to 6–8% in patients treated by PPCI. The overall success rate depends not only on the individual patient's risk (high-risk patients, e.g. patients in shock, or the elderly, usually have not been included into controlled trials)1,2 but also on the optimal organization and the use of the available reperfusion strategies. Although thrombolytic therapy concerns remain with suboptimal patency, early reocclusion, and failure of myocardial reperfusion at the microcirculatory level,3 suboptimal results with PPCI are related to the lack of operator experience and excessive treatment delays.4 The mean door-to-balloon time in registries and the real world often exceeds 110–120 min, depending on the time of the day the patient is presenting and the availability of a cath lab and experienced interventionalists.5 Time to reperfusion plays an important role for myocardial salvage, and the concept of the ‘golden hour’, i.e. the optimal time window for initiation of treatment (within 1 h from the onset of pain), appears now to be applicable to all patients, including those treated by pharmacological reperfusion therapies.4
At present, PPCI is only available on an average for ∼15–20% of patients suffering from STEMI in the western world. Exceptions are well-developed areas where a 24 h availability of catheter facilities has been organized and the PPCI rate in STEMI patients can be up to >80%. Therefore, in most regions in the world in general, and in Europe in particular, thrombolytic therapy is still the fastest and best accessible reperfusion treatment for most patients presenting with acute STEMI, as indicated in the international guidelines.4,6 In addition, thrombolysis and PPCI are not mutually exclusive therapies, and attempts at combining them are ongoing.
Therefore, the main questions today are not whether percutaneous coronary intervention (PCI) is superior to thrombolytic therapy, but (i) what kind of reperfusion therapy is immediately available, dependent on the local situation; (ii) how to reduce time from onset of symptoms to reperfusion; and (iii) whether the combination of both strategies, immediate medical reperfusion followed by PCI (‘facilitated PCI’), will further improve the therapy of STEMI. Another important question is to elucidate which patients should be transferred to tertiary care units for primary PCI and to determine the optimal medical therapy during transport. These aspects are the focus of the this article. However, it should be realized that the most important goal today is still to offer more patients with STEMI any type of reperfusion therapy, because recent investigations have shown that almost one-third of eligible patients is not given reperfusion therapy at all.7
It was not the intent of the authors to make specific recommendations, given the absence of formal guidelines and need for more data. However, the current report should increase the awareness for the need and formulation of specific guidelines concerning pre-hospital reperfusion strategies in the near future. The report is based on the relevant literature after extensive search for related papers (MEDLINE, up to 5 years back) and after extensive communications between the members of the writing committee.
The ‘golden hour’
Thrombolytic therapy
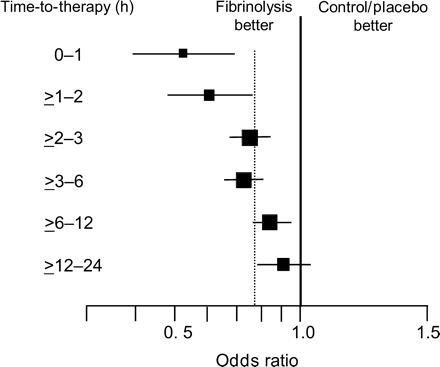
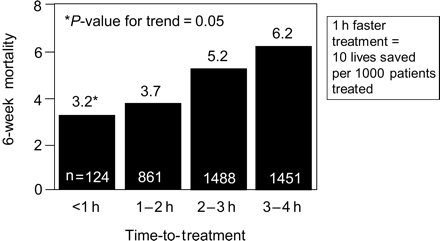
The maximum efficacy of thrombolytic therapy is achieved when the treatment is initiated within the first hour of symptom onset (65 lives saved per 1000 patients treated) (Figure 2).8 In the second hour after the onset of pain, this benefit is already reduced by 50%. Cannon et al.9 showed a 3.2% 6-week mortality when treatment was started within the first hour. A 1 h faster treatment may save about 10 additional patient lives per 1000 patients (Figure 3).9 A retrospective analysis of the pre-hospital Myocardial Infarction Triage and Intervention (MITI trial) showed a 7-fold reduction of 30-day mortality in patients treated within 70 min when compared with patients treated later (1.2 vs. 8.7%).10 More recently, an electrocardiographic (ECG) substudy of the second Assessment of the Safety and Efficacy of a New Thrombolytic Trial (ASSENT-2) revealed an independent association between the 1-year mortality and the ST-segment resolution on a 24–26 h ECG on the one hand, and a prognostic interaction of time-to-treatment and ST-segment resolution on the other. About 55.6% of patients treated within 2 h from symptom onset had complete ST-segment resolution when compared with 52.2% of patients treated within 2–4 h, and only 43% of patients treated between 4 and 6 h.11 In addition, the study also showed a consistent impact of time on mortality, with the lowest mortality rates in the earlier treatment groups, regardless of the extent of ST-segment resolution.11
Primary PCI and transferral for primary PCI
When compared with data generated in more than 58 000 patients treated with thrombolytic therapy, the role of early reperfusion with PPCI is less well-known, mostly owing to the relative small number of patients involved in PPCI trials, the lack of subgroup analyses investigating the impact of time on the outcome of PPCI, and also, partially, to some conflict in results.
The PRAGUE trial randomized 300 patients with ST elevation MI and symptoms of <6 h to treatment with streptokinase, treatment with streptokinase and transferral for ‘facilitated’ PCI, or transferral for PPCI.12 Transfer was safe, and transferral for PPCI was associated with a significantly lower incidence of the composite endpoint of death/re-infarction/stroke at 30 days when compared with the other treatment groups. This difference was mainly due to a very low incidence of re-infarction in the PPCI group.
In the AIRPAMI study, 138 high-risk acute STEMI patients with <12 h from symptom onset were randomized to either t-PA (plus 48 h of unfractionated heparin infusion) in the local hospital or transferred for PPCI.13 The primary endpoint was the combination of death, re-infarction, and disabling stroke within 30 days. The trial was stopped pre-maturely because of difficulties in recruiting patients. The transfer group had a 38% reduction in the primary endpoint, but this difference was not statistically significant. The time-to-treatment was delayed in the PPCI group, mainly due to a delay in the inititation of transport.
PRAGUE-2 was a multicentre trial performed in the Czech Republic.14 Eight-hundred and fifty patients with acute STEMI presenting within 12 h from symptom onset admitted to the nearest community hospital without PCI-facilities were randomized to either thrombolysis (streptokinase) in the local hospital or immediate transport for PPCI. The primary endpoint (30-day mortality) occurred in 6.8% in the PPCI group when compared with 10.0% in the streptokinase group (P=0.12). Among 299 patients who were randomized after >3 h from symptom onset, the 30-day mortality for primary PCI was 6 vs. 15.3% in the thrombolysis group (P<0.02). However, for patients randomized within 3 h from symptom onset (n=551), there was no difference in mortality between the two groups (PPCI: 7.3% and thrombolysis: 7.4%).
DANAMI-2 was a randomized multicentre Danish controlled trial that assigned 1572 patients to alteplase (plus 48 h of unfractionated heparin) or PPCI.15,16 The primary endpoint was a combination of death, clinical re-infarction, and disabling stroke at 30 days. A total of 1129 patients were enrolled at referral hospitals and 443 patients were randomized in centres with PCI facilities. Among patients who underwent randomization at referral centres, the primary endpoint occurred in 8.5% of the patients transferred for PPCI and in 14.2% of the patients treated with alteplase (P=0.002). In patients randomized in the hospitals with PCI-facilities, 6.7% in the PPCI group reached the primary endpoint when compared with 12.3% in the thrombolysis group (P=0.05). Among all patients, the better outcome of PPCI was driven by a reduction in re-infarction (1.6% in the PPCI vs. 6.3% in the thrombolysis group; P<0.001). No significant differences were observed in the rate of death (6.6 vs. 7.8%; P=0.35) or the rate of stroke (1.1 vs. 2.0%; P=0.15). The superiority of PPCI over thrombolysis was present in patient subgroups randomized within <2 h, between 2 and 4 h, and >4 h from the onset of symptoms. Unfortunately, <2% of patients in the alteplase group underwent ‘rescue’ PCI.
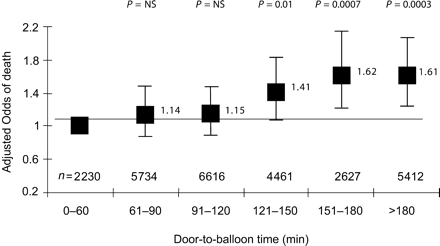
In these studies, besides the beneficial effects shown in patients transferred for PPCI, the loss of time during the organization of PPCI (door-to-needle time) was also demonstrated to be associated with an increase in mortality: the Analyses of the National Registry of Myocardial Infarction-2 trial (NRMI-2) indeed demonstrated a 41–62% increase in mortality with increases in the door-to-balloon time (Figure 4).17 Similar data were obtained from the GUSTO IIb study.18 In an analysis of the randomized controlled trials comparing a fibrin-specific agent with PPCI, Juliard et al.19 demonstrated that mortality increases significantly with each 15 min delay in the time between patient's arrival and restoration of TIMI-3 flow. In addition, time from symptom onset to balloon inflation is significantly correlated with 1-year mortality in patients undergoing PPCI (8% increase of relative risk for each 30 min delay; P=0.04).20,21
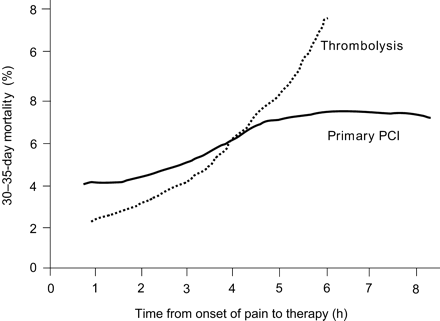
The pathophysiological evidence of the increase in myocardial necrosis wavefront over time and of the related increase in mortality with both treatment options clearly underscores the impact of time delay, regardless of whether pharmacological or mechanical reperfusion is used. The increase in mortality over time in relation with the start of reperfusion therapy with pharmacological vs. mechanical means is shown in Figure 5, compiling data from a meta-analysis of thrombolysis trials9 and the NRMI-2 results for mechanical reperfusion.17 Mortality rates after thrombolysis and PPCI are quite comparable, if both procedures are started within the first 2 h from symptom onset. Afterwards, mortality increases dramatically after thrombolysis, whereas there is a slighter increase of mortality over time with PPCI. This might be explained by the fact that successful PPCI immediately leads to optimal recanalization (TIMI-3 flow) in >90% of patients, whereas thrombolysis is less effective on ‘older’ clots, resulting in a TIMI-3 flow rate of only 50% or less. In addition, if successful, fibrinolysis requires at least an additional 30–45 min from the initiation of treatment until vessel reperfusion.22 This makes the ‘golden hour’ so important for thrombolytic therapy, and still important—albeit less—for PPCI.
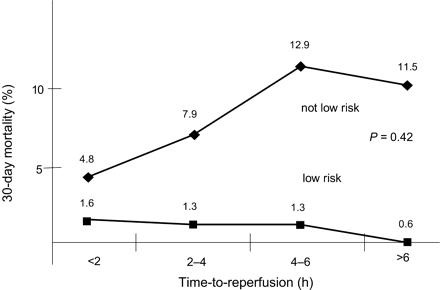
Antoniucci et al.23 have recently shown that the time-dependence of the outcome after PPCI is higher in high-risk STEMI patients than in patients at lower risk (Figure 6). Therefore, the logical conclusion is that STEMI patients with an early presentation (within 2 h from symptom onset) should be recanalized with the method which can be offered fastest (in most cases at present in Europe pre-hospital thrombolytic therapy), followed by transportation to a tertiary centre for ‘facilitated’ PCI of an already recanalized vessel or ‘rescue’ PCI for failed thrombolysis (discussed subsequently).
Components of time delay in reperfusion therapy
The time delay between the onset of chest pain and the initiation of reperfusion therapy on average is 2.5–3 h,24 and is composed of three main delay times: (i) the pre-hospital patient decision delay (1.5–3 h); (ii) the pre-hospital transportation delay (30–130 min); and (iii) the in-hospital delay, either the ‘door-to-needle’ time (up to 60–90 min in non-specialized hospitals) or the ‘door-to-balloon’ time (up to 2 h and more in non-specialized hospitals with catheter facilities).24 As in many cases, patients with an acute MI are initially admitted to a community hospital without catheterization facilities and then transferred to a tertiary medical centre for mechanical intervention, a further delay for transportation between hospitals has to be added.
The first of these three components, the pre-hospital patient decision delay, can be reduced to <1 h by community programmes of improved patient information.25 Unfortunately, the initial benefit seen in these programmes is not sustained, and also its cost-effectiveness has been questioned.26,27 Other investigations based on media campaigns have resulted in increased usage of medical services without significant reduction of the individual patient's delay.28
The last component of treatment delay, in-hospital delay, has been shortened to ∼20–30 min by establishing special in-hospital structures, e.g. by building up emergency departments with special equipment and specifically trained personnel and by allowing emergency physicians to administer thrombolytic therapy.29
Therefore, a further substantial shortening of time delays is only possible by bringing reperfusion therapy to the patient before the patient reaches the hospital. This will be now analysed in greater detail
Pre-hospital reperfusion strategies
Pre-hospital thrombolysis
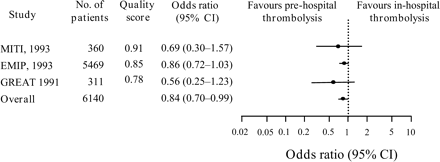
Pre-hospital initiation of thrombolytic therapy in STEMI has led to an additional reduction of the delay time between 33 and 130 min, as demonstrated in three high-quality pre-hospital thrombolysis trials: the EMIP, MITI, and GREAT trials.10,30,31 A meta-analysis of the six randomized trials comparing pre-hospital vs. in-hospital thrombolyis, involving 6434 patients, demonstrated that time-to-treatment was reduced by 58 min with pre-hospital therapy.32 This overall reduction in delay time was associated with a significant 1.7% absolute (17% relative) risk reduction of 30-day mortality, as shown by the three best performed pre-hospital thrombolysis trials (Figure 7). No significant safety risk was demonstrated for pre-hospital vs. in-hospital thrombolysis in these trials.32
Despite these interesting and favourable data, pre-hospital thrombolysis has not been organized on a widespread basis either in Europe or in North America, with the exception of a few countries.33 There are several possible reasons for the underuse of pre-hospital thrombolysis, including the inability of responsible persons or institutions to organize pre-hospital treatment in a quality-controlled fashion, but mostly such reasons revolve around budgetary restriction and selective preference for in-hospital allocations of resources. Pre-hospital thrombolysis has been shown to be beneficial, especially in rural areas with long transportation delays, whereas transportation times are considered to be short in urban areas. Thus, minimization of in-hospital delays appeared to many cardiologists and hospital administrators to be more important and effective, involving fewer logistics and expenditures than implementing pre-hospital thrombolysis.
Efficacy and safety of pre-hospital thrombolysis depend on several prerequisites: (i) STEMI has to be diagnosed by a 12-lead ECG, a diagnostic measure which only adds few minutes to the delay time, but helps to initate treatment appropriately, even in the hands of paramedics with or without computer assistance;34–38 (ii) ambulance personnel (physicians, nurses, and paramedics) has to be trained for symptom recognition and management of STEMI and its early complications; (iii) intravenous (i.v.) access has to be established and i.v. medications have to be administered according to a reperfusion therapy checklist; (iv) during transportation, rhythm monitoring and advanced cardiac life support are mandatory; and (v) finally, early contact with the referring hospital (e.g. by electronic transmission of the 12-lead ECG), appears to be necessary, allowing early preparation of further care, which also contribute to improving the outcome.33
The specific value of a mobile coronary care unit (MCCU), i.e. an ambulance equipped with a defibrillator, which can also record the 12-lead ECG, and with facilities to transmit the ECG digitally to the referring hospital, all manned by a trained, even junior, doctor and a coronary care nurse, has been recently emphasized.39 With such MCCUs, the response time to a call has been shown to be as low as 10 min.40 As mentioned by the authors, the patients managed pre-hospitally showed a significantly shorter median delay time from call for help to receive thrombolytic therapy (1.0 vs. 1.8 h; P<0.001), resulting in a shorter pain-to-needle time (2.3 vs. 4.0 h; P<0.001) and a consequently lower in-hospital mortality rate (7 vs. 13%; P=0.02). Patients aged ≥75 who received pre-hospital thrombolytic therapy had a significantly lower mortality than those first treated in-hospital (21 vs. 43%; P=0.02).
The administration of thrombolytics by infusion, as it was mostly available over the past years, has been, until recently, another obstacle for the implementation of quicker pre-hospital reperfusion strategies. The administration of thrombolysis by infusion resulted in 13.5% medication errors in the streptokinase arm, and 11.5% in the t-PA arm in GUSTO-I, and this was associated with a more than doubling of the 24 h mortality rate and a significant increase in 30-day mortality.41 A similar experience comes from the TIME-II trial, indicating that dosing errors with t-PA infusion were mostly seen in patients with a low body weight and suggesting that the need for weight adjustments in only a fraction of patients for t-PA was the major source of incorrect dosing, resulting in a higher number of intracranial haemorrhages.41 These data provide support for the observation that appropriate dosing of the fibrinolytic agent is important to optimize outcomes in acute MI, and that the use of simpler weight-adjusted dosing regimens, such as those associated with the single-bolus fibrinolytic agent tenecteplase, might improve the accuracy of dosing and, in turn, the therapeutic outcome.
Therefore, the availability of thrombolytic agents suitable for double- or single-bolus injection has led to new interest in the setting-up of clinical pre-hospital thrombolysis trials: The Early Retavase-Thrombolysis in Myocardial Infarction 19 (ER-TIMI-19) trial planned inclusion of 1000 patients, comparing open-label pre-hospital thrombolytic therapy (r-PA, double bolus 10+10 mg) with a retrospective group.40 The trial was stopped after only 315 patients had been recruited. A 31 min reduction of the delay time when compared with a historical control group was demonstrated.42
In the Comparison of Angioplasty and Pre-hospital Thrombolysis in Acute Myocardial Infarction (CAPTIM) trial, 1200 patients were planned to be randomized either to pre-hospital thrombolysis with accelerated t-PA or to pre-hospital triage to PPCI.43 The trial was stopped early due to slow inclusion of patients. Pre-hospital thrombolysis seemed to be advantageous in patients treated early (within 2 h of symptom onset), whereas PPCI had advantages in patients treated later (>2 h). However, it has to be mentioned that the lytic group also received ‘rescue’ PCI in one-third of cases. Advantages for PPCI consisted in fewer re-infarctions (thrombolysis: 3.7% vs. PPCI: 1.7%) and a reduction in disabling stroke (thrombolysis: 1.0% vs. PPCI: 0%). Bleeding complications were not statistically different between the study groups (thrombolysis: 0.5% vs. PPCI: 2.0%).
The third Assessment of the Safety and Efficacy of New Thrombolytic Regimens Pre-hospital Substudy (ASSENT 3+) enrolled more than 1600 patients, with patients randomized to pre-hospital TNK-tPA and either unfractionated heparin or enoxaparin as an adjunct antithrombotic agent.44 The impact of pre-hospital thrombolysis was compared with the results of a similar population from the ASSENT 3 main trial45 in a non-randomized comparison. Time-to-treatment was reduced in the pre-hospital treatment group by ∼45 min. Treatment with enoxaparin resulted in a 3.2% absolute risk reduction (P=0.08). However, intracerebral bleeding rate was significanly higher in enoxaparin treated patients when compared with unfractionated heparin (2.2 vs. 0.97%; P=0.047), which was primarily a function of age (enoxaparin group; >75 years: 6.8% vs. ≤75 years: 1.2%; P=0.01) and gender (higher bleeding rates in women; P=0.02). Accordingly, pre-hospital thrombolytic therapy with TNK-tPA and enoxaparin has been demonstrated to be beneficial and safe in patients ≤75 years of age compared with unfractionated heparin. In older patients (>75 years), the optimal dosage of adjunct enoxaparin is still unknown. The recently published ‘ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarcion’ have taken these results into account: accordingly, low molecular heparin is considered an acceptable alternative to unfractionated heparin as ancillary therapy for patiets of <75 years of age, whereas it is not recommended for patients of >75 years.4 The TIMI group has suggested that in the elderly, the dose of enoxaparin be reduced to 75% of the full-dose (0.75 mg/kg b.i.d. s.c.) and the initial bolus avoided. This regimen is currently being tested in the TIMI-25 ExTRACT trial, which also involves a pre-hospital treatment arm.
Recently, the impact on efficacy and safety of clopidogrel as adjunct to thrombolytic therapy with unfractionated heparin or enoxaparin has been tested in the CLARITY-TIMI-28 trial, which also offered the possibility of performing pre-hospital thrombolysis.46 The trial enrolled 3491 patients aged ≤75 who presented within 12 h after onset of STEMI and were randomly assigned to receive clopidogrel (300 mg loading dose, followed by 75 mg once daily) or placebo. Patients received a fibrinolytic agent (in 70% a fibrin-specific agent and in 30% streptokinase), aspirin, and heparin, and were scheduled to undergo angiography 48–120 h after the initiation of study medication. The primary efficacy endpoint consisted of an occluded infarct-related artery (TIMI flow grade 0 or 1) on angiography or death or recurrent MI before angiography. Clopidogrel-treated patients reached this endpoint in 15.0 vs. 21.7% in the placebo group (36% relative risk reduction; P<0.001). By 30 days, clopidogrel reduced the odds of the composite endpoint of cardiovascular death, recurrent MI, or urgent revascularization due to recurrent ischaemia by 20% (11.6 vs. 14.1%; P=0.03). Bleeding complications (intracerebral or major) were comparable in the two groups.
It is frequently discussed that pre-hospital thrombolysis is a typical and useful reperfusion strategy in rural areas with long transportation delays, and that primary PCI should be the therapy of choice as the best treatment of acute STEMI in urban or metropolitan areas where transportation delays might be of less importance. In contrast, it has been shown that, at present, also in urban areas, time from first medical contact to first balloon inflation with primary PCI often exceeds the optimal pre-intervention period of 90 min47 recommended in the recent guidelines.4,48 As shown in several trials, including the PRAGUE-2, STOPAMI-1 and -2, MITRA, MIR, and CAPTIM trials,14,43,49,50 this is of special interest in patients with a short duration of infarct symptoms (up to 3 h), because of the similar effectiveness of thrombolytic therapy when compared with primary PCI on mortality and infarct size in those patients. A recent study in Vienna clearly demonstrated the advantage of optimal organization of reperfusion strategies for acute STEMI:51 primary PCI was offered to 60% of consecutive patients with acute STEMI, but only 11% of patients receiving primary PCI were treated within 2 h of onset of pain. In contrast, >50% of patients treated with thrombolytic therapy received the treatment within 2 h of symptom onset. Thrombolysis (pre-hospital or in-hospital depending on the individual situation) was performed in 26% of patients with acute STEMI when transportation and organization times for acute PCI most likely seemed to exceed 90 min from first medical contact. The primary endpoint of the trial, total in-hospital mortality, was identical between thrombolytic treatment and primary PCI in patients treated within 3 h of symptom onset (6%). These data add further information to the need of pre-hospital thrombolytic therapy also in urban areas for STEMI patients within the first 3 h after onset of pain when extensive transportation delays cannot be excluded.
Moreover, it could be shown that the mortality benefit associated with primary PCI in acute STEMI may be lost if door-to-balloon time is delayed by >1 h when compared with thrombolytic therapy (tissue plasminogen activator) door-to-needle time. When a substantial delay in initiating primary PCI is likely, reperfusion therapy with second- or third-generation fibrinolytic agents should be strongly considered.52,53
‘Facilitated’ PCI
According to the current evidence (also reflected in the latest ESC and ACC/AHA Guidelines), primary PCI is the preferred treatment option in STEMI, if it can be performed within 90 min after the diagnosis by an experienced team (interventional cardiologists and skilled supporting staff) in a high-volume centre.4,6 Randomized trials comparing timely performed PPCI in experienced centres with thrombolytic therapy have demonstrated higher patency rates, less early reocclusion rates, better residual left ventricular function, and better clinical outcome.12,14,16,43,54–68
Keely et al.69 combined most of the published trials in an overview of fibrinolysis vs. PPCI (n=2909) by additional inclusion of the SHOCK study, which compared medical stabilization with immediate revascularization for cardiogenic shock:70 this analysis showed that PCI-treated patients experienced lower short-term mortality rates, fewer non-fatal MIs, and fewer haemorrhagic strokes than those treated with fibrinolysis, at the cost of an increased risk of major bleeding.69 Transfer for PPCI was also associated with a non-significant trend towards a decrease in death rate when compared with thrombolytic therapy (P=0.057), even when the SHOCK trial was excluded from calculations (PPCI: 5.5% vs. lysis: 6.7%; P=0.081).71
In another meta-analysis, Dalby et al.72 analysed the data from various trials comparing on-site thrombolyis with transferrals for PPCI (n=3750). The combined endpoint of death, re-infarction, and stroke was significantly reduced, by 42%, in the group transferred for PPCI when compared with the group that received on-site thrombolysis. Looking at the endpoints separately, re-infarction was significantly reduced by 68% and stroke was reduced by 56%. There was a trend towards a reduction of all-cause mortality by 19% at 30 days (P=0.08) in the PPCI group, despite the time delay for transferral.
Retrospective analysis of the subgroups revealed the existence of high-risk groups of patients with STEMI who might especially benefit from PPCI: this consisted of patients with >70 years of age, tachycardia at presentation, and an anterior wall infarction,59,73 and patients with cardiogenic shock.70 In this latter group, an absolute 9% reduction in 30-day mortality with coronary revascularization when compared with immediate medical stabilization was reported.70
In contrast to widely held beliefs, total costs after 1 year seem to be comparable between thrombolytic therapy and PPCI.74
The DANAMI-2 study included patients who could be transferred to a tertiary hospital within 3 h.15 However, 96% of the patients were transferred within 2 h.16 As this trial showed that transferral for primary PCI was superior to thrombolytic therapy, it might be argued that the useful window for transfer to a PPCI centre indicated in guidelines might theoretically be extended from 90 to 120 min. However, based on the results of an analysis of the randomized controlled trials, comparison of fibrinolysis with a fibrin-specific agent vs. PPCI showed that the mortality benefit of PPCI only exists when treatment is delayed by ≤60 min,19 which is in agreement with the recent European Guidelines.6 Also the recent ACC/AHA guidelines have reduced the medical contact-to-balloon or door-to-balloon time goal from 120 to 90 min, in an attempt to maximize the benefits of reperfusion with PPCI.4 The writing committee of the recent ACC/AHA Guidelines also openly stated that there is legitimate evidence-based concern that routine PPCI for patients with acute STEMI will result in unacceptable delays in achieving reperfusion in a substantial number of patients and also lead to suboptimal outcomes if performed by less-experienced interventionalists.4 Accordingly, in patients in whom PPCI is performed by non-specialized centres, outcome data for PPCI are not so impressive: TIMI-3 flow rates were <90%;75 in-hospital and 3-year mortality rates were comparable with those of thrombolytic therapy even in high-risk patients, and treatment costs were significantly higher.75 This is because mortality increases with increasing door-to-balloon times.17,18 Other studies have shown smaller infarct size, better left ventricular function, and fewer complications when reperfusion occurs before PCI.76–78
If PPCI is performed, routine stent implantation decreases the need for target vessel revascularization, but this had so far no significant impact on death or re-infarction rates.79–83
Early triage of candidates to PPCI and the pre-hospital initiation of pharmacological reperfusion therapy with subsequent transfer to coronary angiography (and angioplasty) might improve the efficacy of treatment. Therefore, the combination of early pharmacological reperfusion with subsequent mechanical optimization of the initial result (‘facilitated’ PCI) is a very attractive therapeutic concept that deserves further investigation. Facilitation can be achieved by the use of thrombolytic agents, glycoprotein IIb/IIIa antagonists, or a combination of the two. In addition, the administration of low molecular weight heparin, instead of standard heparin, is currently being tested for possible superiority.
Facilitation with thrombolytic agents alone or in conjunction with glycoprotein IIb/IIIa antagonists
Earlier trials combining full-dose thrombolytic therapy with subsequent PCI to improve reperfusion and/or reduce reocclusion all showed an unfavourable trend to more complications and an increased mortality rate.84–86 Owing to improved interventional methods and the availability of stents (for bail-out situations), as well as, possibly, of more potent antiplatelet agents (GP IIb/IIIa blockers) as adjunctive therapy, PCI following thrombolytic therapy has become more effective and relatively safe. However, a combination of different antithrombotic agents may lead to excessive bleeding complications, and careful dosing and patient selection are recommended. A meta-analysis of all published trials using combination therapy of thrombolytic agents and abciximab has shown that combination therapy leads to severe bleeding rates, which are increased by >50% in the combination arm when compared with thrombolytic monotherapy, without improving efficacy outcomes.87 Extremely high bleeding complications have been shown for streptokinase in combination with abciximab (TIMI 14 trial88), which should therefore not be further used.
In the PACT trial, in patients with acute STEMI treated with a bolus of 50 mg t-PA and subsequently undergoing coronary angiography, Ross et al.89 could demonstrate (i) a higher percentage of TIMI-2 and -3 flow at diagnostic angiography; (ii) no excess need for mechanical intervention due to TIMI-3 flow and low residual stenosis after successful thrombolysis; (iii) that PCI, if performed, was unproblematic; and (iv) that complication rates due to the combined treatment (thrombolysis plus PCI) were low and acceptable from the clinical point of view.89
In the GRACIA-2 trial, PPCI (108 patients) was compared with fibrinolysis with TNK-tpA (and enoxaparin) and subsequent PCI (104 patients).90 Primary endpoints were infarct size, time until ST-segment resolution, and the development of left ventricular dysfunction. The secondary combined endpoints consisted of death, non-fatal MI, or acute revascularization after 6 weeks and 6 months. Diagnostic angiography revealed an open infarct-related artery in 59% of patients pre-treated with TNK-tPA, but in only 14% of patients initially randomized to PPCI. Patients in the facilitated PCI group showed at 6 h a significanly faster reduction of initial ST-segement elevation (61 vs. 43%; P=0.03). No significant differences could be demonstrated as to infarct size and the secondary combined endpoints. However, there was a trend towards lower mortality, re-infarctions, and reintervention rates in patients submitted in whom PCI was facilitated with thrombolysis (9 vs. 14%).
Pre-PCI facilitation combining abciximab (full-dose) and reteplase (half-dose) is currently being tested (FINESSE and CARESS trials). In another trial, the ASSENT-4 PCI trial (Assessment of the Safety and Efficacy or a New Treatment Strategy for Acute Myocardial Infarction trial; 4000 patients planned; PPCI vs. TNK-tPA/enoxaparin-facilitated PCI), which was initiated worldwide to investigate the effectiveness and safety of primary PCI facilitated with full-dose TNK-tPA, inclusion of patients has been recently suspended due to a lower mortality rate in patients treated with primary PCI only.
Facilitation with potent antiplatelet agents
GP IIb/IIIa-receptor antagonists
Randomized trials with abciximab as adjunjunctive antiplatelet therapy during PCI of the infarct-related artery demonstrated the usefulness of this GP IIb/IIIa-blocker.
In the RAPPORT trial (482 patients), abciximab significantly improved the immediate clinical outcome (combination of death, MI, and urgent revascularization) and reduced the need for ‘bail-out’ stenting.91 However, bleeding complications also increased significantly in the abciximab group, as a result of the relatively high doses of adjunct i.v. unfractionated heparin.
In the ISAR-2 trial (401 patients), the use of abciximab in combination with a reduced dose of unfractionated heparin resulted in a significant reduction of the combined endpoint (death, re-infarction, and target lesion revascularization after 30 days), but did not reduce angiographic restenosis.92
In the double-blind ADMIRAL trial (300 patients), abciximab was given before sheath insertion in all patients, and one-fourth of the patients received the study drug early, in the emergency room or in the ambulance on the way to the cath lab.93 The drug improved the angiographic and clinical outcomes in the global population, with a particular benefit in the subgroup of patients treated early. Recent data have shown that there is a long-term benefit at 3 years in patients treated early with abciximab.94
However the largest study of this kind, the CADILLAC trial (2082 patients), demonstrated a beneficial effect of abciximab only for patients with balloon dilatation, but not for patients receiving stents.82 There was some benefit of abciximab vs. no abciximab on the combined endpoint of death, re-infarction, and target vessel revascularization at 30 days in this study, but the primary endpoint of death and re-infarction, both at 30 days and 6 months, was reduced non-significantly.95 In this study, a high-risk subgroup which was not included into the main trial (long, complicated lesions, multivessel disease, small vessels, and visible thrombus) showed some advantage of abciximab.
Recently, the ACE trial, performed in 400 patients with acute STEMI, reported on the use of abciximab in high-risk patients (cardiogenic shock, massive coronary thrombus load, main stem lesions, and significant stenoses of side branches related to the culprit lesion) who had been excluded in most other trials.96 The combined primary endpoint (death, re-infarction, urgent target vessel revascularization, and stroke at 30 days) was significanlty reduced in the abciximab group (4.5 vs. 10.5%; P=0.023). Early ST-segment resolution (≥50% after 30 min) occurred significantly more often (85 vs. 68%; P=0.001) in abciximab-treated patients, in whom also some smaller infarct size was shown at 30 days. After 6 months, the rate of the combined endpoint was still significantly lower in the abciximab arm of the trial (5.5 vs. 13.5%). Restenosis rate was comparable in the two groups.
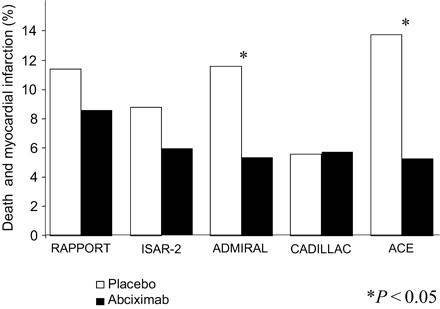
One should remember that inclusion criteria differed markedly in these five trials: patients with shock were only incuded in the ISAR-2, ADMIRAL, and ACE trials, whereas patients in the CADILLAC trial had a relatively low-risk profile, as confirmed by the low event rate in the placebo group. Figure 8 summarizes the combined endpoint of death and MI for the five aforementioned trials: with the exception of CADILLAC, all trials showed some benefit for patients treated with abciximab, which was statistically significant in those trials (ADMIRAL and ACE trials), in which abciximab was initiated early before primary PCI.
Three smaller trials have investigated the parameters which could help in understanding mechanisms underlying the benefit of early abciximab use: Zorman et al.97 demonstrated a higher patency rate at diagnostic angiography in patients with acute MI pre-treated with abciximab when compared with patients without pre-treatment. In the REOMOBILE trial, Arntz et al.98 studied 100 patients randomized to an early administration of abciximab in the mobile intensive care unit vs. the administration at the time of intervention in the cath lab. Only a moderate trend in favour of early initiation of abciximab was shown for ST-segment resolution after 60–90 min, TIMI-2+3 flow and TIMI blush score at diagnostic angiography, and TIMI-3 flow after PCI.98 Finally, the ReoPro-BRIDGING trial99 showed significantly improved surrogate parameters for early reperfusion when abciximab was administered in the emergency room when compared with reperfusion immediately before primary PCI: pre-PPCI ST-segment resolution: 55±21.4 vs. 42.4±18.2%; P=0.005; TIMI-3 flow grade: 29 vs. 7%; P=0.042; corrected TIMI frame count: 58.4±32.7 vs. 78.9±28.4 frames; P=0.018; per cent diameter stenosis (median 76.3 vs. 100; P=0.023).99
Recently, a meta-analysis of the different trials comparing early adminstration of abciximab vs. administration in the cath lab confirmed the favourable outcome of early abciximab use on surrogate parameters and clinical endpoints (especially on re-infarction), but failed to demonstrate a significant reduction of mortality.100 Overall, these data on the use of abciximab before PPCI are promising, but its routine administration before PPCI is still a matter of discussion.
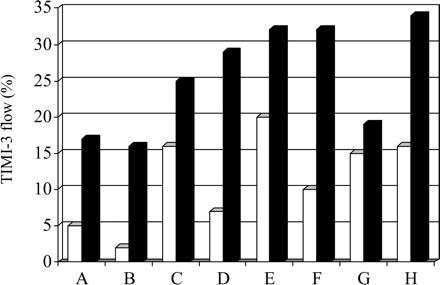
When compared with abciximab, less data on tirofiban (Cutlip et al.,101 TIGER-PA pilot trial,102 and OnTime Trial103) and eptifibatide (INTAMI trial 104) in PPCI are currently available, but have demonstrated similar improvements in the TIMI-3 flow rate at first diagnostic angiogram, when treatment with the respective GP IIb/IIIa-antagonist was initiated early (Figure 9).
Clopidogrel
Clopidogrel as adjunctive antithrombotic agent has been shown to be effective in patients with non-ST-elevation acute coronary syndrome at low, medium, and high risk for thrombo-embolic complications. Steinhubl et al.105 in the CREDO trial have shown a beneficial effect of clopidogrel 300 mg loading dose followed by 75 mg/day if administered >6 h before PCI with stent implantation when compared with later administration.
Data from experimental studies suggest a much faster (within 2 h) maximum action of clopidogrel if the drug is given with a high loading dosage, which has been demonstrated to be safe.106
On the basis of the promising preliminary data,107,108 high-dose clopidogrel (600 mg loading dose, followed by 150 mg/day), given before PCI, has been investigated in chronic stable low-risk patients in the ISAR-REACT-1 trial,109 showing that it may render the use of abciximab before PCI in these patients unnecessary, and is at the moment being tested in patients with NSTEMI in the ongoing ISAR-REACT-2 study, and in patients with STEMI (BRAVE-3 trial) comparing patients receiving such a treatment with patients receiving abciximab immediately before PPCI. The use of clopidogrel in the pre-hospital setting in patients with STEMI is of potential interest but needs further testing in randomized prospective trials.
Summary
Although the medical and technological revolution in the last three decades has improved clinical outcome in patients presenting with acute STEMI, residual morbidity and mortality are still high. The critical role of treatment delay and optimal sustained patency are prerequisites of successful reperfusion. Clinical efficacy of successful reperfusion has been repeatedly demonstrated. Nevertheless, the ideal pharmacological and mechanical reperfusion methods are still a matter of major debate.
Dependent on the local situation, either immediate thrombolytic therapy or fast transfer to an experienced high-volume tertiary care centre for PPCI has to be preferred. According to the mortality data, pre-hospital but also in-hospital thrombolysis has success rates comparable with PPCI when intitiated within the first 2–3 h after the onset of pain. Therefore, in these patients, thrombolytic therapy should not be withheld in favour of mechanical reperfusion if it cannot be offered within 90 min. The situation seems to be somewhat different in patients presenting later than 3 h after the onset of pain. These patients have a mortality rate of ∼6–8% when treated by mechanical reperfusion. Mortality rates increase exponentially with every hour delay, when treated with thrombolysis. In contrast, for patients with a longer delay from symptom onset, a further time delay for transfer to a tertiary care hospital with catheter facilities seems to be acceptable and less deleterious.
Outcomes can be further improved by pre-hospital administration of lytic therapy when transportation and organization delays for PPCI can be expected. Whether or not immediate PCI should be performed following hospital arrival in patients with clinical signs of successful lysis is still not fully clarified. Although the advantages of pre-hospital thrombolysis have been well-known for many years, this therapy has not become a common practice for various reasons. This is a missed opportunity for many patients, especially those in rural areas, requiring long transportation times to the referral hospitals, although thrombolysis, when left alone, is still a suboptimal therapy. Efforts should therefore be made to improve this situation, wherever possible, by organizing quick transportation to centres equipped for PPCI and with an experienced staff. Investigations have clearly shown that a well-organized pre-hospital pharmacological reperfusion strategy can save lives and positively influence other clinical outcomes.
Besides pre-hospital thrombolysis, and because of better overall results, especially pre-hospital ‘facilitation’ of PCI is currently under investigation. Facilitation can be performed theoretically by use of thrombolytic drugs, by use of a combined approach (thrombolytics 1/2 dose+GP IIb/IIIa-inhibition; now fallen mostly out of credit), or by pre-treatment with GP IIb/IIIa-blockers alone. The combined use of pharmacological and mechanical reperfusion might be the optimal treatment principle, but still questions with regard to safety (major bleeding complications), particulary in older patients, and of efficacy remain unsolved. Alterations in the dosages of the various antithrombotic agents have to be further tested before pre-PCI pharmacological treatment can become a general treatment option.
Acknowledgements
The current report was sponsored by a grant of the ESC allowing frequent get-together meetings of the members of the writing committee.
Figure 1 Major trials comparing 30- or 35-day mortality among fibrinolytics vs. primary PCI. SK, streptokinase; t-PA, tissue plasminogen activator (alteplase); r-PA, reteplase; n-PA, lanoteplase; TNK-tPA, tenecteplase. Adapted from Huber and Maurer.24
Figure 2 Influence of time-to-treatment on the Odds ratio (OR) of mortality. Adapted from Boersma et al.8
Figure 3 Effect of time-to-treatment on 6-week mortality. Adapted from Cannon et al.9
Figure 4 Primary PCI: door-to-balloon time vs. in-hospital mortality. Data from the NRMI-2 registry, adapted from Cannon et al.17
Figure 6 Impact of time-to-reperfusion on 30-day mortality in patients with acute MI at low and ‘not low’ risk. Adapted from Antoniucci et al.23
Figure 7 Pre-hospital vs. in-hospital thrombolysis trials. Odds ratio for 30-day mortality. Only data from the three highest-quality trials are given. Adapted from Morrison et al.32
Figure 8 Abciximab in primary PCI: death or MI at 6 months. Data are presented from the five largest trials performed with the adjunctive use of this drug.
Figure 9 Early vs. late use of GP IIb/IIIa-antagonists in patients with ST-elevation MI treated with primary PCI: TIMI-3 flow at diagnostic angiogram. Black bars represent early treatment groups (inititation of treatment in the emergency room or pre-hopsital) and white bars represent late treatment groups (inititation of treatment immediately before PCI). Abciximab was used in trials A–D (A: ADMIRAL;93 B: Zorman et al.;97 C: ReoMobile;98 and D: ReoPro-BRIDGING99), tirofiban in trials E–G (E: Cutlip et al.;101 F: TIGER-PA;102 and G: OnTIME103), and eptifibatide in trial H (INTAMI104), respectively.
References
Granger CB, Goldberg RJ, Dabbous O et al. for the Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the Global Registry of Acute Coronary Events.
Morrow DA, Antman EM, Charlesworth A et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy.
Roe MT, Ohman EM, Maas AC et al. Shifting the open artery hypothesis downstream: the quest for optimal reperfusion.
Antman EM, Anbe DT, Armstrong P et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarcion—executive summary.
Tiefenbrunn AJ, Chandra NC, French WJ, Gore JM, Rogers WJ. Clinical experience with primary percutaneous transluminal angioplasty compared with alteplase (recombinant tissue-type plasminogen activator) in patients with acute myocardial infarction. A report from the Second National Registry of Myocardial Infarction (NRMI-2).
Van de Werf F, Ardissino D, Betriu A et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology.
Barron HV, Bowlby LJ, Breen T et al. Use of reperfusion therapy for acute myocardial infarction in the United States: data from the National Registry of Myocardial Infarction 2.
Boersma E, Maas ACP, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour.
Cannon CP, Antman EM, Walls R, Braunwald E. Time as an adjunctive agent to thrombolytic therapy.
Weaver WD, Cerqueira M, Hallstrom AP et al. for the Myocardial Infarction Triage and Intervention Project Group. Prehospital-initiated vs hospital-initiated thrombolytic therapy. The Myocardial Infarction Triage and Intervention Trial.
Fu Y, Goodman S, Chang WC et al. Time to treatment influences the impact of ST-segment resolution on one-year prognosis: insights from the assessment of the safety and efficacy of a new thrombolytic (ASSENT-2) trial.
Widimsky P, Groch L, Zelizko M et al. Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs. combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE Study.
Grines CL, Westerhausen DRJ, Grines LL et al. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: the Air Primary Angioplasty in Myocardial Infarction study.
Widimsky P, Budesinsky T, Vorac D et al., on behalf of the PRAGUE Study Group Investigators. Long distance transport for primary angioplasty vs. immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2.
Andersen HR, Nielsen TT, Vesterlund T et al. The Danish multicenter randomized study on fibrinolytic therapy versus acute coronary angioplasty in acute myocardial infarction: rationale and design of the DANAMI-2 trial.
Andersen HR, Nielsen TT, Rasmussen K et al. for the DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction.
Cannon CP, Gibson CM, Lambrew CT. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction.
Berger PB, Ellis SG, Holmes DR. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the Global Use of Strategies To Open Occluded Arteries in Acute Coronary Syndromes (GUSTO-IIb) trial.
Juliard JM, Feldman LJ, HGolmard JL et al. Relation of mortality of primary angioplasty during acute myocardial infarction to door-to-Thrombolyis in Myocardial Infarction (TIMI) time.
De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction. Every minute of delay counts.
Antoniucci D, Valenti R, Migliorini A et al. Relation of time to treatment and mortality in patients with acute myocardial infarction undergoing primary coronary angioplasty.
Huber K, Maurer G. Thrombolytic therapy in acute myocardial infarction.
Herlitz J, Hartford M, Blohm M, Risenfors M, Karlsson BW, Holmberg S. Effect of a media campaign on delay times and ambulance use in suspected acute myocardial infarction.
Blohm M, Herlitz J, Hartford M et al. Consequences of a media campaign focusing on the delay in acute myocardial infarction.
Ho MT, Eisenberg MS, Litwin PE et al. Delay between onset of chest pain and seeking medical care: the effect of public education.
Luepker RV, Raczynski JM, Osganian S et al. for the REACT Study Group. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: the Rapid Early Action for Coronary Treatment (REACT) trial.
MacCallum AG, Stafford PJ, Jones C, Vincent R, Perez-Avila C, Chamberlain DA. Reduction in hospital time to thrombolytic therapy by audit of policy guidelines.
European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction.
Rawles J. on behalf of the GREAT Group. Halving of mortality at 1 year by domiciliary thrombolysis in the Grampian Region Early Anistreplase Trial (GREAT).
Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: a meta-analysis.
Welsh RC, Ornato J, Armstrong PW. Prehospital management of acute ST-elevation myocardial infarction: A time for reappraisal in North America.
Canto JG, Rogers WJ, Bowlby U et al. The prehospital electrocardiogram in acute myocardial infarction: Is its full potential being realized? National Registry of Myocardial Infarction 2 Investigators.
Karagounis L, Ipsen SK, Jessop MR et al. Impact of field-transmitted electrocardiography on time to in-hospital thrombolytic therapy in acute myocardial infarction.
Kudenchuk PJ, Ho MT, Weaver WD et al. Accuracy of computer-interpreted electrocardiography in selecting patients for thrombolytic therapy: MITI project investigators.
O'Rourke MF, Cook A, Carroll G et al. Accuracy of a portable interpretive ECG machine in diagnosis of acute evolving myocardial infarction.
Weaver WD, Eisenberg MS, Martin JS et al. Myocardial Infarction Triage and Intervention Project-phase I: patient characteristics and feasibility of pre-hospital initiation of thrombolytic therapy.
Mathew TP, Menown IBA, McCarthy D, Gracey H, Hill L, Adgey AAJ. Impact of pre-hospital care in patients with acute myocardial infarction compared with those first managed in-hospital.
Cannon CP. Exploring the issues of appropriate dosing in the treatment of acute myocardial infarction: potential benefits of bolus fibrinolytic agents.
Morrow DA, Antman EM, Sayah AJ et al. Evaluation of the time saved by pre-hospital initiation of reteplase for ST-elevation myocardial infarction: results of the early retavase-thrombolysis in myocardial infarction (ER-TIMI) 19 trial.
Bonnefoy E, Lapostolle F, Leizorovicz A et al. for the Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial Infarction (CAPTIM) study group. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomized study.
Wallentin L, Goldstein P, Armstrong PW et al. Efficacy and safety of tenecteplase in combination with the low-molecular-weight heparin enoxaparin or unfractionated heparin in the prehospital setting. The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 PLUS Randomized Trial in Acute Myocardial Infarction.
The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomized trial in acute myocardial infarction.
Sabatine MS, Cannon CP, Gibson M et al. for the CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation.
Nallamothu BK, Bates ER, Herrin J, Wang Y, Bradley EH, Krumholz HM. Times to treatment in transfer patients undergoing primary percutaneous coronary intervention in the United States: National Registry of Myocardial Infarction (NRMI)-3/4 analysis.
Silber S, Albertsson P, Aviles F et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.
Schomig A, Ndrepepa G, Mehilli J et al. Therapy-dependent influence of time-to-treatment interval on myocardial salvage in patients with acute myocardial infarction treated with coronary artery stenting or thrombolysis.
Zahn R, Schiele R, Gitt AK et al. Impact of prehospital delay on mortality in patients with acute myocardial infarction treated with primary angioplasty and intravenous thrombolysis.
Kalla K, Kozanli I, Unger G et al. Relation of time to treatment and in-hospital mortality in patients with ST-segment elevation myocardial infarction: Vienna pilot study on mechanical versus medication reperfusion strategies. (Abstract 2511).
Nallamothu BK, Antman EM, Bates ER. Primary percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: does the choice of fibrinolytic agent impact on the importance of time-to-treatment?
Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything?
Akhras F, Ousa AA, Swann G et al. Primary coronary angioplasty or intravenous thrombolysis for patients with acute myocardial infarction? Acute and late follow-up results in a new cardiac unit. (Abstract).
Andersen HR, Nielsen TT, Rasmussen K et al. for the DANAMI-2 Investigators. Thrombolytic therapy vs. primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial.
de Boer MJ, Ottervanger JP, van t' Hof AW et al. for the Zwolle Myocardial Infarction Study Group. Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy.
Garcia E, Elizaga J, Perez-Castellano N et al. Primary angioplasty versus systemic thrombolysis in anterior myocardial infarction.
Gibbons RJ, Holmes DR, Reeder GS, Bailey KR, Hopfenspirger MR, Gersh BJ. Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction.
Grines CL, Browne KF, Marco J et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction.
Grinfield L, Berrocal D, Bellardi J et al. Fibrinolytics versus primary angioplasty in acute myocardial infarction (FAP): a randomized trial in a community hospital in Argentina. (Abstract).
The Global Use of Strategies to Open Occlude Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primars coronary angioplasty with tissue plasminogen activator for acute myocardial infarction.
Kastrati A, Mehilli J, Dirschinger J et al. for the Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction (STOPAMI-2) Study. Myocardial salvage after coronary stenting plus abciximab versus fibrinolysis plus abciximab in patients with acute myocardial infarction: a randomized trial.
Le May MR, Labinaz M, Davies RF et al. Stenting versus thrombolysis in acute myocardial infarction (STAT).
Ribeiro EE, Silva LA, Carneiro R et al. Randomized trial of direct coronary angioplasty versus intavenous streptokinase in acute myocardial infarction.
Ribichini F, Steffenino G, Dellavalle A et al. Comparison of thrombolytic therapy and primary coronary angioplasty with liberal stenting for inferior myocardial infarction with precordial ST-segment depression: immediate and long-term results of a randomized study.
Schömig A, Kastrati A, Dirschinger J et al. for the Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction Study Investigators. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction.
Vermeer F, Oude Ophuis AJ, van den Berg EJ et al. Prospective randomized comparison between thrombolysis, rescue PTCA, and primary PTCA in patients with extensive myocardial infarction admitted to a hospital without PTCA facilities: a safety and feasibility study.
Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction.
Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials.
Hochman JS, Sleeper LA, Webb JG et al. for the SHOCK Investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock.
Dalby M, Bouzamando A, Lechat P et al. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction.
Stone GW, Grines CL, Browne KF et al. Predictors of in-hospital and 6-months outcome after acute myocardial infarction in the reperfusion era: the Primary Angioplasty in Myocardial Infarction (PAMI) Trial.
Reeder GS, Bailey KR, Gersh BJ, Holmes DR, Christianson J, Gibbons RJ. Cost comparison of immediate angioplasty versus thrombolysis followed by conservative therapy for acute myocardial infarction: a randomized prospective trial.
Every NR, Parsons LS, Hlatky M, Martin JS, Weaver WD. A comparison of thrombolytic therapy with primary coronary angioplasty for acute myocardial infarction.
Brodie BR, Stuckey TD, Hansen C et al. Benefit of coronary reperfusion before intervention on outcomes after primary angioplasty for acute myocardial infarction.
Clements IP, Christian TF, Higano ST et al. Residual flow to the infarct zone as a determinant of infarct size after direct angioplasty.
Stone GW, Cox DA, Garcia EJ et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials.
Grines CL, Cox DA, Stone GW et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction.
Maillard L, Hamon M, Khalife K et al. for the STENTIM-2 Investigators. A comparison of systematic stenting and conventional balloon angioplasty during primary percutaneous transluminal coronary angioplasty for acute myocardial infarction.
Scheller B, Hennen B, Severin-Kneib S et al. Long-term follow-up of a randomized study of primary stenting versus angioplasty in acute myocardial infarction.
Stone GW, Grines CL, Cox DA et al. for the CADILLAC Investigators. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction.
Zhu MM, Feit A, Chadow H et al. Primary stent implantation compared with primary balloon angioplasty for acute myocardial infarction: a meta-analysis of randomized clinical trials.
Simoons ML, Arnold AE, Betriu A et al. Thrombolysis with tissue plasminogen activator in acute myocardial infarction: no additional benefit from immediate PTCA.
The TIMI Research Group. Immediate vs delayed catheterization and angioplasty following thrombolytic therapy for acute myocardial infarction.
Topol EJ, Califf RM, George BS et al. A randomized trial of immediate versus delayed elective angioplasty after intravenous tissue plasminogen activator in acute myocardial infarction.
Verheugt FWA, Brouwer MA. Half dose lytic plus abciximab compared to full dose lytic in acute myocardial infarction; a meta-analysis.
Antman EM, Giugliano RP et al. for the TIMI 14 Investigators. Abciximab facilitates the rate and extent of thrombolysis: results of the Thrombolysis In Myocardial Infarction (TIMI) 14 Trial.
Ross AM, Coyne KS, Reiner JS. for the PACT-Investigators. A randomized trial comparing primary angioplasty with a strategy of short-acting thrombolysis and immediate planned rescue angioplasty in acute myocardial infarction: the PACT Trial.
Fernandez-Avilés F, Alonso JJ, Castro-Beiras, A et al. for the GRACIA-2 Investigators. Primary angioplasty versus facilitated intervention (tenecteplase plus stenting) in patients with ST elevation acute myocardial infarction: Final results of the GRACIA-2 trial. (Abstract
Brener SJ, Barr LA, Burchenal JE et al. on behalf of the ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) Investigators. Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction.
Neumann F-J, Kastrati A, Schmitt C. Effect of glycoprotein IIb/IIIa receptor blockade with abciximab on clinical and angiographic restenosis rate after the placement of coronary stents following acute myocardial infarction.
Montalescot G, Barragan P, Wittenberg O. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction (ADMIRAL).
Montalescot G, Barragan P, Wittenberg O et al. Three years follow-up after primary stenting with or without abciximab in acute myocardial infarction (the ADMIRAL trial). (Abstract).
Tcheng JE, Kandzari DE, Grines CL et al. Benefits and risks of abciximab use in primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial.
Antoniucci D, Rodriguez A, Hempel A et al. A randomized trial comparing primary infarct artery stenting with or without abciximab in acute myocardial infarction.
Zorman S, Zorman D, Noc M. Effects of abciximab pretreatment in patients with acute myocardial infarction undergoing primary angioplasty.
Arntz H-R, Schröder J, Schwimmbeck P et al. Is early prehospital administration of abciximab superior to periprocedural therapy in patients with ST-segment elevation myocardial infarction and planned percutaneous coronary intervention? Early and late results from the randomized REOMOBILE pilot study. (Abstract).
Gyöngyösi M, Domanovits H, Benzer W et al. Use of abciximab prior to primary angioplasty in STEMI results in early recanalization of the infarct-related artery and improved myocardial tissue reperfusion. Results of the Austrian multicenter randomized ReoPro-BRIDGING Study.
Montalescot G, Borentain M, Payot L, Collet JPH, Thomas D. Early versus late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction.
Cutlip DE, Ricciardi MJ, Frederick SL et al. Effect of tirofiban before primary angioplasty on initial coronary flow and early ST segment resolution in patients with acute myocardial infarction.
Lee DP, Herity NA, Hiatt BL et al. for the TIrofiban Given in the Emergency Room before Primary Angioplasty Investigators. Adjunctive platelet glycoprotein IIb/IIIa receptor inhibition with tirofiban before primary angioplasty improves angiographic outcomes: results of the TIrofiban Given in the Emergency Room before Primary Angioplasty (TIGER-PA) pilot trial.
van't Hof AW, Ernst N, de Boer MJ et al. for the On-TIME study group. Facilitation of primary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial.
Zeymer U, Zahn R, Schiele R et al. Early eptifibatide improves TIMI 3 patency before primary percutaneous coronary intervention for acute ST elevation myocardial infarction: results of the randomized integrilin in acute myocardial infarction (INTAMI) Pilot trial.
Steinhubl SR, Berger PB, Mann III JT et al. for the CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. A randomized controlled trial.
Müller I, Seyfarth M, Rudiger S et al. Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement.
Müller I, Besta F, Schulz C, Massberg S, Schömig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement.
Pache J, Kastrati A, Mehilli J et al. Clopidogrel therapy in patients undergoing coronary stenting: Value of a high-loading-dose regimen.