-
PDF
- Split View
-
Views
-
Cite
Cite
David F Hutt, Marianna Fontana, Maria Burniston, Ann-Marie Quigley, Aviva Petrie, James C Ross, Joanne Page, Ana Martinez-Naharro, Ashutosh D Wechalekar, Helen J Lachmann, Candida C Quarta, Tamer Rezk, Shameem Mahmood, Sajitha Sachchithanantham, Taryn Youngstein, Carol J Whelan, Thirusha Lane, Janet A Gilbertson, Dorota Rowczenio, Philip N Hawkins, Julian D Gillmore, Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid, European Heart Journal - Cardiovascular Imaging, Volume 18, Issue 12, December 2017, Pages 1344–1350, https://doi.org/10.1093/ehjci/jew325
Close - Share Icon Share
Abstract
High-grade (Perugini grade 2 or 3) cardiac uptake on bone scintigraphy with 99mTechnetium labelled 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) has lately been confirmed to have high diagnostic sensitivity and specificity for cardiac transthyretin (ATTR) amyloidosis. We sought to determine whether patient stratification by Perugini grade on 99mTc-DPD scintigraphy has prognostic significance in ATTR amyloidosis.
Patient survival from time of 99mTc-DPD scintigraphy was determined in 602 patients with ATTR amyloidosis, including 377 with wild-type ATTR (ATTRwt) and 225 with mutant ATTR (ATTRm) amyloidosis. Patients were stratified according to Perugini grade (0-3) on 99mTc-DPD scan. The prognostic significance of additional patient and disease-related factors at baseline were determined. In the whole cohort, the finding of a Perugini grade 0 99mTc-DPD scan (n = 28) was invariably associated with absence of cardiac amyloid according to consensus criteria as well as significantly better patient survival compared to a Perugini grade 1 (n = 28), 2 (n = 436) or 3 (n = 110) 99mTc-DPD scan (P < 0.005). There were no differences in survival between patients with a grade 1, grade 2 or grade 3 99mTc-DPD scan in ATTRwt (n = 369), V122I-associated ATTRm (n = 92) or T60A–associated ATTRm (n = 59) amyloidosis. Cardiac amyloid burden, determined by equilibrium contrast cardiac magnetic resonance imaging, was similar between patients with Perugini grade 2 and Perugini grade 3 99mTc-DPD scans but skeletal muscle/soft tissue to femur ratio was substantially higher in the latter group (P < 0.001).
99mTc-DPD scintigraphy is exquisitely sensitive for identification of cardiac ATTR amyloid, but stratification by Perugini grade of positivity at diagnosis has no prognostic significance.
Introduction
Cardiac transthyretin (ATTR) amyloidosis is a rarely diagnosed infiltrative cardiomyopathy with an inexorably progressive clinical course and poor prognosis. It is characterized by the relentless deposition and accumulation of fibrillar amyloid deposits in the extracellular space of the myocardium. ATTR amyloid fibrils are composed of either wild-type or variant transthyretin, the latter associated with a multitude of mutations in the transthyretin (TTR) gene.1,2 Wild-type ATTR (ATTRwt) amyloidosis is usually diagnosed in elderly males,3,4 and mutant ATTR (ATTRm) cardiac amyloidosis is particularly prevalent in certain populations such as individuals of African descent and from North West Ireland in whom the respective pathogenic V122I and T60A TTR variants are present in 4%5 and 1%6 of individuals, respectively. Certain TTR variants, such as V30M, which is particularly prevalent in Swedish, Japanese, and Portuguese populations, may result in a clinical phenotype of ATTR amyloidosis that is characterized by predominant autonomic and peripheral polyneuropathy in which the heart is spared.7
The traditional gold standard for diagnosis of cardiac ATTR amyloidosis is demonstration of cardiac amyloid deposits in an endomyocardial biopsy in the context of a characteristic echocardiogram or cardiac magnetic resonance (CMR) scan. Recently however, cardiac uptake on bone scintigraphy with tracers such as 99mTechnetium 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) and pyrophosphate (99mTc-PYP) has been confirmed to have high diagnostic sensitivity and specificity for cardiac ATTR amyloidosis.8 Rapezzi and colleagues devised a simple four-stage grading system based on the intensity of uptake in the myocardium relative to that in the bones (Perugini grade 0-3).9
The aim of our study was to determine whether patient stratification by Perugini grade on 99mTc-DPD scintigraphy has prognostic significance in ATTR amyloidosis, and to further investigate the biological basis for the different 99mTc-DPD scan appearances across the Perugini grades.
Methods
Patients
Six hundred and two patients with ATTR amyloidosis underwent 99mTc-DPD scintigraphy at the UK National Amyloidosis Centre between 2010 and 2016. Both ATTRwt and ATTRm amyloidosis were diagnosed in accordance with previously published criteria.8 Patients were managed symptomatically, as previously described.3 Deaths were recorded by the UK Office for National Statistics (ONS) and death certificate data was obtained from the NHS Health and Social Care Information Centre (HSCIC).10 Censor date was 5th October 2016.
All patients were managed in accordance with the Declaration of Helsinki and provided informed consent for publication of their data. The study was approved by the Royal Free Hospital ethics committee.
99mTc-DPD scintigraphy
All patients were administered 700 MBq of 99mTc-DPD intravenously and imaged 3 hrs later on either a General Electric (GE) Infinia Hawkeye 4 or GE Discovery 670 hybrid gamma camera. Whole body images were acquired at a scan speed of 10 cm/min using low energy high resolution collimators and were immediately followed by a SPECT-CT (single photon emission computed tomography with a low-dose, non-contrast CT scan) of the heart, as previously described.11 The expected radiation dose from the entire procedure was 6.7 mSv per patient.
Intensity of myocardial uptake on planar 99mTc-DPD scan was categorized as 0-3 according to the widely used Perugini grading system.9 Briefly, this comprises: grade 0–no cardiac uptake and normal bone uptake; grade 1–cardiac uptake which is less intense than the bone signal; grade 2–cardiac uptake with intensity similar or greater than bone signal; and grade 3–cardiac uptake with much attenuated or absent bone signal.
Soft tissue to femur ratio was calculated in the whole cohort by placing a line profile region of interest across the mid-thighs on the anterior whole body image and subsequently dividing the number of counts at the level of the skeletal muscle/soft tissue region by those at the adjacent femur level, as previously reported.11
Echocardiography
All patients underwent echocardiography at the UK National Amyloidosis Centre, as previously described.12 Presence or absence of cardiac ATTR amyloidosis was determined on the basis of consensus criteria, initially established for cardiac AL amyloidosis,13 with the addition in selected cases of gadolinium enhanced CMR imaging.14,15 Briefly, presence of cardiac amyloidosis was defined by positive Congo red histology on endomyocardial biopsy and/or mean left ventricular wall thickness of >12 mm on echocardiography and/or presence of delayed gadolinium enhancement on CMR imaging.
Cardiac magnetic resonance imaging
A subset of 122 patients with cardiac ATTR amyloidosis from the cohort underwent equilibrium contrast CMR imaging within 24 hrs of their 99mTc-DPD scan, on a 1.5-T clinical scanner (Avanto or Aera, Siemens Healthcare, Erlangen, Germany), reflecting the limited availability of this additional service. Cardiac amyloid burden was estimated by calculation of extracellular volume (ECV), as previously described.15 Patients were stratified according to Perugini grade on 99mTc-DPD scan and cardiac amyloid burden by CMR imaging was compared between groups.
Skeletal muscle histology
Skeletal muscle biopsies were obtained in six of the patients from the cohort. All muscle biopsies were stained with Congo red by the method of Puchtler et al.16 Amyloid deposits identified in these biopsies were typed immunohistochemically as previously described.17
Statistical analyses of survival
Patient survival from time of the baseline 99mTc-DPD scan was analysed by Kaplan Meier analysis using GraphPad Prism v5.03 software. Patients were stratified according to Perugini grade on 99mTc-DPD scan and patient survival was compared using the log-rank test. Patient and disease-related factors significantly associated with death by univariate analyses to a 0.1 level, were investigated in the whole cohort by Cox proportional hazards regression analysis to establish those that were independently associated with death to a significance level of 0.05 using IBM SPSS Statistics 23 software. ECV and soft tissue to femur ratios were compared between groups by Mann–Whitney U test.
Results
Patients
The cohort of 602 patients comprised 377 patients with ATTRwt amyloidosis, and 225 patients with ATTRm amyloidosis. Among patients with ATTRm amyloidosis, the most prevalent variants were V122I (n = 92) and T60A (n = 59), both of which are associated with a phenotype dominated by cardiac amyloidosis. Cardiac ATTR amyloidosis, identified on the basis of echocardiography, histology and/or CMR scan findings was present in a total of 563/602 (94%) patients. Thirty-five patients had V30M–associated ATTR amyloidosis, which is predominantly a neuropathic disease, and in which cardiac involvement is often absent. It is noteworthy that patients with V30M-associated ATTR amyloidosis had lower median LV wall thickness and NT-proBNP values in keeping with this clinical phenotype. Patient demographics for the whole cohort and in each individual cohort at the time of the baseline 99mTc-DPD scan are shown in Table 1.
Baseline characteristics
| Characteristic . | All patients N=602 . | Wild-type ATTR N = 377 . | V122I ATTRm N = 92 . | T60A ATTRm N = 59 . | V30M ATTRm N = 35 . | Other ATTRm N = 39 . |
|---|---|---|---|---|---|---|
| Age (median, range) (years) | 75 (23–91) | 77 (56–91) | 76 (57–88) | 69 (58–84) | 50 (23–80) | 55 (41–79) |
| Male (%) | 86 | 94 | 73 | 71 | 74 | 69 |
| NT-proBNP (median, range) (ng/L) | 2765 (17–34910) | 3188 (127–34910) | 2791 (135–32272) | 2250 (237–30657) | 186 (17–2799) | 1869 (17–15975) |
| Troponin T (median, range) (ng/L) | 63 (3–753) | 62 (4–582) | 87 (20–753) | 55 (13–161) | 12 (3–91) | 42 (5–109) |
| eGFR (median, range) (mL/min) | 57 (10–100) | 56 (18–100) | 48 (10–100) | 71 (10–100) | 95 (45–100) | 88 (28–100) |
| Supine systolic BP (median, range) (mmHg) | 121 (72–180) | 122 (72–179) | 120 (99–175) | 114 (85–171) | 124 (102–180) | 119 (81–162) |
| LV ejection fraction (median, range) (%) | 49 (15–75) | 48 (21–75) | 43 (15–68) | 50 (34–69) | 60 (45–73) | 55 (25–70) |
| IVSd (median, range) (mm) | 16 (8–25) | 17 (9–25) | 17 (12–22) | 16 (12–23) | 11 (9–20) | 15 (8–23) |
| 6MWT distance (median, range) (metres) | 345 (0–665) | 346 (0–607) | 272 (0–567) | 394 (0–499) | 368 (0–665) | 385 (0–506) |
| Cardiac amyloidosis‡ | ||||||
| Yes | 563 | 364 | 92 | 59 | 13 | 35 |
| No | 39 | 13 | 0 | 0 | 22 | 4 |
| ECOG performance status | ||||||
| 0 | 54 | 33 | 5 | 4 | 6 | 6 |
| 1 | 148 | 99 | 16 | 22 | 4 | 7 |
| 2 | 114 | 78 | 17 | 6 | 4 | 9 |
| 3 | 37 | 18 | 8 | 6 | 2 | 3 |
| Missing | 249 | 149 | 46 | 21 | 19 | 14 |
| NYHA functional class | ||||||
| 1 | 95 | 57 | 7 | 12 | 13 | 6 |
| 2 | 211 | 130 | 30 | 26 | 6 | 19 |
| 3 | 93 | 64 | 16 | 10 | 0 | 3 |
| 4 | 3 | 1 | 1 | 1 | 0 | 0 |
| Missing | 200 | 125 | 38 | 10 | 16 | 11 |
| DPD scintigraphy grade | ||||||
| Grade 0 | 28 | 8 | 0 | 0 | 19 | 1 |
| Grade 1 | 28 | 14 | 0 | 0 | 4 | 10 |
| Grade 2 | 436 | 316 | 57 | 36 | 12 | 15 |
| Grade 3 | 110 | 39 | 35 | 23 | 0 | 13 |
| Characteristic . | All patients N=602 . | Wild-type ATTR N = 377 . | V122I ATTRm N = 92 . | T60A ATTRm N = 59 . | V30M ATTRm N = 35 . | Other ATTRm N = 39 . |
|---|---|---|---|---|---|---|
| Age (median, range) (years) | 75 (23–91) | 77 (56–91) | 76 (57–88) | 69 (58–84) | 50 (23–80) | 55 (41–79) |
| Male (%) | 86 | 94 | 73 | 71 | 74 | 69 |
| NT-proBNP (median, range) (ng/L) | 2765 (17–34910) | 3188 (127–34910) | 2791 (135–32272) | 2250 (237–30657) | 186 (17–2799) | 1869 (17–15975) |
| Troponin T (median, range) (ng/L) | 63 (3–753) | 62 (4–582) | 87 (20–753) | 55 (13–161) | 12 (3–91) | 42 (5–109) |
| eGFR (median, range) (mL/min) | 57 (10–100) | 56 (18–100) | 48 (10–100) | 71 (10–100) | 95 (45–100) | 88 (28–100) |
| Supine systolic BP (median, range) (mmHg) | 121 (72–180) | 122 (72–179) | 120 (99–175) | 114 (85–171) | 124 (102–180) | 119 (81–162) |
| LV ejection fraction (median, range) (%) | 49 (15–75) | 48 (21–75) | 43 (15–68) | 50 (34–69) | 60 (45–73) | 55 (25–70) |
| IVSd (median, range) (mm) | 16 (8–25) | 17 (9–25) | 17 (12–22) | 16 (12–23) | 11 (9–20) | 15 (8–23) |
| 6MWT distance (median, range) (metres) | 345 (0–665) | 346 (0–607) | 272 (0–567) | 394 (0–499) | 368 (0–665) | 385 (0–506) |
| Cardiac amyloidosis‡ | ||||||
| Yes | 563 | 364 | 92 | 59 | 13 | 35 |
| No | 39 | 13 | 0 | 0 | 22 | 4 |
| ECOG performance status | ||||||
| 0 | 54 | 33 | 5 | 4 | 6 | 6 |
| 1 | 148 | 99 | 16 | 22 | 4 | 7 |
| 2 | 114 | 78 | 17 | 6 | 4 | 9 |
| 3 | 37 | 18 | 8 | 6 | 2 | 3 |
| Missing | 249 | 149 | 46 | 21 | 19 | 14 |
| NYHA functional class | ||||||
| 1 | 95 | 57 | 7 | 12 | 13 | 6 |
| 2 | 211 | 130 | 30 | 26 | 6 | 19 |
| 3 | 93 | 64 | 16 | 10 | 0 | 3 |
| 4 | 3 | 1 | 1 | 1 | 0 | 0 |
| Missing | 200 | 125 | 38 | 10 | 16 | 11 |
| DPD scintigraphy grade | ||||||
| Grade 0 | 28 | 8 | 0 | 0 | 19 | 1 |
| Grade 1 | 28 | 14 | 0 | 0 | 4 | 10 |
| Grade 2 | 436 | 316 | 57 | 36 | 12 | 15 |
| Grade 3 | 110 | 39 | 35 | 23 | 0 | 13 |
NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; BP, blood pressure; LV, left ventricular; IVSd, interventricular septal thickness at diastole; 6MWT, six-minute walk test; ECOG, Eastern Cooperative Oncology Group; NYHA, New York Heart Association; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid.
presence or absence of cardiac amyloidosis defined by echocardiography and/or CMR and/or endomyocardial histology.
Baseline characteristics
| Characteristic . | All patients N=602 . | Wild-type ATTR N = 377 . | V122I ATTRm N = 92 . | T60A ATTRm N = 59 . | V30M ATTRm N = 35 . | Other ATTRm N = 39 . |
|---|---|---|---|---|---|---|
| Age (median, range) (years) | 75 (23–91) | 77 (56–91) | 76 (57–88) | 69 (58–84) | 50 (23–80) | 55 (41–79) |
| Male (%) | 86 | 94 | 73 | 71 | 74 | 69 |
| NT-proBNP (median, range) (ng/L) | 2765 (17–34910) | 3188 (127–34910) | 2791 (135–32272) | 2250 (237–30657) | 186 (17–2799) | 1869 (17–15975) |
| Troponin T (median, range) (ng/L) | 63 (3–753) | 62 (4–582) | 87 (20–753) | 55 (13–161) | 12 (3–91) | 42 (5–109) |
| eGFR (median, range) (mL/min) | 57 (10–100) | 56 (18–100) | 48 (10–100) | 71 (10–100) | 95 (45–100) | 88 (28–100) |
| Supine systolic BP (median, range) (mmHg) | 121 (72–180) | 122 (72–179) | 120 (99–175) | 114 (85–171) | 124 (102–180) | 119 (81–162) |
| LV ejection fraction (median, range) (%) | 49 (15–75) | 48 (21–75) | 43 (15–68) | 50 (34–69) | 60 (45–73) | 55 (25–70) |
| IVSd (median, range) (mm) | 16 (8–25) | 17 (9–25) | 17 (12–22) | 16 (12–23) | 11 (9–20) | 15 (8–23) |
| 6MWT distance (median, range) (metres) | 345 (0–665) | 346 (0–607) | 272 (0–567) | 394 (0–499) | 368 (0–665) | 385 (0–506) |
| Cardiac amyloidosis‡ | ||||||
| Yes | 563 | 364 | 92 | 59 | 13 | 35 |
| No | 39 | 13 | 0 | 0 | 22 | 4 |
| ECOG performance status | ||||||
| 0 | 54 | 33 | 5 | 4 | 6 | 6 |
| 1 | 148 | 99 | 16 | 22 | 4 | 7 |
| 2 | 114 | 78 | 17 | 6 | 4 | 9 |
| 3 | 37 | 18 | 8 | 6 | 2 | 3 |
| Missing | 249 | 149 | 46 | 21 | 19 | 14 |
| NYHA functional class | ||||||
| 1 | 95 | 57 | 7 | 12 | 13 | 6 |
| 2 | 211 | 130 | 30 | 26 | 6 | 19 |
| 3 | 93 | 64 | 16 | 10 | 0 | 3 |
| 4 | 3 | 1 | 1 | 1 | 0 | 0 |
| Missing | 200 | 125 | 38 | 10 | 16 | 11 |
| DPD scintigraphy grade | ||||||
| Grade 0 | 28 | 8 | 0 | 0 | 19 | 1 |
| Grade 1 | 28 | 14 | 0 | 0 | 4 | 10 |
| Grade 2 | 436 | 316 | 57 | 36 | 12 | 15 |
| Grade 3 | 110 | 39 | 35 | 23 | 0 | 13 |
| Characteristic . | All patients N=602 . | Wild-type ATTR N = 377 . | V122I ATTRm N = 92 . | T60A ATTRm N = 59 . | V30M ATTRm N = 35 . | Other ATTRm N = 39 . |
|---|---|---|---|---|---|---|
| Age (median, range) (years) | 75 (23–91) | 77 (56–91) | 76 (57–88) | 69 (58–84) | 50 (23–80) | 55 (41–79) |
| Male (%) | 86 | 94 | 73 | 71 | 74 | 69 |
| NT-proBNP (median, range) (ng/L) | 2765 (17–34910) | 3188 (127–34910) | 2791 (135–32272) | 2250 (237–30657) | 186 (17–2799) | 1869 (17–15975) |
| Troponin T (median, range) (ng/L) | 63 (3–753) | 62 (4–582) | 87 (20–753) | 55 (13–161) | 12 (3–91) | 42 (5–109) |
| eGFR (median, range) (mL/min) | 57 (10–100) | 56 (18–100) | 48 (10–100) | 71 (10–100) | 95 (45–100) | 88 (28–100) |
| Supine systolic BP (median, range) (mmHg) | 121 (72–180) | 122 (72–179) | 120 (99–175) | 114 (85–171) | 124 (102–180) | 119 (81–162) |
| LV ejection fraction (median, range) (%) | 49 (15–75) | 48 (21–75) | 43 (15–68) | 50 (34–69) | 60 (45–73) | 55 (25–70) |
| IVSd (median, range) (mm) | 16 (8–25) | 17 (9–25) | 17 (12–22) | 16 (12–23) | 11 (9–20) | 15 (8–23) |
| 6MWT distance (median, range) (metres) | 345 (0–665) | 346 (0–607) | 272 (0–567) | 394 (0–499) | 368 (0–665) | 385 (0–506) |
| Cardiac amyloidosis‡ | ||||||
| Yes | 563 | 364 | 92 | 59 | 13 | 35 |
| No | 39 | 13 | 0 | 0 | 22 | 4 |
| ECOG performance status | ||||||
| 0 | 54 | 33 | 5 | 4 | 6 | 6 |
| 1 | 148 | 99 | 16 | 22 | 4 | 7 |
| 2 | 114 | 78 | 17 | 6 | 4 | 9 |
| 3 | 37 | 18 | 8 | 6 | 2 | 3 |
| Missing | 249 | 149 | 46 | 21 | 19 | 14 |
| NYHA functional class | ||||||
| 1 | 95 | 57 | 7 | 12 | 13 | 6 |
| 2 | 211 | 130 | 30 | 26 | 6 | 19 |
| 3 | 93 | 64 | 16 | 10 | 0 | 3 |
| 4 | 3 | 1 | 1 | 1 | 0 | 0 |
| Missing | 200 | 125 | 38 | 10 | 16 | 11 |
| DPD scintigraphy grade | ||||||
| Grade 0 | 28 | 8 | 0 | 0 | 19 | 1 |
| Grade 1 | 28 | 14 | 0 | 0 | 4 | 10 |
| Grade 2 | 436 | 316 | 57 | 36 | 12 | 15 |
| Grade 3 | 110 | 39 | 35 | 23 | 0 | 13 |
NT-proBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; BP, blood pressure; LV, left ventricular; IVSd, interventricular septal thickness at diastole; 6MWT, six-minute walk test; ECOG, Eastern Cooperative Oncology Group; NYHA, New York Heart Association; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid.
presence or absence of cardiac amyloidosis defined by echocardiography and/or CMR and/or endomyocardial histology.
Median follow-up from baseline for the whole cohort was 29.6 months during which time 202 patients died. Cause of death in almost all (>95%) patients was from ‘progressive amyloidosis’, usually associated with pneumonia or end-stage cardiac failure.
99mTc-DPD scintigraphy in relation to survival
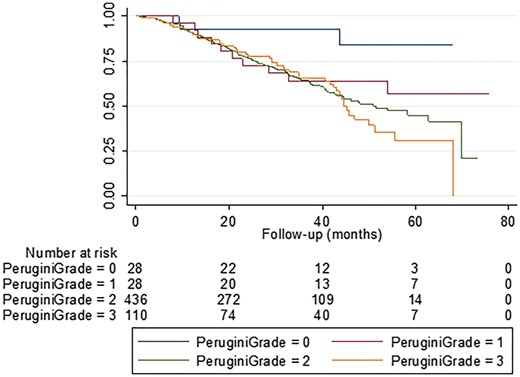
Among 602 patients with ATTR amyloidosis, 28 had Perugini grade 0 99mTc-DPD scans, 28 Perugini grade 1, 436 Perugini grade 2 and 110 had Perugini grade 3 99mTc-DPD scans. Ninety five percent of the whole cohort had abnormal cardiac uptake on 99mTc-DPD scintigraphy, the vast majority (91%) having a Perugini grade 2 or grade 3 99mTc-DPD scan. Survival was significantly longer (median not reached) in patients with a Perugini grade 0 99mTc-DPD scan compared to those with a Perugini grade 1, Perugini grade 2 or Perugini grade 3 99mTc-DPD scan by Kaplan Meier analysis (log rank test, P = <0.04 for grade 0 vs. each other grade). There was no significant difference in survival between patients with Perugini grade 1, Perugini grade 2 or Perugini grade 3 scans (Figure 1, log rank test, P = 0.39 for Perugini grade 1 vs. Perugini grade 2; P = 0.51 for Perugini grade 2 vs. Perugini grade 3). Median estimated survival in those with a Perugini grade 1 99mTc-DPD scan was not reached, in those with a Perugini grade 2 99mTc-DPD scan was 55.3 months, and in those with a Perugini grade 3 99mTc-DPD scan was 46.5 months.
Survival of all patients with ATTR amyloidosis stratified by Perugini grade on 99mTc-DPD scintigraphy. Patients with no cardiac uptake on 99mTc-DPD scan (Perugini grade 0) survived for significantly longer than those with abnormal cardiac uptake on 99mTc-DPD scan (log rank test, P < 0.02 for grade 0 vs. 1, P < 0.002 for grade 0 vs. 2, and P = 0.0003 for grade 0 vs. 3). There was no significant difference in survival between patients with a Perugini grade 1, 2, or 3 99mTc-DPD scan (P = 0.39 for grade 1 vs. 2, P = 0.19 for grade 1 vs. 3, P = 0.51 for grade 2 vs. 3).
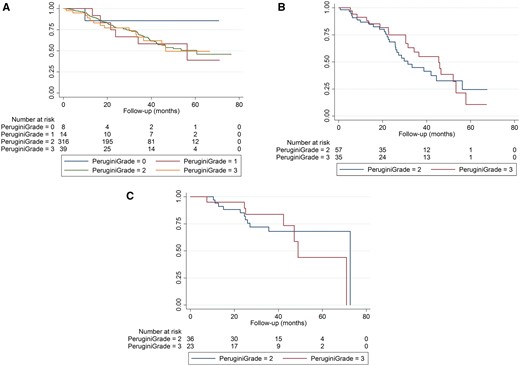
Survival, stratified according to a Perugini grade 1, Perugini grade 2 and Perugini grade 3 99mTc-DPD scan, was separately compared in three different cohorts of patients; those with ATTRwt amyloidosis (n = 377), those with V122I-associated ATTR amyloidosis (n = 92), and those with T60A–associated ATTR amyloidosis (n = 59). There was no difference in survival on the basis of 99mTc-DPD scan grade of positivity in any of these patient cohorts (Figure 2A–C). In 35 patients with V30M-associated ATTR amyloidosis, survival was significantly longer among the 19 patients without cardiac uptake of 99mTc-DPD, none of whom had evidence of cardiac amyloidosis by consensus criteria, compared to 16 patients with positive 99mTc-DPD scans (log rank test, P < 0.02).
Survival in individual cohorts stratified by Perugini grade 1, 2, or 3 on 99mTc-DPD scintigraphy. (A) Survival in patients with ATTRwt amyloidosis stratified by Perugini grade 1, 2, or 3 99mTc-DPD scan (log rank test, P = 0.89 for grade 1 vs. 2, P = 0.88 for grade 2 vs. 3). (B) Survival in patients with V122I-associated ATTR amyloidosis stratified by Perugini grade 2 or 3 99mTc-DPD scan (log rank test, P = 0.49). There were no patients with a Perugini grade 1 scan. (C) Survival in patients with T60A-associated ATTR amyloidosis stratified by Perugini grade 2 or 3 99mTc-DPD scan (log rank test, P = 0.75). There were no patients with a Perugini grade 1 scan.
Additional factors influencing survival
The following additional factors were significant predictors of mortality in the whole cohort by univariable analyses; age (P < 0.001), 6 min walk test distance (P < 0.001), left ventricular ejection fraction (P = 0.002), Troponin T concentration (P < 0.001), NT-proBNP concentration (P < 0.001), ECOG performance status (P < 0.001), supine systolic blood pressure (P < 0.001), estimated glomerular filtration rate (eGFR) (P < 0.001), and NYHA class (P < 0.001).
The only factors independently associated with mortality in a multivariable Cox proportional hazards model with age, ECOG performance status, left ventricular ejection fraction, 99mTc-DPD scan grade (0 vs. 1/2/3), NT-proBNP concentration, supine systolic blood pressure (≤100 mmHg vs. >100 mmHg), and eGFR as predictor variables were ECOG performance status (HR for 3 vs. 0 of 9.5 [CI 1.9–47.4], P = 0.006) and eGFR (HR 0.98 [CI 0.96–0.99], P = 0.002) (Table 2).
Multivariable analysis of baseline factors influencing survival
| Factor . | Hazard ratio . | P-value . | Confidence interval . |
|---|---|---|---|
| Perugini grade | |||
| 0 | 1 | ||
| 1/2/3 | 0.68 | 0.715 | 0.089–5.235 |
| Age (per yr) | 1.00 | 0.854 | 0.973–1.034 |
| ECOG performance | |||
| 0 | 1 | ||
| 1 | 2.06 | 0.338 | 0.469–9.048 |
| 2 | 4.04 | 0.066 | 0.914–17.838 |
| 3 | 9.53 | 0.006 | 1.914–47.432 |
| Supine systolic BP (mmHg) | |||
| >100 | 1 | ||
| ≤100 | 0.76 | 0.617 | 0.265–2.197 |
| eGFR (per mL) | 0.98 | 0.002 | 0.962–0.992 |
| LVEF (per percentage point) | 1.00 | 0.895 | 0.978–1.025 |
| NT-proBNP (per ng/L) | 1.00 | 0.695 | 0.999–1.000 |
| Factor . | Hazard ratio . | P-value . | Confidence interval . |
|---|---|---|---|
| Perugini grade | |||
| 0 | 1 | ||
| 1/2/3 | 0.68 | 0.715 | 0.089–5.235 |
| Age (per yr) | 1.00 | 0.854 | 0.973–1.034 |
| ECOG performance | |||
| 0 | 1 | ||
| 1 | 2.06 | 0.338 | 0.469–9.048 |
| 2 | 4.04 | 0.066 | 0.914–17.838 |
| 3 | 9.53 | 0.006 | 1.914–47.432 |
| Supine systolic BP (mmHg) | |||
| >100 | 1 | ||
| ≤100 | 0.76 | 0.617 | 0.265–2.197 |
| eGFR (per mL) | 0.98 | 0.002 | 0.962–0.992 |
| LVEF (per percentage point) | 1.00 | 0.895 | 0.978–1.025 |
| NT-proBNP (per ng/L) | 1.00 | 0.695 | 0.999–1.000 |
Multivariable analysis of baseline factors influencing survival
| Factor . | Hazard ratio . | P-value . | Confidence interval . |
|---|---|---|---|
| Perugini grade | |||
| 0 | 1 | ||
| 1/2/3 | 0.68 | 0.715 | 0.089–5.235 |
| Age (per yr) | 1.00 | 0.854 | 0.973–1.034 |
| ECOG performance | |||
| 0 | 1 | ||
| 1 | 2.06 | 0.338 | 0.469–9.048 |
| 2 | 4.04 | 0.066 | 0.914–17.838 |
| 3 | 9.53 | 0.006 | 1.914–47.432 |
| Supine systolic BP (mmHg) | |||
| >100 | 1 | ||
| ≤100 | 0.76 | 0.617 | 0.265–2.197 |
| eGFR (per mL) | 0.98 | 0.002 | 0.962–0.992 |
| LVEF (per percentage point) | 1.00 | 0.895 | 0.978–1.025 |
| NT-proBNP (per ng/L) | 1.00 | 0.695 | 0.999–1.000 |
| Factor . | Hazard ratio . | P-value . | Confidence interval . |
|---|---|---|---|
| Perugini grade | |||
| 0 | 1 | ||
| 1/2/3 | 0.68 | 0.715 | 0.089–5.235 |
| Age (per yr) | 1.00 | 0.854 | 0.973–1.034 |
| ECOG performance | |||
| 0 | 1 | ||
| 1 | 2.06 | 0.338 | 0.469–9.048 |
| 2 | 4.04 | 0.066 | 0.914–17.838 |
| 3 | 9.53 | 0.006 | 1.914–47.432 |
| Supine systolic BP (mmHg) | |||
| >100 | 1 | ||
| ≤100 | 0.76 | 0.617 | 0.265–2.197 |
| eGFR (per mL) | 0.98 | 0.002 | 0.962–0.992 |
| LVEF (per percentage point) | 1.00 | 0.895 | 0.978–1.025 |
| NT-proBNP (per ng/L) | 1.00 | 0.695 | 0.999–1.000 |
Cardiac MRI vs. 99mTc-DPD scintigraphy
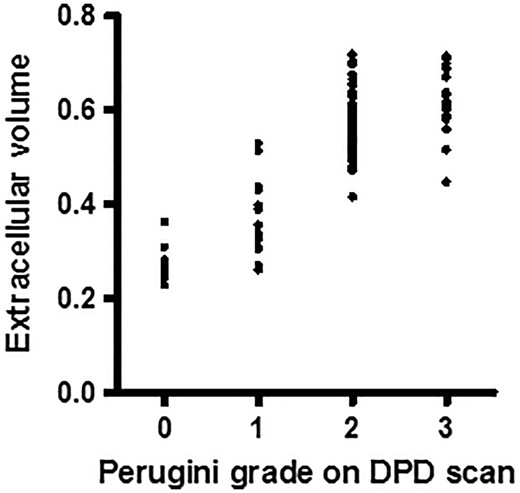
An analysis of cardiac amyloid burden by CMR imaging in a subset of 122 patients from the cohort showed a significant difference in cardiac amyloid burden between patients with a Perugini grade 0 99mTc-DPD scan compared to those with a Perugini grade 1 99mTc-DPD scan (Mann Whitney U test, P < 0.004). There was also a statistically significant difference in cardiac amyloid burden between those with Perugini grade 1 compared to Perugini grade 2 or 3 99mTc-DPD scans, but with considerable overlap between the groups (Figure 3). Comparison of cardiac amyloid burden between those with a Perugini grade 0 and those with a Perugini grade 2 or 3 99mTc-DPD scan showed no overlap between the groups and was highly significantly different (Mann Whitney U test, P = <0.0001).
Cardiac amyloid burden in relation to Perugini grade by 99mTc-DPD scintigraphy in a subset of 122 patients. There is a significant difference in cardiac amyloid burden between patients with a Perugini grade 0 99mTc-DPD scan compared to those with a Perugini grade 1 99mTc-DPD scan (Mann Whitney U test, P < 0.004). There is also a statistically significant difference in cardiac amyloid burden between those with Perugini grade 1 compared to Perugini grade 2 or 3 99mTc-DPD scans, but with considerable overlap between the groups. Comparison of cardiac amyloid burden between those with a Perugini grade 0 and those with a Perugini grade 2 or 3 99mTc-DPD scan was highly significantly different with no overlap between the groups (Mann Whitney U test, P = <0.0001).
Soft tissue to femur ratio by 99mTc-DPD scintigraphy
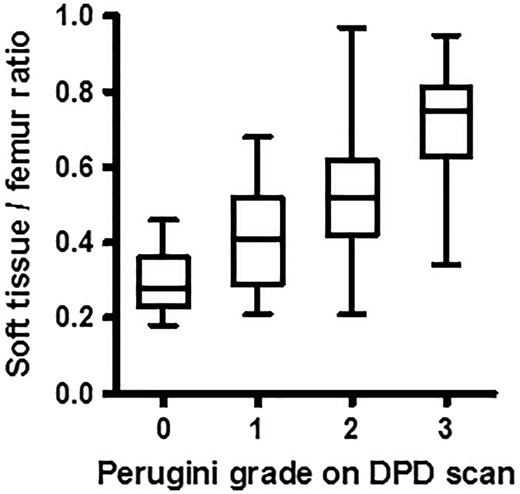
Soft tissue to femur ratio was significantly different between each Perugini grade of 99mTc-DPD scan (P = 0.002) with a particularly marked increase between Perugini grades 2 and 3 (P < 0.001) (Figure 4).
Soft tissue to femur ratio of counts in relation to Perugini grade by 99mTc-DPD scintigraphy. There was a significant increase in soft tissue to femur ratio between all Perugini grades of 99mTc-DPD scan (P = 0.002), with little overlap between those with a grade 2 and those with a grade 3 99mTc-DPD scan (P < 0.001).
Muscle biopsies
ATTR amyloid was present in 3/3 skeletal muscle biopsies from patients with a Perugini grade 3 99mTc-DPD scan but in 0/1 with a Perugini grade 1 scan and 0/2 patients with a Perugini grade 2 99mTc-DPD scan.
Discussion and conclusions
The findings presented in this large study of patients with ATTR amyloidosis, 91% of whom had a Perugini grade 2 or 3 99mTc-DPD scan at the time of diagnosis, indicate that there is no difference in prognosis between patients with different grades of abnormal cardiac uptake (i.e. Perugini grade 1, 2 or 3) by 99mTc-DPD scan, but that patients with no abnormal cardiac uptake (Perugini grade 0) on 99mTc-DPD scan, indicating absence of cardiac ATTR cardiac amyloidosis, do fare better. These data show that despite the previously reported high diagnostic sensitivity of abnormal cardiac uptake on 99mTc-DPD scintigraphy for cardiac ATTR amyloidosis and its utility in disease diagnosis,8 the specific grade of positivity according to the Perugini classification provides little prognostic information in ATTR amyloidosis. Although our centre has no experience with 99mTc-PYP scintigraphy, which is widely used in North America for diagnosis of cardiac ATTR amyloidosis, our findings are in keeping with those reported by Castano et al.,18 who performed a similar analysis in a cohort of patients injected with PYP tracer. They also reported a non-significant Hazard ratio for grade 3 vs. 2 (HR = 3.543 [95% CI of 0.474–26.455], P = 0.22). They did however, find that myocardial retention of 99mTc-PYP using the heart to contralateral ratio was independently associated with survival. This raises the intriguing possibility that quantitative or semi quantitative measures may provide prognostic information not provided by the Perugini grading system.
Ninety-seven percent (546/563) of patients in this cohort who were identified to have cardiac ATTR amyloidosis on the basis of a left ventricular wall thickness >12 mm by echocardiography and/or characteristic CMR scan findings and/or positive endomyocardial histology, presented with a Perugini grade 2 or Perugini grade 3 99mTc-DPD scan. The difference between a Perugini grade 2 and a Perugini grade 3 99mTc-DPD scan is based on attenuation of the bone signal. Whilst the latter has previously been attributed to competitive uptake of 99mTc-DPD in the heart vs. the bones, we have previously demonstrated on planar whole body imaging that the apparent attenuation of bone signal reflects uptake of tracer in the skeletal muscle and/or soft tissue overlying the bones,11 further corroborated here by the highly significant increase in soft tissue to femur ratio observed in patients with a grade 3 99mTc-DPD scan compared to a Perugini grade 2 99mTc-DPD scan. Amyloid in the soft tissues and muscle has previously been reported as a clinical manifestation of ATTR amyloidosis,19 and muscle biopsies performed in a small subset of patients in this cohort confirmed skeletal muscle amyloid in all 3 patients with Perugini grade 3 99mTc-DPD scans, but none of 3 patients with Perugini grade 1 or 2 99mTc-DPD scans. Whilst skeletal muscle amyloid deposits may be relatively scanty and of limited clinical significance, the total bulk of skeletal muscle in a patient may easily exceed 25 kg, compared to a typical myocardial mass of less than 0.5 kg. Skeletal muscle amyloid may therefore represent a substantial compartment in terms of localisation of the 99mTc-DPD tracer, potentially competing with uptake into amyloid in the heart and thereby complicating its quantification by the Perugini grading method. This is also the mechanism by which tracer uptake into the bones is obscured on visualization of planar whole body imaging, resulting in the appearance of a Perugini grade 3 99mTc-DPD scan.
Survival was superior among patients with Perugini grade 0 99mTc-DPD scans compared to those with Perugini grade 1, 2, or 3 99mTc-DPD scans. Cardiac amyloid burden measured independently by equilibrium contrast CMR imaging was similar among patients with Perugini grade 2 or 3 99mTc-DPD scans, and was substantially greater than among patients with grade 0 99mTc-DPD scans with no overlap (P < 0.0001). Although the cardiac amyloid burden by CMR imaging in those with Perugini grade 1 99mTc-DPD scans was significantly less than in those with Perugini grade 2 or 3 99mTc-DPD scans, there was considerable overlap between these groups and no survival difference was detected. Given that <5% of patients in the cohort had Perugini grade 1 99mTc-DPD scans, the comparison between patients with grade 1 and patients with grades 2 or 3 99mTc-DPD scans should be interpreted with a degree of caution. Nonetheless, these data serve to re-affirm the diagnostic sensitivity of 99mTc-DPD scintigraphy for cardiac ATTR amyloidosis, and corroborate previously reported findings which show that presence of cardiac involvement by amyloid among patients with ATTR amyloidosis confers a poor prognosis.2,20 It is noteworthy that a substantial proportion of patients (19/28) with Perugini grade 0 99mTc-DPD scans, none of whom had evidence of cardiac amyloid on either CMR scan or echocardiography, had V30M-associated ATTR amyloidosis and that their median age was 39 years. It is well established that younger V30M-associated ATTR amyloidosis patients often have a phenotype characterized by polyneuropathy without cardiomyopathy.21 Multivariable analyses on the whole cohort showed that the only independent predictors of mortality were eGFR and ECOG performance status at baseline.
In summary, 99mTc-DPD scintigraphy is exquisitely sensitive for identifying the presence of cardiac ATTR amyloid at the time of diagnosis. Although a Perugini grade 2 or 3 99mTc-DPD scan has a high diagnostic sensitivity and specificity for cardiac ATTR amyloidosis, it is the presence of cardiac amyloid indicated by abnormal cardiac uptake of 99mTc-DPD into the heart rather than the Perugini grade of uptake that confers prognostic significance in patients with ATTR amyloidosis. Uptake of tracer into skeletal muscle and soft tissue amyloid deposits is the chief cause of the attenuated bone signal among patients with grade 3 99mTc-DPD scans.
Acknowledgements
We thank our many physician colleagues for referring and caring for the patients. Core support for the National Amyloidosis Centre is provided by NHS England, the UK National Institute for Health Research Biomedical Research Centre and Unit Funding Scheme. DFH, MF and JDG were responsible for conceiving the study, interpreting the results and drafting the manuscript. MB, AMQ, JCR, AP, JP, AMN, ADW, HJL, TR, CCQ, SM, SS, TY, CJW, TL, JAG, and DR were responsible for data collection and interpretation of results. PNH contributed to interpretation of results and critical review of the manuscript. CCQ was granted a Research award from the Italian Ministry of Health–GR-2011-02352282.
Conflict of interest: None declared.