-
PDF
- Split View
-
Views
-
Cite
Cite
F. Tustumi, W. M. Bernardo, J. R. M. da Rocha, S. Szachnowicz, F. C. Seguro, E. T. Bianchi, R. A. A. Sallum, I. Cecconello, Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis, Diseases of the Esophagus, Volume 30, Issue 10, October 2017, Pages 1–8, https://doi.org/10.1093/dote/dox072
Close - Share Icon Share
Summary
Achalasia of the cardia is associated with an increased risk of esophageal carcinoma. The real burden of achalasia at the malignancy genesis is still a controversial issue. Therefore, there are no generally accepted recommendations on follow-up evaluation for achalasia patients. This study aims to estimate the risk of esophageal adenocarcinoma and squamous cell carcinoma in achalasia patients. We searched for association between carcinoma and esophageal achalasia in databases up to January 2017 to perform a systematic review and meta-analysis. A total of 1,046 studies were identified from search strategy, of which 40 were selected for meta-analysis. A cumulative number of 11,978 esophageal achalasia patients were evaluated. The incidence of squamous cell carcinoma was 312.4 (StDev 429.16) cases per 100,000 patient-years at risk. The incidence of adenocarcinoma was 21.23 (StDev 31.6) cases per 100,000 patient-years at risk. The prevalence for esophageal carcinoma was 28 carcinoma cases in 1,000 esophageal achalasia patients (CI 95% 2, 39). The prevalence for squamous cell carcinoma was 26 cases in 1,000 achalasia patients (CI 95% 18, 39) and for adenocarcinoma was 4 cases in 1,000 achalasia patients (CI 95% 3, 6).The absolute risk increase for squamous cell carcinoma was 308.1 and for adenocarcinoma was 18.03 cases per 100,000 patients per year. To the best of our knowledge, this is the first meta-analysis estimating the burden of achalasia as an esophageal cancer risk factor. The high increased risk rate for cancer in achalasia patients points to a strict endoscopic surveillance for these patients. Also, the increased risk for developing adenocarcinoma in achalasia patients suggests fundoplication after myotomy, to avoid esophageal reflux and Barret esophagus, a known risk factor for adenocarcinoma.
INTRODUCTION
Several factors leading to an increased risk of esophageal carcinoma in achalasia patients have been described. Continuous chemical irritation due to saliva and food decomposition in the esophageal stasis could induce chronic hyperplastic esophagitis, dysplasia, and eventually carcinoma.1 Pajecki et al.2 attribute to volatile nitrosamines an important role in carcinogenesis, since nitrosamines have shown strong carcinogenic effects in experimental models.3,4 Overgrowth of nitrate-reducing bacteria due to esophageal food stasis would lead to increased nitrite concentration in the esophageal lumen.
Genetic studies pointed some mutations that could be associated with advanced Chagasic megaesophagus. Silent mutation in codon 88 of exon 7 of the FHIT gene5 and a mutation involving exon 6 of the TP53 gene, as well as mutations in exons 5 and 7 of this gene6 were reported. Aneuploidies in chromosome 7, 11, and 17 might as well be associated with an increased risk of esophageal squamous cell carcinoma (SCC).7
A recent study8 demonstrates that esophageal SCC emerges as a disease that brings genetic similarity with other SCCs. Still, esophageal adenocarcinoma (EA) resembles chromosomal instability of the gastric adenocarcinoma. However, there are no studies investigating genetics characteristics of the EA in achalasia patients. It is assumed that achalasia treatment with dilatation or myotomy could be a carcinogenesis-producing mechanism. These procedures may lead to esophageal reflux, and hence metaplastic transformation of squamous mucosa to Barrett's esophagus and, eventually, to EA.9
While several studies evidence reasons for carcinoma and esophageal achalasia association, the real burden of achalasia at the malignancy genesis is still a controversial issue. Hence, there are no generally accepted recommendations on follow-up evaluation for achalasia patients.10
This study aims to perform a systematic review and meta-analysis to estimate the risk of esophageal carcinoma in achalasia patients and to report the demographics and clinical information of these patients.
METHODS
The construction and modeling of this study was guided by PRISMA statement.11
Database search
Literature search was performed in MEDLINE, with search algorithm: ((esophageal OR esophagus) AND (neoplasm OR neoplasia OR tumor OR cancer) AND (achalasia OR cardiospasm OR megaesophagus)). Others databases searched were LILACS/BVS, Cochrane, and gray literature.
No attempts were made to locate unpublished material. When more than one publication of a single trial existed, only the publication with the most complete data was included.
Inclusion criteria
Patients with documented esophageal achalasia;
Studies that evaluate prevalence or incidence of esophageal carcinoma;
Prospective or retrospective and cohort or cross-sectional studies;
Studies selected by both reviewers.
Exclusion criteria
Data that could not be extracted from pooled results;
Patients previously submitted to esophagectomy;
Case reports, animal models, conference proceedings, editorials and letters;
Review papers were excluded from meta-analysis;
Studies with no fulltext.
Languages
No restriction.
Search period
No restriction. Search was performed up to January 2017.
Outcomes
Prevalence of esophageal carcinoma;
Incidence of esophageal carcinoma;
Mortality;
Carcinoma esophageal topography.
Subgroup analysis was performed to assess risk of bias. Outcomes evaluated include studies with exclusion of synchronous diagnosis of achalasia and cancer; studies with a minimum follow-up of 5 years; by time of cancer diagnosis; for comparison between cardia dilatation and myotomy treatments; and for comparison of different geographic places.
Statistical analysis
Absolute numbers for the outcomes parameters were extracted and analyzed with comprehensive meta-analysis software version 2.12 ANOVA test was used for means comparison and Taylor series were performed for analysis risk difference on 2 × 2 table.
RESULTS
Studies characteristics
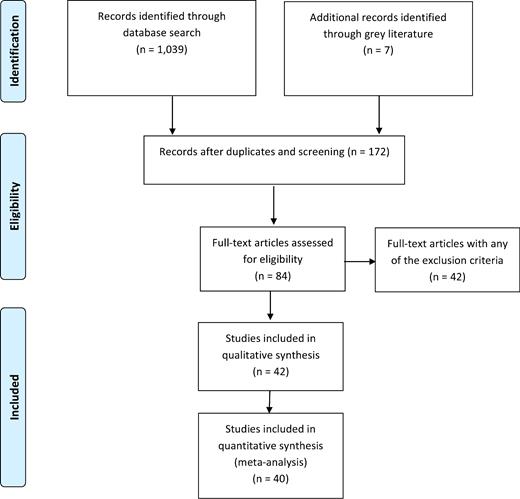
In this study, 4213–54 eligible trials were eventually identified and reviewed and 246,47 were excluded from meta-analysis due to the lack of sufficient data (see Fig. 1). A cumulative number of 11,978 esophageal achalasia patients were evaluated. Only four studies reported cases of Chagas disease among the patients.19,21,31,32
Flow chart of the studies selection. In this study, 42 eligible trials were eventually identified and reviewed and 2 were excluded from meta-analysis due to the lack of sufficient data.
Data were collected from 17 countries. The mean follow-up across all studies, when this information was available,14–16,20,23,25,26,32,36,40,42,43,48–50,52–54 ranged from 3.33 to 23.2 years.
For the evaluation of the methodological quality of studies, we used JBI critical appraisal checklist for case series55 (see Table 1).
JBI55 critical appraisal checklist for case series
| . | No . | Unclear . | Yes . |
|---|---|---|---|
| JBI critical appraisal checklist for case series . | (%) . | (%) . | (%) . |
| 1. Were there clear criteria for inclusion in the case series? | 4.7 | 0 | 95.3 |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | 0 | 0 | 100 |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | 0 | 0 | 100 |
| 4. Did the case series have consecutive inclusion of participants? | 4.7 | 2.4 | 92.9 |
| 5. Did the case series have complete inclusion of participants? | 9.5 | 2.4 | 88.1 |
| 6. Was there clear reporting of the demographics of the participants in the study? | 2.4 | 66.6 | 31 |
| 7. Was there clear reporting of clinical information of the participants? | 2.4 | 54.7 | 43.9 |
| 8. Were the outcomes or follow up results of cases clearly reported? | 4.7 | 73.8 | 21.5 |
| 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | 0 | 0 | 100 |
| 10. Was statistical analysis appropriate? | 0 | 0 | 100 |
| . | No . | Unclear . | Yes . |
|---|---|---|---|
| JBI critical appraisal checklist for case series . | (%) . | (%) . | (%) . |
| 1. Were there clear criteria for inclusion in the case series? | 4.7 | 0 | 95.3 |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | 0 | 0 | 100 |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | 0 | 0 | 100 |
| 4. Did the case series have consecutive inclusion of participants? | 4.7 | 2.4 | 92.9 |
| 5. Did the case series have complete inclusion of participants? | 9.5 | 2.4 | 88.1 |
| 6. Was there clear reporting of the demographics of the participants in the study? | 2.4 | 66.6 | 31 |
| 7. Was there clear reporting of clinical information of the participants? | 2.4 | 54.7 | 43.9 |
| 8. Were the outcomes or follow up results of cases clearly reported? | 4.7 | 73.8 | 21.5 |
| 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | 0 | 0 | 100 |
| 10. Was statistical analysis appropriate? | 0 | 0 | 100 |
JBI55 critical appraisal checklist for case series
| . | No . | Unclear . | Yes . |
|---|---|---|---|
| JBI critical appraisal checklist for case series . | (%) . | (%) . | (%) . |
| 1. Were there clear criteria for inclusion in the case series? | 4.7 | 0 | 95.3 |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | 0 | 0 | 100 |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | 0 | 0 | 100 |
| 4. Did the case series have consecutive inclusion of participants? | 4.7 | 2.4 | 92.9 |
| 5. Did the case series have complete inclusion of participants? | 9.5 | 2.4 | 88.1 |
| 6. Was there clear reporting of the demographics of the participants in the study? | 2.4 | 66.6 | 31 |
| 7. Was there clear reporting of clinical information of the participants? | 2.4 | 54.7 | 43.9 |
| 8. Were the outcomes or follow up results of cases clearly reported? | 4.7 | 73.8 | 21.5 |
| 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | 0 | 0 | 100 |
| 10. Was statistical analysis appropriate? | 0 | 0 | 100 |
| . | No . | Unclear . | Yes . |
|---|---|---|---|
| JBI critical appraisal checklist for case series . | (%) . | (%) . | (%) . |
| 1. Were there clear criteria for inclusion in the case series? | 4.7 | 0 | 95.3 |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | 0 | 0 | 100 |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | 0 | 0 | 100 |
| 4. Did the case series have consecutive inclusion of participants? | 4.7 | 2.4 | 92.9 |
| 5. Did the case series have complete inclusion of participants? | 9.5 | 2.4 | 88.1 |
| 6. Was there clear reporting of the demographics of the participants in the study? | 2.4 | 66.6 | 31 |
| 7. Was there clear reporting of clinical information of the participants? | 2.4 | 54.7 | 43.9 |
| 8. Were the outcomes or follow up results of cases clearly reported? | 4.7 | 73.8 | 21.5 |
| 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | 0 | 0 | 100 |
| 10. Was statistical analysis appropriate? | 0 | 0 | 100 |
Development of carcinoma
The mean age across the studies by the time esophageal carcinoma was diagnosed was 56.9 (StDev 12.9) years old for SCC and 68.3 (StDev 16.5) years old for EA.
The mean period after symptoms onset of achalasia until developing esophageal carcinoma across studies16,20–22,24,26,28,30,33,34,40,43,44,50 was 22.22 (StDev 11.1) years.
The mean period after myotomy or esophageal dilatation until developing esophageal carcinoma across studies16,18,20,25–28,30,34,38,42,43,48,50,53 was 11.5 (StDev 9.5) years for SCC; and 12 (StDev 4.9) years for EA.26,37,48
Prevalence
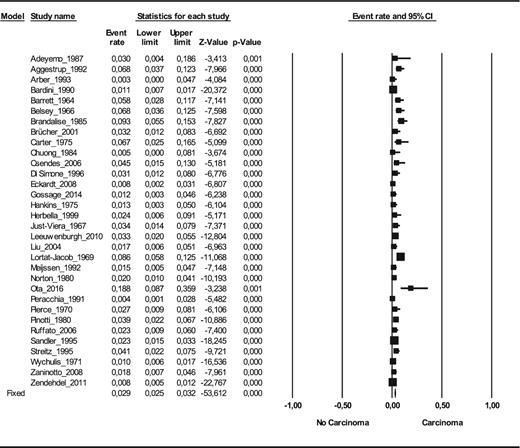
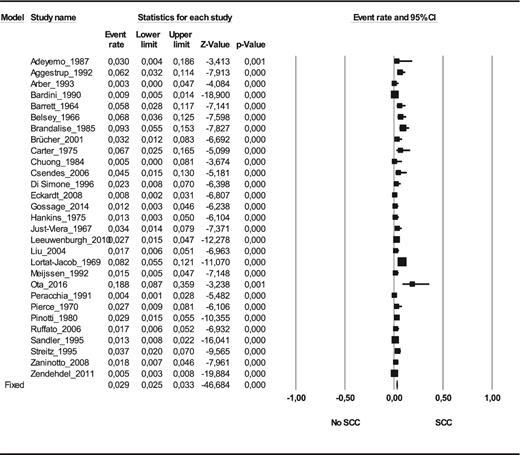
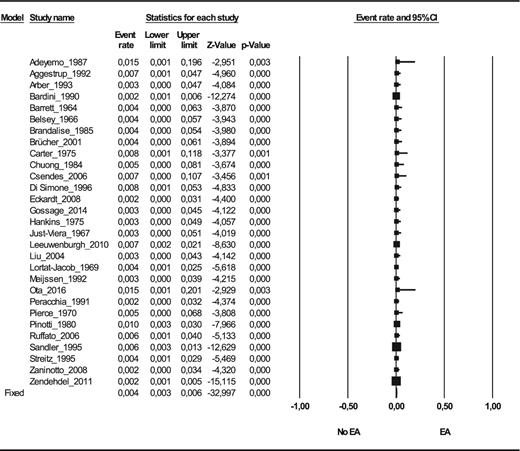
The prevalence of esophageal carcinoma in esophageal achalasia patients was 28 carcinoma cases per 1,000 achalasia patients on random effect model (CI 95% 2, 39; n = 11,899) and 29 on fixed effect model (CI 95% 25, 32; n = 11,899)13–20,22,23,25–28,30,32,35,36,38–45,48–50,52–54 (see Fig. 2). The prevalence of squamous cell carcinoma was 26 SCC cases per 1,000 achalasia patients on random effect model (CI 95% 18, 39; n = 10,148) and 29 on fixed effect model (CI 95% 25, 33; n = 10,148)13–20,22,23,25–28,30,35,36,38–40,42–45,48–50,53,54 (see Fig. 3); and the prevalence of adenocarcinoma was 4 EA cases per 1,000 achalasia patients on random effect model (CI 95% 3, 6; n = 10,158) and 4 on fixed effect model (CI 95% 3, 6; n = 10,158)13–20,22,23,25–28,30,35,36,38–40,42–45,48–50,53,54 (see Fig. 4).
The prevalence of esophageal carcinoma in esophageal achalasia patients (number of carcinoma cases among achalasia patients) (n = 11,899).
The in prevalence of SCC (squamous cell carcinoma) in esophageal achalasia patients (number of SCC cases among achalasia patients) (n = 10,148).
The prevalence of esophageal adenocarcinoma (EA) in achalasia patients (number of EA among achalasia patients) (n = 10,158).
Subgroup analysis
Patients who have cancer at the diagnosis of achalasia were excluded to be certain that achalasia is not secondary to cancer (pseudoachalasia).Then, the prevalence of carcinoma was 21 carcinoma cases per 1,000 achalasia patients (CI 95% 14, 33; n = 7,997).13–15,20,23,25–28,32,36,38,40,42,43,48,49,52–54
When considering only studies that had a mean follow-up of at least 5 years, the prevalence was 25 carcinoma cases per 1,000 achalasia patients (CI 95% 15, 42; n = 7,018).14,15,20,23,25,26,36,38,42,48,49,52–54
For subgroup analysis, studies evaluating exclusively results after endoscopic cardia dilatation,36,40 the prevalence for EA was 6 cases per 1,000 achalasia patients (CI 95% 2, 17; n = 643); for studies evaluating exclusively patients after myotomy,13–15,19,25,26,28,38,42,45,48,53 prevalence was 7 cases per 1,000 achalasia patients (CI 95% 4, 12; n = 1,721).The prevalence ratio of myotomy over dilatation was 1.34 (CI 95% 0.34, 5.33; p = 0.71).
Considering only casuistry from South American countries,19,25,32 where Chagas disease is endemic, the prevalence for carcinoma was 56 cases per 1,000 achalasia patients (25, 120; n = 290). For the rest of the world13–18,20,22,23,26–28,30,35,36,38–44,48–50,52–54 the prevalence for carcinoma was 26 cases per 1,000 achalasia patients (23, 30; n = 11,333). The prevalence ratio was 3.35 (CI 95% 2.1, 5.34; p < 0.01).
Incidence rate
The full number of esophageal achalasia patient-years14,16,20,23,25,36,40,41,42,49,50,53,54 was 60,547.4. For SCC, mean carcinoma per 100,000 patient-years at risk was 312.4 (StDev 428.16). Mean cases per 100,000 patient-years at risk was 21.23 (StDev 31.6) for EA (see Table 2).
Incidence rate of esophageal cancer in achalasia patients
| . | SCC . | EA . |
|---|---|---|
| Cases/100,000/year | 312.4 ± 428.16 | 21.23 ± 31.6 |
| Risk ratio† | 72.65 | 6.63 |
| Absolute risk increase† | 308.1 | 18.03 |
| NNH† | 324.57 | 5546.31 |
| . | SCC . | EA . |
|---|---|---|
| Cases/100,000/year | 312.4 ± 428.16 | 21.23 ± 31.6 |
| Risk ratio† | 72.65 | 6.63 |
| Absolute risk increase† | 308.1 | 18.03 |
| NNH† | 324.57 | 5546.31 |
†Considering 4.3 cases/100,000 patient-years at risk in a general population for SCC and 3.2 cases/100,000 patient-years at risk for EA.56
EA, esophageal adenocarcinoma; NNH, number needed to harm; SCC, squamous cell carcinoma.
Incidence rate of esophageal cancer in achalasia patients
| . | SCC . | EA . |
|---|---|---|
| Cases/100,000/year | 312.4 ± 428.16 | 21.23 ± 31.6 |
| Risk ratio† | 72.65 | 6.63 |
| Absolute risk increase† | 308.1 | 18.03 |
| NNH† | 324.57 | 5546.31 |
| . | SCC . | EA . |
|---|---|---|
| Cases/100,000/year | 312.4 ± 428.16 | 21.23 ± 31.6 |
| Risk ratio† | 72.65 | 6.63 |
| Absolute risk increase† | 308.1 | 18.03 |
| NNH† | 324.57 | 5546.31 |
†Considering 4.3 cases/100,000 patient-years at risk in a general population for SCC and 3.2 cases/100,000 patient-years at risk for EA.56
EA, esophageal adenocarcinoma; NNH, number needed to harm; SCC, squamous cell carcinoma.
Cumulative incidence
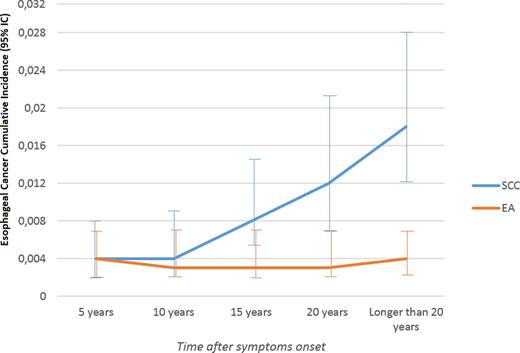
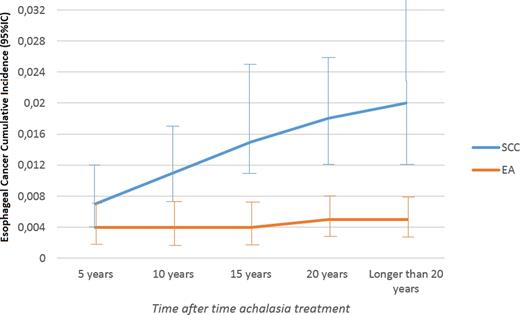
The cumulative incidence for SCC increases for long-standing achalasia, mainly after 10 years after achalasia symptoms onset. For EA, the cumulative incidence remains around 4 cases per 1,000 achalasia patients over the years (see Figs. 5 and 6).13–20,22,23,25–28,30,32,35,36,38–45,48–50,52–54
Esophageal cancer cumulative incidence (carcinoma cases over achalasia patients): 95% confidence interval by time after achalasia symptoms onset. The prevalence of squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EA) increase over the time in curves with different slopes.
Esophageal cancer cumulative incidence (carcinoma cases over achalasia patients): 95% confidence interval by time after achalasia treatment (myotomy or cardia dilatation). The prevalence of squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EA) increase over the time, in curves with different slopes.
Carcinoma topography
For SCC in achalasia patients,13,16,19,21,22,24,25,28–31,33–35,40,43,44,50,51 42 cases (42%) were diagnosed in the lower third of the esophagus, 40 (40%) in the middle third of the esophagus and 17 (17%) in the upper third of the esophagus (p = 0.0013).
Survival
The pooled data of network13,16,19,21,22,25,27,29–31,33–35,40,43,44,50 showed that the mean survival rate after cancer diagnosis was 12.7 (StDev 4.9) months. Only 4.54% of the patients survived longer than 5 years.
DISCUSSION
Usually, carcinoma has a late diagnosis in achalasia due to many years of dysphagia attributed to the achalasia disease.57 Consequently, achalasia-associated cancer is often diagnosed in advanced stages.43
To the best of our knowledge, this is the first meta-analysis estimating the burden of achalasia as esophageal cancer risk factor.
In this study, for EA, the mean cases per 100,000 patient-years at risk was 21.23 (StDev 31.6), with a risk ratio of 6.63, considering a 3.2 cases/100,000 patient-years at risk in a general population for EA.56 Therefore, the risk for developing Barrett's esophagus and consequently EA points out to a strong recommendation for prophylactic fundoplication post myotomy. Nevertheless, although our review includes studies from several countries, prevalence, and incidence indices vary worldwide and the risk ratio for EA in achalasia patients may also change in different countries.
We compared South American countries with the rest of the world to assess the difference in carcinoma prevalence. Although South American countries had a higher prevalence of carcinoma in achalasia patients, few data are available concerning Chagas disease, which is endemic in South America and few data are available concerning time of symptoms onset in these studies.19,25,32 Future studies are needed for evaluating the Chagas carcinogenic role, if there is any.
The risk for achalasia-associated SCC seems to be higher than that for EA and malignancy tends to occur no sooner than 5 years after achalasia symptoms onset, and studies with short-term follow-up may underestimate the malignancy risk. Ergo, as limitation of our review, some studies had a short-term mean follow-up or these data were not available. To work around this problem, we performed a subgroup analysis including only studies with a mean follow-up of at least 5 years. Also, we performed a subgroup analysis excluding cases of simultaneous diagnosis of cancer and achalasia, to avoid possible cases of pseudoachalasia. Another information bias of our review is that studies encompass different methods or techniques for achalasia treatment. Subgroup analysis was performed to evaluate results of myotomy and cardia endoscopic dilatation. Comparing these two methods, no statistically significant difference for esophageal cancer development was found. Nevertheless, few studies report fundoplication techniques and no data are available concerning esophagitis after achalasia treatment.
We advocate surveillance with esophageal endoscopy, mainly after 10 years of achalasia symptoms onset, since the risk for squamous cell carcinoma is much higher. Surveillance should also be indicated after myotomy or cardia dilatation, since, as we assume, cancer genesis pathologic pathway occurs through esophageal reflux, which is secondary to the achalasia treatment, although there are few data in the literature available for follow-up in cases after myotomy and fundoplication. In these cases, endoscopy should be performed even without striking symptoms, because obstructive cancer dysphagia mimics achalasia's symptoms.
References
Author notes
Specific author contributions: Acquisition, analysis and interpretation of the data: Francisco Tustumi, Wanderley Marques Bernardo; Revising critically the work: Julio Rafael Mariano da Rocha; Revising critically the work: Sérgio Szachnowicz; Revising critically the work and statistical analysis and interpretation of the data: Francisco Carlos Bernal da Costa Seguro; Statistical analysis and interpretation of the data: EdnoTales Bianchi; Drafting of the study: Rubens Antonio Aissar Sallum; Drafting and supervision of the study: Ivan Cecconello.