-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Tenenbaum, Michael Motro, Enrique Z. Fisman, Yehuda Adler, Joseph Shemesh, David Tanne, Jonathan Leor, Valentina Boyko, Ehud Schwammenthal, Solomon Behar, Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients, European Heart Journal, Volume 26, Issue 19, October 2005, Pages 2032–2038, https://doi.org/10.1093/eurheartj/ehi310
Close - Share Icon Share
Aims To assess the effect of fibric acid derivative bezafibrate on incidence of type 2 diabetes in obese patients over a median 6.3 years follow-up period.
Methods and results The study sample comprised 339 non-diabetic obese patients (body mass index ≥30.0 kg/m2) aged 42–74. Patients received either bezafibrate retard 400 mg (178 patients) or placebo (161 patients) once daily. Development of new diabetes was recorded in 98 patients: in 56 (37.0%) from the placebo group vs. 42 (27.1%) from the bezafibrate group, (P log-rank=0.01). The median time (interquartile range) until onset of new diabetes was significantly delayed in patients on bezafibrate when compared with those on placebo: 4.0 (2.1–5.0) vs. 2.0 (0.5–3.5) years, P=0.002. Multivariable analysis identified bezafibrate treatment as an independent predictor of reduced risk of new diabetes with hazard ratio (HR) 0.59 [95% confidence interval (CI) 0.39–0.91]. Other significant variables associated with future overt type 2 diabetes in obese patients were triglycerides (50 mg/dL increment) with HR 1.15 (95% CI 1.02–1.28) and fasting glucose (10 mg/dL increment) with HR 2.27 (95% CI 1.83–2.81).
Conclusion Bezafibrate, when compared with placebo, reduced the incidence and delayed the onset of type 2 diabetes in obese patients over a long-term follow-up period.
Introduction
Type 2 diabetes mellitus and obesity, both major health problems worldwide, are considered to be closely related.1–7 In the majority of cases, type 2 diabetes is now widely thought to be an important component in a group of disorders called the metabolic syndrome.8–11
People who develop type 2 diabetes as a part of metabolic syndrome usually pass through the phases of excessive adipogenesis, nuclear peroxisome proliferator activated receptors (PPAR) modulation, insulin resistance (IR), and beta-cell dysfunction.10–14
Recent studies have shown that type 2 diabetes is preventable by lifestyle interventions and by some medications which influence primary glucose metabolism.15–19 There are limited experimental and clinical data regarding prevention of type 2 diabetes by pharmacological interventions which influence primary lipid metabolism.20–23
Fibric acid derivative bezafibrate is a non-selective ligand/activator for PPAR alpha24 with triglyceride-lowering and HDL-cholesterol raising effects resulting in decreased systemic availability of fatty acid, diminished fatty acid uptake by muscle, and improvement of insulin sensitization.24–27 In patients with overt diabetes, bezafibrate reduces plasma glucose concentrations.25,28 Recently, we have shown that bezafibrate can reduce the incidence of diabetes in patients with impaired fasting glucose (IFG) levels.22 We hypothesized that this effect may be present also among obese patients with normal fasting glucose level. The present analysis aimed to evaluate the effect of bezafibrate on the development of type 2 diabetes in obese patients enrolled in the Bezafibrate Infarction Prevention (BIP) Study over a median 6.3 years follow-up period.
Methods
Patients
The major inclusion and exclusion criteria for the BIP study, as well as the ethical guidelines, have been previously reported.29 Briefly, inclusion criteria for men and women were: 45–74 years of age, history of myocardial infarction not less than 6 months and not more than 5 years prior to enrolment into the study and/or stable angina pectoris confirmed by coronary angiography, and/or radio-nuclear studies or standard exercise tests.
The major exclusion criteria for the BIP study were: permanent pacemaker implantation, cerebrovascular disease, chronic hepatic or renal disease, peripheral vascular disease, malignant diseases, oestrogen replacement therapy, insulin dependent diabetes mellitus, and current use of a lipid modifying drug.
After an initial 2 months of lipid lowering diet, 3122 eligible patients were included in the BIP study between May 1990 and January 1993. The follow-up period lasted until May 1998 [median 6.3, interquartile range (lower and upper quartile) 5.6–7.0 years]. The study was a multicentre prospective trial, performed at 18 university-affiliated hospitals. After randomization, intervention (administration of bezafibrate or placebo) and follow-up periods were the same. There was no significant difference in the distribution of all-cause and cardiac mortality between the bezafibrate and placebo study groups. However, the reduction in the primary endpoint (fatal or non-fatal myocardial infarction or sudden death) was impressive in the subgroup of patients with high baseline triglycerides (≥200 mg/dL).30
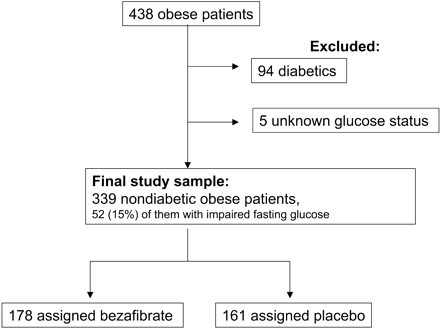
Among the BIP study patients, 438 met the World Health Organization criterion for obesity, i.e. a body mass index (BMI) of ≥30.0 kg/m2.2,31 Among them, there were 58 diagnosed diabetics, 36 patients with a fasting blood glucose level of ≥126 mg/dL (7 mmol/L; 1 mmol/L=18 mg/dL) (undiagnosed diabetics), and five patients with unknown glucose metabolism status: all these patients were excluded from this analysis. Thus, the final study sample for this post hoc analysis comprised 339 non-diabetic obese patients without any antihyperglycaemic treatment on baseline (Figure 1). Among them, there were 287 (85%) patients with fasting blood glucose level of <110 mg/dL (6.1 mmol/L).
In accordance with the American Diabetic Association (ADA) classification,32 we defined the detection of a fasting blood glucose level at baseline ≥110 mg/dL as IFG and ≥126 mg/dL during the follow-up period as the criterion for new diabetes (or launch of antidiabetic medications).
The standard lipid lowering diet advice (based on National Heart, Lung, and Blood Institute recommendations at that time) was provided for all patients in the form of initial 15 min individual session that emphasized the importance of a healthy diet. Two months after the screening initiation (on randomization, the third visit) and again in 1994, additional reinforcements of dietary advice were performed. In addition, the patients received either 400 mg of bezafibrate retard or placebo once a day. Patients continued their prescribed medications for cardiac and other conditions except lipid lowering drugs. Routine visits to the clinics were scheduled bimonthly for study medication distribution and compliance assessment by tablet count, every 4 months for clinical evaluation and every year for blood analyses.
Laboratory methods
Detailed data on laboratory methods were given in a previous report.30 Briefly, blood samples, collected in the 18 participating medical centres using standardized equipment and procedures, were transferred in cooled containers to a central laboratory. Blood samples were drawn after at least 12 h of fasting for determination of serum levels of cholesterol, HDL-cholesterol, and triglycerides. Laboratory measurements were carried out using standard automated procedures using commercially available kits (Roche Diagnostics).
Fasting blood glucose values (serum) were determined by the enzymatic glucose oxidase–peroxidase amino phenazone phenol (GOD–PAP) method, employing a BM/Hitachi 717/911 analyzer.30 For the purpose of the present study, serum samples, which had been taken at baseline from each study participant and stored at −70°C, were thawed and assayed for insulin level by routine radio-immunoassay (Insik 5; Sorin Biomedica, Saluggia, Italy). The homeostatic indexes of IR were calculated according to the homeostasis model of assessment (HOMA) as follows:34–36
Determination of additional variables
Criteria for the diagnosis of myocardial infarction, anginal syndrome, hypertension, and congestive heart failure have been previously reported.30 Functional capacity classes were evaluated according to the New York Heart Association (NYHA) classification. Smoking habits were determined on the basis of self-reporting by the patient during an interview held with a study physician.
Statistical analysis
Data were analysed using the SAS software.37 Continuous variables were presented as mean values±SD. Baseline characteristics were compared by χ2 test for discrete variables and t-test for continuous variables. The time until onset of new diabetes was presented as median [interquartile range (lower and upper quartile)] and compared using Wilcoxon rank sum test. A value of P<0.05 (two-sided) was considered as statistically significant. No adjustments for multiplicity were made to the significance level.
Kaplan–Meier curves and estimates of diabetic incidence and other outcomes were produced using the LIFETEST procedure.37 The log-rank test was used for comparing the curves and outcomes. Seven annual measurements of glucose, BMI, HDL-cholesterol, and triglycerides among the patients allocated to bezafibrate or placebo were compared using analysis of variance for repeated measures. We employed general linear model (GLM) procedure which included factors for treatment, time, and their interaction. The P-values for the interaction test are presented.
Multivariable analysis of incidence of new diabetes was performed using the Cox proportional hazard model (PHREG procedure).37 Estimated HRs and their 95% CI are presented. The linearity assumption for the continuous variables was assessed by fitting the models with added quadratic terms. This method showed that the assumption of linearity was satisfied. We tested for fulfillment of the assumption of proportional hazards using the method based on time-dependent co-variates. The proportional hazards assumption for treatment (placebo vs. bezafibrate) was not exactly satisfied. Therefore, the obtained HR (CI) reflected an average effect of treatment over the range of times observed in the data.38 Variables included in the model were age, gender, total cholesterol, triglycerides, fasting glucose, hypertension, previous myocardial infarction, heart failure, anginal syndrome, and smoking status.
To assess whether the association between bezafibrate treatment and reduced rate of new diabetes persisted in diverse categories of metabolic status, diabetes mellitus incidence was determined in patients according to the level of glucose at baseline (using cut point for IFG), LDL-cholesterol, triglycerides, or the presence of angiotensin converting enzyme-inhibitors (ACE-I).
Results
Baseline data
Our population included two groups: (i) bezafibrate group, 178 patients; (ii) placebo group, 161 patients.
Patients in the placebo and bezafibrate groups were well balanced in terms of clinical and laboratory baseline characteristics and concomitant medications (Table 1). The study groups were similar in regard to age, gender, and the prevalence of the most relevant cardiovascular diseases and risk factors (myocardial infarction in the past, hypertension, heart failure, anginal syndrome). The majority of the patients in both groups were men who had sustained a myocardial infarction in the past. Systolic blood pressure was somewhat higher among patients on bezafibrate. No significant differences between the groups were found for all types of cholesterol, apolipoproteins, diastolic blood pressure, heart rate, BMI, fasting glucose, triglycerides, fibrinogen, and creatinine levels. The fasting insulin level and indices of insulin sensitivity (HOMA IR and QUICKI) were determined in 108 (32%) randomly selected of 339 study patients: in 55 patients on placebo and in 53 patients on bezafibrate. No significant differences between the groups were found for these parameters: fasting insulin level, 14.9±5.7 µU/mL in patients from the placebo group vs. 17.3±8.0 µU/mL in patients from the bezafibrate group (P=0.07); HOMA IR, 3.8±1.6 in patients from the placebo group vs. 4.3±2.4 in patients from the bezafibrate group (P=0.17); QUICKI, 0.32±0.02 in patients from the placebo group vs. 0.31±0.02 in patients from the bezafibrate group (P=0.2). There were no significant differences between the groups in the proportion of patients receiving cardiovascular drugs. Nitrates, calcium antagonists, beta-blockers, and antiplatelet drugs (mainly aspirin) were the most commonly used medications at baseline. The use of ACE-I increased significantly during the follow-up period.
Changes in glucose, BMI, and lipids during follow-up
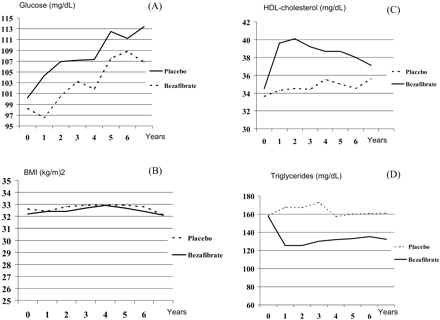
No significant differences between the groups were found for fasting glucose levels at baseline (Figure 2A). As a whole, there was a significant difference in glucose levels over the time between bezafibrate and placebo groups (P=0.003 for the interaction test).
There was no significant change in mean BMI values in either the bezafibrate or the placebo group during the follow-up (Figure 2B). Average changes in mean HDL-cholesterol and triglyceride levels are given in Figure 2C and D. No significant differences between the groups were found for lipid level at baseline. There was a significant difference in mean HDL-cholesterol (P<0.001) and triglyceride levels (P<0.001) over the time between bezafibrate and placebo groups.
Development of new diabetes and other outcomes
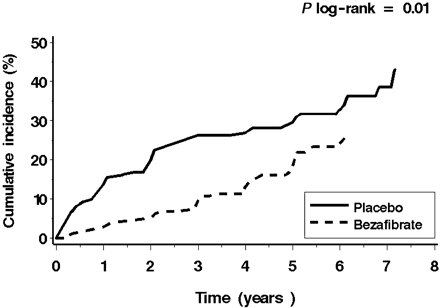
According to the definition, there were no patients with diabetes among the study groups at the beginning of the follow-up. During the follow-up period, development of new diabetes was recorded in 98 patients: in 56 (37.0%) patients from the placebo group vs. 42 (27.1%) from the bezafibrate group, (Kaplan–Meier%, P log-rank=0.01). The total mortality and rate of the fatal or non-fatal myocardial infarction in obese patients on bezafibrate tended to be lower than in their counterparts on placebo, but this tendency did not reach statistical significance (Table 2).
In addition to reducing the incidence of disease, the median time (interquartile range) until onset of new diabetes was significantly delayed in patients on bezafibrate in comparison with patients on placebo: 4.0 (2.1–5.0) vs. 2.0 (0.5–3.5) years, P=0.002.
Oral antihyperglycaemic drugs were initiated during follow-up in 50 (51%) of 98 patients with new diabetes. There was no difference in the proportion of patients on those medications between the study groups.
Kaplan–Meier curves of diabetes incidence (in accordance with the time of diagnosis following annual fasting blood glucose level measurements) for the two study groups are presented in Figure 3. The incidence rate of diabetes among patients on placebo was significantly higher than in their bezafibrate-treated counterparts (P log-rank=0.01).
Multivariable analysis identified bezafibrate treatment as an independent predictor of reduced risk of new diabetes development with HR 0.59 (95% CI 0.39–0.91).
Other significant variables associated with future overt type 2 diabetes in obese patients were triglycerides (50 mg/dL increment) with HR 1.15 (95% CI 1.02–1.28) and fasting glucose (10 mg/dL increment) with HR 2.27 (95% CI 1.83–2.81).
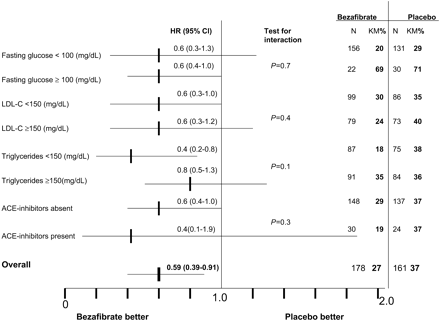
The association between bezafibrate treatment and the rate of new diabetes in diverse categories of metabolic status are presented in Figure 4. A lower incidence of diabetes mellitus was found in the patients on bezafibrate, regardless of IFG or ACE-I, and for different levels of LDL cholesterol and triglycerides.
Discussion
Overweight and obesity have reached epidemic dimensions worldwide, mainly due to consumption of high-energy diets and increased sedentary behaviour. Obesity is clearly associated with cardiovascular diseases and type 2 diabetes.1–7 The Diabetes Prevention Programme (DPP) results have shown that individualized, systematic, and intensive lifestyle interventions (including dietary changes, increased physical activity, and weight loss) are the most effective means of prevention of type 2 diabetes in general high-risk populations (unfortunately, they are not easily applied in general practice).15 Pharmacological interventions employing some medications which influence primary glucose metabolism (metformin and acarbose) or induce weight loss (orlistat, combined with dietary intervention) can also effectively delay the progression to type 2 diabetes,15,18,19 but the magnitude of the benefit seems to be somewhat less (58% for DPP lifestyle changes vs. 31% for metformin, 37% for orlistat, and 25% for acarbose). Recently, we have shown that a pharmacological intervention (bezafibrate) which influences primary lipid metabolism can reduce incidence of type 2 diabetes in patients with IFG levels.22 The data from the present study have shown that this effect may be present in different category of high-risk population—obese patients even with normal fasting glucose level.
There have been a number of reports of prevention of diabetes with non-glucose lowering agents in post-study analysis. In the WOSCOPS Study, there was a 30% risk reduction in development of diabetes in those in the pravastatin group and, although pravastatin was not an independent risk factor, when triglyceride levels were included in the model this raised the role of statin therapy in the prevention of diabetes as an issue.39 However, these findings were not supported in the Heart Protection Study.40 In the HOPE Study, treatment with ramipril compared with placebo showed a relative risk reduction of 34% in the development of diabetes,41 but the study was potentially flawed because it was based solely on self-reported cases. Furthermore, a 25% risk reduction was also noted in the LIFE Study with losartan.42 However, the control group received atenolol and one could argue whether it was the losartan protecting against diabetes or the atenolol increasing the risk of diabetes.43
The factor dominating in obesity is the permanent elevation of plasma free fatty acid with augmented utilization of lipids by the muscle inducing a diminution of glucose uptake and IR. Currently, an insulin-resistant state, as the key phase of metabolic syndrome, constitutes the major risk factor for the development of diabetes mellitus.10,11,44 Hyperinsulinaemia appears to be a compensatory mechanism that responds to the increased levels of circulating glucose. If beta-cell dysfunction occurs, it leads to the fall in insulin secretion and to hyperglycaemia and, in fact, separates the obese patients with metabolic syndrome from those with or without overt diabetes.44
On the basis of the current concept of the evolution of obesity toward overt type 2 diabetes, decrease in plasma free fatty acid and improvement in insulin sensitization seem to be a valuable goal for therapy.
Bezafibrate is a non-selective pharmacological ligand for PPAR-alpha, controlling primarily the expression of genes involved in lipid metabolism. However, PPAR-alpha (in addition to PPAR-gamma) also plays a role in glucose homeostasis and in the development of IR.24,45 Moreover, bezafibrate activates all three PPAR subtypes (alpha, gamma, and delta) at comparable doses.46,47 Therefore, bezafibrate has the potential to directly improve insulin sensitization via PPAR-gamma activation.
Our data suggest that bezafibrate can slow down progression to overt type 2 diabetes in obese patients with an extent of benefit (42%) comparable with other medications already recommended for secondary prevention. Therefore, pharmacological interventions which influence primary lipid metabolism can be effective in this context.
Study limitations
Our study has several important limitations. Development of diabetes was not a declared endpoint of the original BIP study. Therefore, caution should be used in interpreting our finding, as it was identified in post hoc subgroup (obesity) analysis. Glucose tolerance test was not been used in the BIP study. Whereas the ADA recommended using fasting blood glucose for more aggressive IFG and diabetes screening, most large prospective prevention trials deal with patients with impaired glucose tolerance. However, impaired glucose tolerance and IFG do not totally overlap. Thus, our data may be biased by unrecognized diabetes at baseline and during follow-up.
Conclusion
Bezafibrate decreased the incidence and delayed the onset of type 2 diabetes in obese patients over a long-term follow-up period.
Figure 1 Patients' flow chart.
Figure 2 Changes in mean fasting blood glucose (A, mg/dL), BMI (B, kg/m2), mean HDL-cholesterol (C, mg/dL), and triglyceride (D, mg/dL) values throughout the study period (bezafibrate vs. placebo), following annual measurements. Each data point represents the mean value for all participants who remained at that time.
Figure 3 Kaplan–Meier curves of diabetes incidence (in accordance with the time of diagnosis following annual fasting blood glucose measurements) for the study groups (bezafibrate vs. placebo).
Figure 4 Subgroup analyses for diabetes mellitus incidence rate among the study patients according to the level of glucose on baseline, LDL-cholesterol, triglycerides, or the presence of ACE-I. KM%, Kaplan–Meier%.
Baseline characteristics of the study population
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | P-value . |
|---|---|---|---|
| Age (years) | 59.6±6.3 | 58.5±6.6 | 0.15 |
| BMI (kg/m2) | 32.2±2.0 | 32.7±2.8 | 0.1 |
| Weight (kg) | 91.4±10.7 | 92.2±11.5 | 0.5 |
| Height (cm) | 168±8.5 | 168±8.3 | 0.6 |
| Women (%) | 29 (16) | 22 (14) | 0.5 |
| Past myocardial infarction (%) | 140 (79) | 122 (76) | 0.5 |
| Angina (%) | 113 (64) | 102 (64) | 1.0 |
| NYHA Class ≥2* (%) | 63 (36) | 43 (27) | 0.1 |
| Hypertension (%) | 80 (45) | 61 (38) | 0.2 |
| Current or past smokers (%) | 133 (75) | 129 (80) | 0.2 |
| Systolic blood pressure (mmHg) | 138±17 | 134±19 | 0.05 |
| Diastolic blood pressure (mmHg) | 84±10 | 83±9 | 0.6 |
| Heart rate (b.p.m.) | 70.8±8.8 | 69.3±10 | 0.1 |
| Fasting glucose (mg/dL) | 98.2±10.7 | 100.2±10.4 | 0.1 |
| Total cholesterol (mg/dL) | 214±18 | 214±18 | 0.8 |
| HDL-cholesterol (mg/dL) | 34.5±5.5 | 33.6±5.5 | 0.1 |
| LDL-cholesterol (mg/dL) | 148±17 | 149±17 | 0.8 |
| Triglycerides (mg/dL) | 157±55 | 158±52 | 0.9 |
| APO A (mg/dL) | 103±13 | 101±13 | 0.2 |
| APO B (mg/dL) | 101±13 | 103±12 | 0.15 |
| Fibrinogen (mg/dL) | 360±74 | 348±70 | 0.1 |
| Creatinine (mg/dL) | 1.1±0.2 | 1.1±0.1 | 0.5 |
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | P-value . |
|---|---|---|---|
| Age (years) | 59.6±6.3 | 58.5±6.6 | 0.15 |
| BMI (kg/m2) | 32.2±2.0 | 32.7±2.8 | 0.1 |
| Weight (kg) | 91.4±10.7 | 92.2±11.5 | 0.5 |
| Height (cm) | 168±8.5 | 168±8.3 | 0.6 |
| Women (%) | 29 (16) | 22 (14) | 0.5 |
| Past myocardial infarction (%) | 140 (79) | 122 (76) | 0.5 |
| Angina (%) | 113 (64) | 102 (64) | 1.0 |
| NYHA Class ≥2* (%) | 63 (36) | 43 (27) | 0.1 |
| Hypertension (%) | 80 (45) | 61 (38) | 0.2 |
| Current or past smokers (%) | 133 (75) | 129 (80) | 0.2 |
| Systolic blood pressure (mmHg) | 138±17 | 134±19 | 0.05 |
| Diastolic blood pressure (mmHg) | 84±10 | 83±9 | 0.6 |
| Heart rate (b.p.m.) | 70.8±8.8 | 69.3±10 | 0.1 |
| Fasting glucose (mg/dL) | 98.2±10.7 | 100.2±10.4 | 0.1 |
| Total cholesterol (mg/dL) | 214±18 | 214±18 | 0.8 |
| HDL-cholesterol (mg/dL) | 34.5±5.5 | 33.6±5.5 | 0.1 |
| LDL-cholesterol (mg/dL) | 148±17 | 149±17 | 0.8 |
| Triglycerides (mg/dL) | 157±55 | 158±52 | 0.9 |
| APO A (mg/dL) | 103±13 | 101±13 | 0.2 |
| APO B (mg/dL) | 101±13 | 103±12 | 0.15 |
| Fibrinogen (mg/dL) | 360±74 | 348±70 | 0.1 |
| Creatinine (mg/dL) | 1.1±0.2 | 1.1±0.1 | 0.5 |
HDL, high density lipoproteins; Apo A and B, apolipoproteins A and B.
Data are mean±SD or n (%).
Baseline characteristics of the study population
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | P-value . |
|---|---|---|---|
| Age (years) | 59.6±6.3 | 58.5±6.6 | 0.15 |
| BMI (kg/m2) | 32.2±2.0 | 32.7±2.8 | 0.1 |
| Weight (kg) | 91.4±10.7 | 92.2±11.5 | 0.5 |
| Height (cm) | 168±8.5 | 168±8.3 | 0.6 |
| Women (%) | 29 (16) | 22 (14) | 0.5 |
| Past myocardial infarction (%) | 140 (79) | 122 (76) | 0.5 |
| Angina (%) | 113 (64) | 102 (64) | 1.0 |
| NYHA Class ≥2* (%) | 63 (36) | 43 (27) | 0.1 |
| Hypertension (%) | 80 (45) | 61 (38) | 0.2 |
| Current or past smokers (%) | 133 (75) | 129 (80) | 0.2 |
| Systolic blood pressure (mmHg) | 138±17 | 134±19 | 0.05 |
| Diastolic blood pressure (mmHg) | 84±10 | 83±9 | 0.6 |
| Heart rate (b.p.m.) | 70.8±8.8 | 69.3±10 | 0.1 |
| Fasting glucose (mg/dL) | 98.2±10.7 | 100.2±10.4 | 0.1 |
| Total cholesterol (mg/dL) | 214±18 | 214±18 | 0.8 |
| HDL-cholesterol (mg/dL) | 34.5±5.5 | 33.6±5.5 | 0.1 |
| LDL-cholesterol (mg/dL) | 148±17 | 149±17 | 0.8 |
| Triglycerides (mg/dL) | 157±55 | 158±52 | 0.9 |
| APO A (mg/dL) | 103±13 | 101±13 | 0.2 |
| APO B (mg/dL) | 101±13 | 103±12 | 0.15 |
| Fibrinogen (mg/dL) | 360±74 | 348±70 | 0.1 |
| Creatinine (mg/dL) | 1.1±0.2 | 1.1±0.1 | 0.5 |
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | P-value . |
|---|---|---|---|
| Age (years) | 59.6±6.3 | 58.5±6.6 | 0.15 |
| BMI (kg/m2) | 32.2±2.0 | 32.7±2.8 | 0.1 |
| Weight (kg) | 91.4±10.7 | 92.2±11.5 | 0.5 |
| Height (cm) | 168±8.5 | 168±8.3 | 0.6 |
| Women (%) | 29 (16) | 22 (14) | 0.5 |
| Past myocardial infarction (%) | 140 (79) | 122 (76) | 0.5 |
| Angina (%) | 113 (64) | 102 (64) | 1.0 |
| NYHA Class ≥2* (%) | 63 (36) | 43 (27) | 0.1 |
| Hypertension (%) | 80 (45) | 61 (38) | 0.2 |
| Current or past smokers (%) | 133 (75) | 129 (80) | 0.2 |
| Systolic blood pressure (mmHg) | 138±17 | 134±19 | 0.05 |
| Diastolic blood pressure (mmHg) | 84±10 | 83±9 | 0.6 |
| Heart rate (b.p.m.) | 70.8±8.8 | 69.3±10 | 0.1 |
| Fasting glucose (mg/dL) | 98.2±10.7 | 100.2±10.4 | 0.1 |
| Total cholesterol (mg/dL) | 214±18 | 214±18 | 0.8 |
| HDL-cholesterol (mg/dL) | 34.5±5.5 | 33.6±5.5 | 0.1 |
| LDL-cholesterol (mg/dL) | 148±17 | 149±17 | 0.8 |
| Triglycerides (mg/dL) | 157±55 | 158±52 | 0.9 |
| APO A (mg/dL) | 103±13 | 101±13 | 0.2 |
| APO B (mg/dL) | 101±13 | 103±12 | 0.15 |
| Fibrinogen (mg/dL) | 360±74 | 348±70 | 0.1 |
| Creatinine (mg/dL) | 1.1±0.2 | 1.1±0.1 | 0.5 |
HDL, high density lipoproteins; Apo A and B, apolipoproteins A and B.
Data are mean±SD or n (%).
Outcome of the study population during follow-up
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | Log-rank P-value . |
|---|---|---|---|
| New diabetes | 42 (27.1) | 56 (37.0) | 0.01 |
| Total death | 16 (10.7) | 17 (11.8) | 0.66 |
| Fatal myocardial infarction | 2 (1.2) | 4 (2.6) | 0.34 |
| Non-fatal myocardial infarction | 17 (11.1) | 20 (14.1) | 0.41 |
| Primary endpointa | 24 (15.4) | 27 (18.1) | 0.41 |
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | Log-rank P-value . |
|---|---|---|---|
| New diabetes | 42 (27.1) | 56 (37.0) | 0.01 |
| Total death | 16 (10.7) | 17 (11.8) | 0.66 |
| Fatal myocardial infarction | 2 (1.2) | 4 (2.6) | 0.34 |
| Non-fatal myocardial infarction | 17 (11.1) | 20 (14.1) | 0.41 |
| Primary endpointa | 24 (15.4) | 27 (18.1) | 0.41 |
Data are number (Kaplan–Meier%) of patients.
aThe primary endpoint of the BIP study was fatal or non-fatal myocardial infarction or sudden death.
Outcome of the study population during follow-up
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | Log-rank P-value . |
|---|---|---|---|
| New diabetes | 42 (27.1) | 56 (37.0) | 0.01 |
| Total death | 16 (10.7) | 17 (11.8) | 0.66 |
| Fatal myocardial infarction | 2 (1.2) | 4 (2.6) | 0.34 |
| Non-fatal myocardial infarction | 17 (11.1) | 20 (14.1) | 0.41 |
| Primary endpointa | 24 (15.4) | 27 (18.1) | 0.41 |
| Characteristics . | Bezafibrate (n=178) . | Placebo (n=161) . | Log-rank P-value . |
|---|---|---|---|
| New diabetes | 42 (27.1) | 56 (37.0) | 0.01 |
| Total death | 16 (10.7) | 17 (11.8) | 0.66 |
| Fatal myocardial infarction | 2 (1.2) | 4 (2.6) | 0.34 |
| Non-fatal myocardial infarction | 17 (11.1) | 20 (14.1) | 0.41 |
| Primary endpointa | 24 (15.4) | 27 (18.1) | 0.41 |
Data are number (Kaplan–Meier%) of patients.
aThe primary endpoint of the BIP study was fatal or non-fatal myocardial infarction or sudden death.
Manson JE, Skerrett PJ, Greenland P, VanItallie TB. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition.
Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States.
Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome.
Bonow RO, Gheorghiade M. The diabetes epidemic: a national and global crisis.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey.
Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease.
Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension.
Groop LC. Insulin resistance: the fundamental trigger of type 2 diabetes.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men.
Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors.
Porte D Jr, Kahn SE. Beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin.
Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study.
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial.
Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients.
Auer J, Berent R, Weber T, Eber B. Clinical significance of pleiotropic effects of statins: lipid reduction and beyond.
Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation.
Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, Leor J, Boyko V, Mandelzweig L, Behar S. Peroxisome proliferator-activated receptors ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease
Fruchart JC, Staels B, Duriez P. The role of fibric acids in atherosclerosis.
Rovellini A, Sommariva D, Branchi A, Maraffi F, Montalto C, Gandini R, Fasoli A. Effects of slow release bezafibrate on the lipid pattern and on blood glucose of type 2 diabetic patients with hyperlipidaemia.
Taniguchi A, Fukushima M, Sakai M, Tokuyama K, Nagata I, Fukunaga A, Kishimoto H, Doi K, Yamashita Y, Matsuura T, Kitatani N, Okumura T, Nagasaka S, Nakaishi S, Nakai Y. Effects of bezafibrate on insulin sensitivity and insulin secretion in non-obese Japanese type 2 diabetic patients.
Jonkers IJ, Mohrschladt MF, Westendorp RG, van der Laarse A, Smelt AH. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial.
Jones IR, Swai A, Taylor R, Miller M, Laker MF, Alberti KG. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM.
Goldbourt U, Behar S, Reicher-Reiss H, Agmon J, Kaplinsky E, Graff E, Kishon Y, Caspi A, Weisbort J, Mandelzweig L, Abinader E, Aharon L, Braun S, David D, Flich M, Friedman Y, Kristal N, Leil N, Markiewicz W, Marmor A, Palant A, Pelled B, Rabinowitz B, Reisin L, Roguin N, Rosenfeld T, Schlesinger Z, Sclarovsky S, Sherf L, Tzivoni D, Zahavi I, Zion M, Brunner D, for the Bezafibrate Infarction Prevention Study Group. Rationale and design of a secondary prevention trial of increasing serum high-density lipoprotein cholesterol and reducing triglycerides in patients with clinically manifest atherosclerotic heart disease (the Bezafibrate Infarction Prevention Trial).
The Bezafibrate Infarction Prevention (BIP) Study Group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study.
American Diabetes Association. Report of the expert committees on the diagnosis and classification of diabetes mellitus.
Scandinavian Simvastatin Survival Study (4S) Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S).
Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio heart study.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans.
Lichnovska R, Gwozdziewiczova S, Hrebicek J. Gender differences in factors influencing insulin resistance in elderly hyperlipemic non-diabetic subjects.
Allison PD.
Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 individuals: a randomised placebo-controlled trial.
Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B; HOPE Study Investigators. Ramipril and the development of diabetes.
Lindholm LH, Ibsen H, Borch-Johnsen K, Olsen MH, Wachtell K, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristianson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman JM, Snapinn S; for the LIFE study group. Risk of new-onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study.
Davies MJ, Tringham JR, Troughton J, Khunti KK. Prevention of Type 2 diabetes mellitus. A review of the evidence and its application in a UK setting.
Tenenbaum A, Fisman EZ, Motro M. Metabolic syndrome and type 2 diabetes mellitus: focus on peroxisome proliferator activated receptors (PPAR).
Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B. PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance.
Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery.