-
PDF
- Split View
-
Views
-
Cite
Cite
Julien De Wolf, Jocelyn Bellier, Francoise Lepimpec-Barthes, Francois Tronc, Christophe Peillon, Alain Bernard, Jean-Philippe Le Rochais, Olivier Tiffet, Edouard Sage, Alain Chapelier, Henri Porte, Exhaustive preoperative staging increases survival in resected adrenal oligometastatic non-small-cell lung cancer: a multicentre study, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 698–703, https://doi.org/10.1093/ejcts/ezx193
Close - Share Icon Share
Abstract
Adrenal oligometastatic non-small-cell lung cancer is rare, and surgical management remains controversial.
We performed a multicentre, retrospective study from January 2004 to December 2014. The main objective was to evaluate survival in patients who had undergone adrenalectomy after resection of primary lung cancer. Secondary objectives were to determine prognostic, survival and recurrence factors.
Fifty-nine patients were included. Forty-six patients (78%) were men. The median age was 58 years [39–75 years]. Twenty-six cases (44%) showed synchronous presentation, and 33 cases (56%) had a metachronous presentation. The median time to onset of metastasis was 18.3 months [6–105 months]. The 5-year overall survival rate was 59%; the median survival time was 77 months [0.6–123 months]. A recurrence was observed in 70% of the population. Mediastinal lymph node invasion (P = 0.035) is a detrimental prognostic factor of survival.
After exhaustive staging, patients with adrenal oligometastatic non-small-cell lung cancer benefit from bifocal surgery.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths. Despite an expanding therapeutic arsenal, the 5-year survival rate remains low, being estimated as only 14% over all stages [1].
The poor prognosis of lung cancer is largely explained by delays in diagnosis, with metastasis having occurred in 11–36% of patients by the time of diagnosis, thereby limiting the curative therapeutic possibilities [2, 3]. Currently, patients with metastatic presentation are almost exclusively offered palliative treatment.
The early management of patients with lesions accessible to a potentially curative treatment is therefore a major challenge in lung cancer.
The term ‘metastatic’ covers multiple clinical presentations, ranging from a metastasis to metastatic efflorescence. In 1995, Hellman and Weichselbaum described the concept of ‘oligometastatic’, defined as a state of limited metastatic spread in terms of number (maximum of 5 lesions), the locations of which enable radical and comprehensive curative care [4]. The concept of oligometastatic has since been refined in terms of the timing of the metastasis. In the literature, when a diagnosis of metastasis is made during the first 6 months after the primary cancer diagnosis, it is called ‘synchronous’. After 6 months, the metastasis is called ‘metachronous’. Although patients with oligometastatic tumours are rare among patients with lung cancer (7% according Pfannschmidt and Dienemann, 2010), they could benefit from the multimodal curative treatment of both primary and metastatic lung lesions [5].
In 2012, the European Society for Medical Oncology published recommendations for the treatment of oligometastatic non-small-cell lung cancer (NSCLC), advising potentially curative care only as part of a personalized treatment plan, in which general status and patient comorbidities, time of onset of secondary injury and the extent of the primary tumour should be taken into account. These recommendations limited the curative support of patients in Stage IV with a single metastasis if that metastasis was located in the brain or adrenal gland.
Through a retrospective multicentre study, we wanted to evaluate survival rates in patients with NSCLC with adrenal metastases who had undergone bifocal resection after exhaustive staging. We also wanted to identify prognostic factors for survival and recurrence.
METHODS
We performed a retrospective multicentre study from January 2004 to December 2014. Eight university hospitals participated in the study: Caen, Dijon, Lille, Lyon, Paris (European Hospital Georges Pompidou), Rouen, Saint-Etienne and Suresnes (Foch Hospital).
Inclusion criteria
Patients with resected NSCLC and an adrenal lesion who satisfied the following criteria were included:
exhaustive preoperative staging including bronchoscopy, positron emission tomography imaging with injection of fluorodeoxyglucose 18F and brain imaging with computed tomography or magnetic resonance imaging (MRI);
pathological evidence affirming the diagnosis of NSCLC;
radical lung surgery associated with radical lymph node dissection; and
adrenal metastasis resection during the period of inclusion with histological confirmation of the metastatic nature of the lesion.
The following parameters were recorded: age, sex, smoking (the patient was considered a weaned smoker if the smoking was interrupted for at least 4 weeks before the thoracic operation), chronic obstructive pulmonary disease defined by a Tiffeneau–Pinelli index (FEV1/FCV) <70%, diabetes, high blood pressure, history of ischaemic heart disease, vascular history of peripheral arterial disease or a stroke.
For lung resections, we recorded:
the type of surgical resection and its possible extension to adjacent structures;
the location of the primary bronchial tumour;
the tumour, node and matastasis (TNM) classification according to the ‘TNM Classification of Malignant Tumours’, seventh edition;
the administration of perioperative treatment and if so which therapy (radiotherapy, chemotherapy or the combination of both);
histological type as classified by the World Health Organization. Small-cell lung cancers were excluded from the cohort; and
pathological type of resection:
R0: microscopically complete excision,
R1: microscopically incomplete resection and
R2: macroscopically incomplete resection.
Adjuvant chemotherapy after lung resection
Chemotherapy was administered according to the standards of lung cancer management in patients in whom the adrenal metastasis is diagnosed after lung surgery. Chemotherapy was always validated in a meeting of multidisciplinary staff.
On the other hand, when the adrenal metastasis was known before the operation, the decision was made in a meeting of multidisciplinary staff based on the status of the patient’s cancer.
For adrenalectomy, we recorded:
time to onset of the lesion: synchronous or metachronous;
type of surgical approach: laparotomy, minimally invasive procedure (laparoscopy and robotic surgery), phrenotomy;
administration of perioperative treatment and if so which;
laterality relative to the pulmonary tumour;
pathological type of resection; and
postoperative complications after adrenalectomy.
The first recurrence site (loco-regional or distant) was recorded.
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute 9.3) and GraphPad Prism 6 software to generate the survival curves. Continuous variables are described as the median with minimum and maximum. The variables are described in a binary manner by means of a distribution percentage. Each variable was analysed separately. Survival was analysed using the Kaplan–Meier plot. The log-rank test for categorical variables and the Cox model for quantitative variables were used to identify the prognostic factors for overall survival and recurrence in these patients. The proportional hazards assumption was checked by Schoenfeld residuals, and log-linearity was evaluated for quantitative variables. Significant factors in univariate analysis with P < 0.10 were included in a multivariate Cox model regression. P-value <0.05 was considered statistically significant with a 95% confidence interval.
RESULTS
During the study period, 59 patients in the 8 participating centres had surgery for a bifocal adrenal metastasis from an NSCLC. The cohort included 46 men (78%) and 13 women (22%). The median age was 58 years [39–75 years]. At least 1 comorbidity was present in each of 42 patients (71%). The most common comorbidities were chronic obstructive pulmonary disease in 36 patients (62%), vascular antecedent in 20 patients (34.5%) and a history of cancer in 6 patients (10%). Comorbidities are summarized in Table 1.
Population characteristics
| Variable . | n . | n miss . | Lower quartile . | Median . | Upper quartile . |
|---|---|---|---|---|---|
| Age (year) | 59 | 0 | 50 | 56 | 62 |
| Body mass index (kg/m2) | 47 | 12 | 21 | 24 | 26 |
| Pack-years of cigarettes | 55 | 4 | 20 | 34 | 45 |
| Disease-free interval (months) | 59 | 0 | 1 | 11 | 21 |
| Overall survival (months) | 59 | 0 | 11 | 30 | 51 |
| Variable . | n . | n miss . | Lower quartile . | Median . | Upper quartile . |
|---|---|---|---|---|---|
| Age (year) | 59 | 0 | 50 | 56 | 62 |
| Body mass index (kg/m2) | 47 | 12 | 21 | 24 | 26 |
| Pack-years of cigarettes | 55 | 4 | 20 | 34 | 45 |
| Disease-free interval (months) | 59 | 0 | 1 | 11 | 21 |
| Overall survival (months) | 59 | 0 | 11 | 30 | 51 |
Population characteristics
| Variable . | n . | n miss . | Lower quartile . | Median . | Upper quartile . |
|---|---|---|---|---|---|
| Age (year) | 59 | 0 | 50 | 56 | 62 |
| Body mass index (kg/m2) | 47 | 12 | 21 | 24 | 26 |
| Pack-years of cigarettes | 55 | 4 | 20 | 34 | 45 |
| Disease-free interval (months) | 59 | 0 | 1 | 11 | 21 |
| Overall survival (months) | 59 | 0 | 11 | 30 | 51 |
| Variable . | n . | n miss . | Lower quartile . | Median . | Upper quartile . |
|---|---|---|---|---|---|
| Age (year) | 59 | 0 | 50 | 56 | 62 |
| Body mass index (kg/m2) | 47 | 12 | 21 | 24 | 26 |
| Pack-years of cigarettes | 55 | 4 | 20 | 34 | 45 |
| Disease-free interval (months) | 59 | 0 | 1 | 11 | 21 |
| Overall survival (months) | 59 | 0 | 11 | 30 | 51 |
Lung resection involved a lobectomy in 36 patients (61%), an enlarged lobectomy in 15 patients (25.4%) and pneumonectomy in 8 patients (13.6%). Extended resection included lobectomies extending to adjacent lobes in 5 patients (8.5%), 2 (3.4%) bilobectomies, 3 (5%) bronchial sleeve lobectomies, 4 (6.8%) lobectomies with vascular reconstruction (1 upper vena cava replacement, 1 subclavian artery reconstruction and 2 resections of the left atrium) and 6 (10.2%) lobectomies extending to the wall.
The predominant histological type was an adenocarcinoma, accounting for 42 cases (71.2%). A squamous cell carcinoma was found in 11 cases (18.6%); a large-cell carcinoma in 4 cases (6.8%); and a sarcomatoid carcinoma and carcinoid in 1 case each (1.7%).
The majority of pulmonary tumours (n = 32, 54.2%) were classified as pT2 (Table 2). Twenty-five patients (42.4%) were free from mediastinal lymph nodes. Almost a third (n = 19) of patients had mediastinal lymph node invasion type N2.
Predictive factor of survival
| Variables . | n (%) . | Univariate analysis (P-value) . | Multivariate analysis (RR [95% CI] P-value) . |
|---|---|---|---|
| Lung tumour localization: upper lobes/inferior lobes | 39 (66)/11 (19) | 0.70 | |
| Adrenal metastasis: ipsilateral/contralateral | 22 (37)/34 (58) | 0.36 | |
| N0–1/N2 | (68)/(27) | 0.07 | 2.73 [1.08–6.93] 0.04 |
| Lung resection | |||
| Lobectomy | 18 (64) | 0.24 | |
| Enlarged lobectomy Pneumonectomy | 9 (73) 6 (75) | ||
| Radiotherapy on the adrenal resection site | 8 (72.7) | 0.31 | |
| Chemotherapy after adrenalectomy | 14 (73.7) | 0.30 | |
| Squamous cell carcinoma/adenocarcinoma | 11 (18.6)/42 (71.2) | 0.08 | 2.72 [0.92–8.01] 0.07 |
| Synchronous/metachronous | 26 (49)/33 (56) | 0.39 |
| Variables . | n (%) . | Univariate analysis (P-value) . | Multivariate analysis (RR [95% CI] P-value) . |
|---|---|---|---|
| Lung tumour localization: upper lobes/inferior lobes | 39 (66)/11 (19) | 0.70 | |
| Adrenal metastasis: ipsilateral/contralateral | 22 (37)/34 (58) | 0.36 | |
| N0–1/N2 | (68)/(27) | 0.07 | 2.73 [1.08–6.93] 0.04 |
| Lung resection | |||
| Lobectomy | 18 (64) | 0.24 | |
| Enlarged lobectomy Pneumonectomy | 9 (73) 6 (75) | ||
| Radiotherapy on the adrenal resection site | 8 (72.7) | 0.31 | |
| Chemotherapy after adrenalectomy | 14 (73.7) | 0.30 | |
| Squamous cell carcinoma/adenocarcinoma | 11 (18.6)/42 (71.2) | 0.08 | 2.72 [0.92–8.01] 0.07 |
| Synchronous/metachronous | 26 (49)/33 (56) | 0.39 |
Predictive factor of survival
| Variables . | n (%) . | Univariate analysis (P-value) . | Multivariate analysis (RR [95% CI] P-value) . |
|---|---|---|---|
| Lung tumour localization: upper lobes/inferior lobes | 39 (66)/11 (19) | 0.70 | |
| Adrenal metastasis: ipsilateral/contralateral | 22 (37)/34 (58) | 0.36 | |
| N0–1/N2 | (68)/(27) | 0.07 | 2.73 [1.08–6.93] 0.04 |
| Lung resection | |||
| Lobectomy | 18 (64) | 0.24 | |
| Enlarged lobectomy Pneumonectomy | 9 (73) 6 (75) | ||
| Radiotherapy on the adrenal resection site | 8 (72.7) | 0.31 | |
| Chemotherapy after adrenalectomy | 14 (73.7) | 0.30 | |
| Squamous cell carcinoma/adenocarcinoma | 11 (18.6)/42 (71.2) | 0.08 | 2.72 [0.92–8.01] 0.07 |
| Synchronous/metachronous | 26 (49)/33 (56) | 0.39 |
| Variables . | n (%) . | Univariate analysis (P-value) . | Multivariate analysis (RR [95% CI] P-value) . |
|---|---|---|---|
| Lung tumour localization: upper lobes/inferior lobes | 39 (66)/11 (19) | 0.70 | |
| Adrenal metastasis: ipsilateral/contralateral | 22 (37)/34 (58) | 0.36 | |
| N0–1/N2 | (68)/(27) | 0.07 | 2.73 [1.08–6.93] 0.04 |
| Lung resection | |||
| Lobectomy | 18 (64) | 0.24 | |
| Enlarged lobectomy Pneumonectomy | 9 (73) 6 (75) | ||
| Radiotherapy on the adrenal resection site | 8 (72.7) | 0.31 | |
| Chemotherapy after adrenalectomy | 14 (73.7) | 0.30 | |
| Squamous cell carcinoma/adenocarcinoma | 11 (18.6)/42 (71.2) | 0.08 | 2.72 [0.92–8.01] 0.07 |
| Synchronous/metachronous | 26 (49)/33 (56) | 0.39 |
Adrenal metastasis was synchronous in 26 cases (44%) and metachronous in 33 (56%). The median time to onset of metastasis was 18.3 months [6–105 months]. The overall median time from pulmonary resection to adrenalectomy was 10.5 months [0–105 months].
Adrenal metastasis was ipsilateral in 22 cases (37.3%), contralateral in 34 cases (57.6%) and bilateral in 3 cases (5.1%).
Adrenalectomy was performed using a minimally invasive approach in 31 patients (52.5%; 30 laparoscopies and 1 robot assisted) and a laparotomy in 23 patients (39%). In 5 cases (8.5%), it was carried out via a transdiaphragmatic approach (left thoracotomy in 4 cases and a right thoracotomy in 1 case).
Adrenal resection was R0 in 57 cases (96.6%) and R1 in 2 (3.4%). Incomplete resection was performed through a laparoscopy in 1 and a laparotomy for the other.
Additional treatment was administered to 24 patients (40.7%) after adrenalectomy: radiotherapy on the adrenalectomy site in 15 cases (25.4%; including the 2 patients with incomplete resection), chemotherapy for 19 patients (35.9%) and combined treatment (radiotherapy and chemotherapy) in 10 patients (16.9%).
Postoperative mortality at 3 months was 1.7% (n = 2): 1 patient with a voluminous 12-cm metastasis died 19 days after adrenalectomy of a haemorrhage. One patient died of secondary adrenal insufficiency 3 months after a bilateral adrenalectomy.
The NSCLC was located in the upper lobes in 39 patients (66%) and in the lower lobes in 11 patients (19%), with no differences in the overall survival (P = 0.70). The median survival estimated by the Kaplan–Meier method was 77 months (95% confidence interval = 43–110) for upper lobe involvement and 121 months (95% confidence intervaI = 0–265) for lower lobe involvement.
Adrenal metastasis was ipsilateral in 22 patients (37.3%) and contralateral in 34 patients (57.6%). Median survival was 48.3 months (2.9–120.6 months) for ipsilateral involvement and 77.1 months (0.6–122.6 months) for contralateral involvement, with no significant differences between the two (P = 0.358).
Lung resection type was not correlated with a difference in survival rate (P = 0.24); neither was perioperative treatment (P = 0.31).
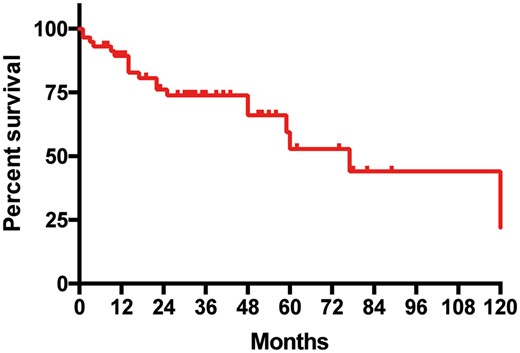
The 5-year overall survival rate was 59% with a median survival estimated at 77 months (0.6–123 months) (Fig. 1). After multivariate analysis, only the N2 versus N0–1 status appeared to be a risk factor for death (P = 0.04) (Table 2).
Kaplan–Meier survivor curve: overall survival after resection of a solitary adrenal metastasis from resected non-small-cell lung cancer.
Thirty-six patients (61%) had a metastatic recurrence during the follow-up period.
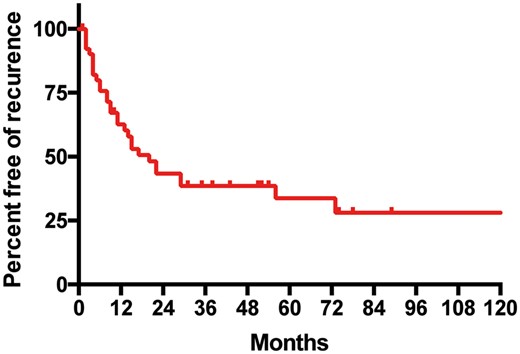
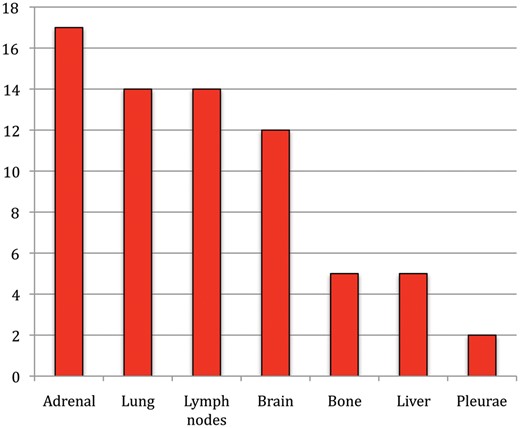
The estimated percentage of patients free from recurrence for 5 years was 30.3% (Fig. 2), with a median time without recurrence of 15 months (range 0–93). Sites of recurrence were adrenal in 17 patients (28.8%), lung in 14 patients (23.7%), lymph nodes (mediastinal or retroperitoneal) in 14 patients (23.7%), the brain in 12 patients (20.3%), bone and liver in 5 patients each (8.5%) and the pleura in 2 patients (3.4%) (Fig. 3).
Kaplan–Meier recurrence curve: evolution of the population without recurrences after resection of solitary adrenal metastasis from resected non-small-cell lung cancer.
We tested the factors influencing recurrence. Table 3 presents the analysis of the risk of recurrence according to the parameters studied, highlighting the influence of the N2 status in the tendency for risk of recurrence.
Predictive factors of recurrences
| Variables . | n (%) . | P-value . |
|---|---|---|
| Sex: women/men | 10 (77)/26 (57) | 0.15 |
| Age | 0.35 | |
| Tobacco: active smoking/weaned | 26 (66)/5 (80) | 0.90 |
| Chronic obstructive pulmonary disease | 19 (52.8) | 0.22 |
| Adrenal metastasis: ipsilateral/ contralateral | 15 (68)/20 (58) | 0.77 |
| N0–1/N2 | 21 (53)/15 (79) | 0.05 |
| Lung resection: lobectomy/enlarged lobectomy/pneumonectomy | 18 (64)/9 (73)/6 (75) | 0.49 |
| Radiotherapy | 8 (72.7) | 0.63 |
| Chemotherapy | 14 (73.7) | 0.76 |
| Abdominal lymph node dissection | 6 (75) | 0.30 |
| Synchronous/metachronous | 22 (67)/14 (54) | 0.46 |
| Variables . | n (%) . | P-value . |
|---|---|---|
| Sex: women/men | 10 (77)/26 (57) | 0.15 |
| Age | 0.35 | |
| Tobacco: active smoking/weaned | 26 (66)/5 (80) | 0.90 |
| Chronic obstructive pulmonary disease | 19 (52.8) | 0.22 |
| Adrenal metastasis: ipsilateral/ contralateral | 15 (68)/20 (58) | 0.77 |
| N0–1/N2 | 21 (53)/15 (79) | 0.05 |
| Lung resection: lobectomy/enlarged lobectomy/pneumonectomy | 18 (64)/9 (73)/6 (75) | 0.49 |
| Radiotherapy | 8 (72.7) | 0.63 |
| Chemotherapy | 14 (73.7) | 0.76 |
| Abdominal lymph node dissection | 6 (75) | 0.30 |
| Synchronous/metachronous | 22 (67)/14 (54) | 0.46 |
Predictive factors of recurrences
| Variables . | n (%) . | P-value . |
|---|---|---|
| Sex: women/men | 10 (77)/26 (57) | 0.15 |
| Age | 0.35 | |
| Tobacco: active smoking/weaned | 26 (66)/5 (80) | 0.90 |
| Chronic obstructive pulmonary disease | 19 (52.8) | 0.22 |
| Adrenal metastasis: ipsilateral/ contralateral | 15 (68)/20 (58) | 0.77 |
| N0–1/N2 | 21 (53)/15 (79) | 0.05 |
| Lung resection: lobectomy/enlarged lobectomy/pneumonectomy | 18 (64)/9 (73)/6 (75) | 0.49 |
| Radiotherapy | 8 (72.7) | 0.63 |
| Chemotherapy | 14 (73.7) | 0.76 |
| Abdominal lymph node dissection | 6 (75) | 0.30 |
| Synchronous/metachronous | 22 (67)/14 (54) | 0.46 |
| Variables . | n (%) . | P-value . |
|---|---|---|
| Sex: women/men | 10 (77)/26 (57) | 0.15 |
| Age | 0.35 | |
| Tobacco: active smoking/weaned | 26 (66)/5 (80) | 0.90 |
| Chronic obstructive pulmonary disease | 19 (52.8) | 0.22 |
| Adrenal metastasis: ipsilateral/ contralateral | 15 (68)/20 (58) | 0.77 |
| N0–1/N2 | 21 (53)/15 (79) | 0.05 |
| Lung resection: lobectomy/enlarged lobectomy/pneumonectomy | 18 (64)/9 (73)/6 (75) | 0.49 |
| Radiotherapy | 8 (72.7) | 0.63 |
| Chemotherapy | 14 (73.7) | 0.76 |
| Abdominal lymph node dissection | 6 (75) | 0.30 |
| Synchronous/metachronous | 22 (67)/14 (54) | 0.46 |
Five patients with a single brain recurrence were treated surgically. All other patients with recurrences (loco-regional or distant) with a performance status allowing chemotherapy had chemotherapy.
DISCUSSION
In this 10-year French multicentre retrospective study, we showed that, with a careful selection process, even patients with adrenal oligometastatic NSCLC could have a 5-year overall survival rate of up to 50%. Our report includes the largest cohort reported to date over a 10-year collection period [6–8].
The previous decade ended with the feeling that operative treatment was appropriate only for NSCLC localized in the lung. Indeed, researchers argued against operating on IIIA-N2 disease [9, 10]. However, in 2013, the American College of Chest Physicians recommended (Grade 1A) treatment by induction chemotherapy, followed by surgery or chemoradiotherapy in the population with advanced N2. In oligometastatic adrenal metastasis, the treatment procedure remains controversial, with several publications evaluating operations yielding a 5-year survival rate of 10–34% [6, 11–13]. In our series, survival at 5 years was 59% with a median of 77 months with bifocal surgery. Therefore, we recommend that, in patients with advanced NSCLC, careful selection with extensive preoperative imaging including positron emission tomography imaging with injection of fluorodeoxyglucose 18F and brain MRI should be performed before proposing surgery as part of a multimodal aggressive management.
In 2001, Porte et al. reported a high rate of early recurrence in their series (38% at 3 months and 56% at 6 months, with the brain as the preferential site of recurrence), highlighting the need for systematic positron emission tomography imaging with injection of fluorodeoxyglucose 18F and brain imaging upon presentation, in order not to miss other metastatic sites [8]. In this study, we confirmed that a better selection process can improve survival in a carefully selected population.
Several prognostic factors for survival have been reported. Mercier et al. showed that patients with metachronous disease had a better survival outcome than patients with a synchronous presentation, with a 2-year survival rate of 38% vs 0% (P = 0.005) [7]. In our series, the type of presentation did not adversely affect survival (P = 0.39).
Over the past decade, perioperative treatment has improved but did not influence survival in our series.
As reported in the literature, the dominant histological type of tumour was adenocarcinoma (71%). As for single brain metastases, adenocarcinoma seemed to have a better prognosis. Indeed, in our study, the 5-year survival rate was 58% for adenocarcinoma but only 39% for squamous cell carcinoma (P = 0.08). A new histological classification of adenocarcinoma has recently been described, in which grades are correlated with overall survival [14]. Unfortunately, the current study began several years before the description of this classification, and thus pathology reports did not mention the adenocarcinoma subtype. In addition, the management of adenocarcinoma is currently guided by molecular biology, e.g. the epidermal growth factor receptor and anaplastic lymphoma kinase mutations. In our study, mutation data were available for very few patients. Targeted therapies are now used as first-line therapy for patients with non-squamous NSCLC depending on the epidermal growth factor receptor mutation [15]. With the development of targeted therapies based on mutations of NSCLC, the next TNM classification should take into these mutations account. ‘Molecular’ TNM would complete the histological classifications. Indeed, the oligometastatic disease treatment strategy will be guided by future molecular TNM.
Lymph node invasion is an important detrimental prognostic factor in patients with NSCLC. With 19 patients (32.2%), our series has a high rate of N2-classified tumours [8]. Indeed, only 1 other series has reported a higher percentage of N2-classified lesions (44%). To date, only Raz et al., in 2011, found that mediastinal lymph node invasion influenced survival in oligometastatic adrenal disease [6]. Five-year survival was 0% in the N2 population but 52% for N0 and N1 (P = 0.008). However, in that particular study, only 7 patients were N2, limiting any generalization. In our population, the 5-year survival rate of the N2 population was 27% vs 68% for N0 and N1. After multivariate analysis, mediastinal lymph node involvement emerged as an independent factor of mortality (P = 0.035). Our results on lymph node involvement are comparable to those of Raz et al. Improving mediastinal evaluation will likely help improve survival. Two studies published in 2007 and 2009 suggested no benefit of surgery compared with radiochemotherapy for 5-year survival in patients with Stage IIIA-N2 lung cancer [9, 10]. In 2013, the American College of Chest Physicians recommended (Grade 1A) treatment by induction chemotherapy, followed by surgery or chemoradiotherapy in the N2 population. However, with an estimated median survival of 50 months in our cohort, patients with N2 lymph node status should be considered for bifocal surgery.
An anatomical study conducted in 1958 by Meyer described direct lymphatic drainage from the lower lung lobes and abdomen (especially retroperitoneal) [16]. Therefore, loco-regional extension should be suspected when lung cancer starts in the lower lobes and extension to the blood when starting in the upper lobes. Based on the same anatomical findings, an ipsilateral adrenal metastasis could be correlated with a better prognosis. However, our series did not show any effect of lung cancer localization or of metastasis laterality.
In our study, the type of surgical resection was not a prognostic factor for survival. We believe that an extended resection should be considered in a few carefully selected patients even in the event of NSCLC metastatic disease.
There was no evidence that radiation therapy had any impact on the adrenalectomy site or that chemotherapy had any influence. This treatment probably has a place as part of the management of metastatic disease, as shown for brain metastases [17, 18]. Our study found that the majority of patients had recurrences (70% at 5 years) and that they were loco-regional. We believe there is interest in the treatments surrounding the management of adrenal metastasis from NSCLC, but it will be difficult to establish a treatment of choice because of the rarity of this condition.
N2 status seems to influence both survival and recurrence, with a tendency for recurrence due to N2 (P = 0.053). Porte et al. reported 56% of patients had recurrences at 6 months, with the brain as the most common site of recurrence. In their series, 9 patients (26.5%) had brain recurrences. Our series had a recurrence rate of 33% at 6 months. A rate of recurrence of 56% was reached in our population 2 years after surgery. Furthermore, the most common site of recurrence was the adrenals (25%) and then the brain (18%). Cerebral assessment seems essential in order to avoid overlooking occult brain disease. Brain MRI identifies more and smaller lesions than the scanner [19, 20]. However, the clinical relevance and impact of brain MRI on long-term survival is still under debate [21]. Also, in the specific case of oligometastatic disease, performing a routine brain MRI may be recommended.
To date, because it is difficult to treat a patient according to a ‘general’ guideline, the treatment decision falls to multidisciplinary staff for each patient based on the status of his or her cancer.
Unlike in metachronous disease (a single adrenal metastases), synchronous adrenal oligometastatic presentation is defined as true bifocal lung cancer disease.
We believe that a patient who presents with synchronous adrenal NSCLC oligometastatic disease should be evaluated for induction chemotherapy, more particularly, for tumour responsiveness. Chemotherapy as a first step of a multimodal treatment strategy is also a way to select candidates for bifocal resections. Indeed, the goal with this strategy is to let time take its course and distinguish the highly aggressive tumour that is inoperable from the tumours that would be responsive to aggressive multimodal treatment.
CONCLUSION
Bifocal resection of adrenal oligometastatic NSCLC is suitable with acceptable long-term survival rates. The absence of mediastinal lymph node involvement is a protective prognostic factor, as is the adenocarcinoma histological type. Careful selection of patients, including optimal brain assessment, is the cornerstone for choosing an appropriate therapeutic strategy.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 30th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 1–5 October 2016.