-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Förg, Jakob Hein, Katharina Volkmar, Martin Winter, Christoph Richter, Andreas Heinz, Christian A. Müller, Efficacy and Safety of Pregabalin in the Treatment of Alcohol Withdrawal Syndrome: A Randomized Placebo-Controlled Trial, Alcohol and Alcoholism, Volume 47, Issue 2, March/April 2012, Pages 149–155, https://doi.org/10.1093/alcalc/agr153
Close - Share Icon Share
Abstract
Aims: The objective of this study was to collect preliminary data on the efficacy and safety of pregabalin in attenuating the severity of alcohol withdrawal symptoms during detoxification treatment in alcohol dependence. Methods: Forty-two alcohol-dependent patients with an alcohol withdrawal syndrome (AWS) were included in the prospective randomized double-blind placebo-controlled trial during inpatient alcohol detoxification. For 6 days, participants either received pregabalin or placebo according to a fixed dose schedule starting with 300 mg/day. Depending on the score of the AWS Scale (AWSS), diazepam was additionally administered as a rescue medication. The primary endpoint was the total amount of diazepam required from Day 2 to 6 of detoxification treatment in each of the two groups. Secondary outcome variables were the difference in AWSS and Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scores between Day 2 and 6, tolerability and safety data, drop-out rates as well as changes in the neuropsychological scales. Results: Pregabalin and placebo were equally safe and well tolerated. However, no statistically significant difference was found comparing the total amount of additional diazepam medication required in the two study groups. Pregabalin and placebo also showed similar efficacy according to alterations of scores of the AWSS, CIWA-Ar and neuropsychological scales. The frequency of adverse events and drop-outs did not differ between the both treatment groups. Conclusions: The study demonstrates the relative safety of pregabalin in the treatment of AWS. However, the results do not provide evidence in favor of pregabalin compared with placebo concerning its efficacy in the treatment of AWS.
INTRODUCTION
Chronic alcohol exposure causes an imbalance in excitatory and inhibitory activity of the central nervous system (Tsai and Coyle, 1998; Williams and McBride, 1998; Chen et al., 2011). Owing to the alcohol-induced increase of inhibitory neurotransmission, a counter-regulatory response is shown by reducing gamma-aminobutyric acid (GABA)ergic and increasing glutamatergic functions in the brain in order to maintain normal function (Nevo and Hamon, 1995). The decreased responsiveness of inhibitory receptors and elevated sensitivity of excitatory receptors lead to nervous system hyperactivity when chronic alcohol intake is terminated like during detoxification treatment (Tsai et al., 1995; Tsai and Coyle, 1998; De Witte et al., 2003; Amato et al., 2011). Thus, most alcohol-dependent patients develop symptoms of withdrawal such as anxiety, tremor, tachycardia, hypertension, agitation, nausea, sweating, insomnia etc. within 6–24 h after cessation of drinking (Hall and Zador, 1997; Heinz et al., 2001; Beck et al., 2009). According to the kindling hypothesis, the intensity of these symptoms becomes greater the more detoxifications a patient experiences (De Witte et al., 2003). Besides severe symptoms, potentially life-threatening complications of an alcohol withdrawal syndrome (AWS) such as withdrawal seizures and delirium tremens can occur and therefore indicate a pharmacological treatment (Amato et al., 2011). GABAergic medications like benzodiazepines are widely used to decrease and prevent symptoms of withdrawal (Ntais et al., 2005) and to encourage patients to enter further treatment programs. Several studies have demonstrated their efficacy and safety in the treatment of the AWS (Ntais et al., 2005; de Millas et al., 2010; Amato et al., 2011). However, various side effects such as liver toxicity as well as excessive sedation, memory deficits and respiratory depression in patients with pre-existing liver impairment (Leggio et al., 2008), abuse liabilities and dependence have been reported (Williams and McBride, 1998; Becker et al., 2006). Therefore, studies have started focusing on non-benzodiazepine GABAergic medications, such as carbamazepine, gabapentin and valproic acid as well as baclofen and newer anticonvulsant drugs such as levetiracetam and pregabalin as promising medications in the treatment of AWS (Malcolm et al., 2001; Becker et al., 2006; Leggio et al., 2008; Müller et al., 2010; Lyon et al., 2011). Pregabalin is a GABA analog without direct agonistic activity at GABA A and GABA B receptors (Ben-Menachem, 2004). It binds to the alpha-2-delta regulatory subunit of voltage-sensitive calcium channels, inhibiting activity-dependent calcium influx in nerve terminals and reducing the release of neurotransmitters such as glutamate and norepinephrine (Dooley et al., 2002; Fink et al., 2002; Cunningham et al., 2004; Stahl, 2004). A possible benefit of pregabalin in the treatment of the AWS can be expected due to its anticonvulsant, analgesic, anxiolytic and antidepressant effects (Field et al., 2001; André et al., 2003; Chesler et al., 2003; Feltner et al., 2003; French et al., 2003; Pande et al., 2003; Arroyo et al., 2004; Brodie, 2004; Becker et al., 2006; Stein et al., 2008; Lydiard et al., 2010; Baidya et al., 2011). Pregabalin has also been reported to improve subjective sleep quality in benzodiazepine-dependent patients during detoxification treatment (Rubio et al., 2011). Given the high prevalence of hepatic disease associated with chronic alcohol use and other comorbidities requiring pharmacological treatment, medications like pregabalin lacking hepatic metabolism as well as drug interactions and offering predictable and linear pharmacokinetics (Ben-Menachem, 2004) may be preferential substances in detoxification treatment (Becker et al., 2006).
Only a few studies have been conducted concerning the efficacy of pregabalin in the treatment of alcohol dependence. Different preclinical studies by Becker et al. (2006) showed the ability of pregabalin to reduce central nervous system hyperexcitability associated with alcohol withdrawal in mice and lead to the conclusion that pregabalin might be effective in the treatment of the AWS, in particular of withdrawal seizures, and also in blocking kindling processes.
Di Nicola et al. (2010) demonstrated the efficacy and safety of pregabalin in outpatient detoxification in a small sample of alcohol-dependent patients with a mild-to-moderate AWS, yet lacking a randomized placebo-controlled study design. A further randomized, single-blind trial compared pregabalin, tiapride and lorazepam without a placebo control in the treatment of AWS in 111 alcohol-dependent patients. It was shown that all medications significantly reduced withdrawal symptoms according to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) over time, with a significantly better performance of pregabalin regarding the symptoms ‘headache’ and ‘orientation’ (Martinotti et al., 2010a). The same research group also presumes pregabalin to be an equally efficacious treatment to naltrexone for the prevention of alcohol relapse prior to detoxification. Furthermore, it was demonstrated that more patients treated with pregabalin stayed completely abstinent for a larger number of days, and that pregabalin was beneficial considering the reduction of symptoms of anxiety, hostility and psychoticism and showed better results in patients with comorbid psychiatric disorders. Additionally, patients receiving naltrexone showed a larger number of adverse events and thus poorer compliance (Martinotti et al., 2010b).
Based on these encouraging findings including the probable positive effects of pregabalin on withdrawal symptoms, mood disorders, anxiety and its favorable side effect profile, we conducted a prospective, randomized, double-blind and placebo-controlled trial to examine the efficacy and tolerability of pregabalin in the treatment of AWS.
METHODS
Study subjects
Inclusion criteria
Included were men and women aged 18–65 years who met DSM-IV (APA, 1994) and ICD-10 (WHO, 1994) criteria for alcohol dependence. They were required to have sufficient German language skills, a negative drug urine screening, a score of at least 4 on the AWS Scale (AWSS) (Wetterling et al., 1997) and to give written informed consent. Women had to have at least one year menopause, to be sterilized or be using a birth control method with a Pearl Index greater than 1.
Exclusion criteria
Patients suffering from acute delirium, other drug addictions (not including nicotine or caffeine addiction and cannabis abuse) as well as epileptic seizures (not including alcohol withdrawal-related seizures) were excluded from this study. Furthermore, patients with a psychic trauma or neurological disorders in their medical history (other than alcohol-related neurological disorders such as polyneuropathy) and patients taking medication, which has an influence on withdrawal symptoms were not included. Subjects with contraindications and known side effects from diazepam and pregabalin were also excluded. Further exclusion criteria were pregnancy or lactation period, suicidal tendencies, other psychiatric disorders requiring treatment, severe internal medicinal illnesses, kidney or liver damage.
The study was approved by the local ethics committee and Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) and was conducted in accordance with the Declaration of Helsinki (WMA, accessed on 24th October 2011) and Good Clinical Practice guidelines (GCP) (European-Medicines-Agency, 2002).
Study design
This study had a prospective, randomized, double-blind and placebo-controlled design. After obtaining written informed consent, 42 participants were enrolled in the trial and were randomized in one of two treatment conditions receiving either pregabalin or placebo. The medication was given orally in following daily dosage, with one tablet in the morning and one in the evening: Day 1 and 2: 300 mg, Day 3 and 4: 200 mg, Day 5 and 6: 100 mg. Diazepam was additionally administered as a rescue medication in a symptom-triggered regimen according to the AWSS, which was completed every 4 h: 5 mg in case of a score of 4 or 5, 10 mg for a score of 6–9 and 20 mg if the score was 10 or higher. The daily maximum dosage was 60 mg of diazepam. Data on the use of diazepam, alcohol withdrawal symptoms [AWSS, CIWA-Ar Scale (Sullivan et al., 1989), Visual Analog Scale Withdrawal, VASW)], craving [(VAS Craving (VASC) (Mottola, 1993)] adverse events and safety measures were collected daily for 1 week.
Owing to hospitalization and discharge at different times of the day, the number of measurement points for the AWSS and CIWA-Ar varied on Days 1 and 7. Thus, the time frame for the primary outcome analysis was defined as from Day 2 to 6. Other assessments such as breathalyzer, the patient's smoking status, mental state [Mini-International Neuropsychiatric Interview (Sheehan et al., 1998)] and general health status [Clinical Global Impression (CGI) (Guy, 1976), Global Assessment of Function (GAF) Scale (Endicott et al., 1976)] were conducted at the beginning of the study. Hamilton Anxiety and Depression Rating Scales [HAM-A (Hamilton, 1976a), HAM-D (Hamilton, 1976b)], Alcohol Dependence Scale [ADS (Skinner and Allen, 1982)], Charlston-Comorbidity Index [CCI (Charlson et al., 1987)], electrocardiogram (ECG) and biochemical changes such as alcohol abuse hepatic indices [aspartate aminotranferases (AST), alanine aminotranferases (ALT), δ-glutamyl-tranpeptidases (GGT) and mean corpuscular volume (MCV)] were assessed at screening and termination visits.
Outcome measures
The primary outcome measure was the total amount of diazepam administered as rescue medication from Day 2 to 6 of detoxification treatment in each of the two groups: pregabalin and placebo.
Secondary outcome criteria were the difference in AWSS and CIWA-Ar scores between Day 2 and 6, the tolerability and safety data, the drop-out rates as well as changes in the neuropsychological scales from the beginning to the end of the study (CGI, VASC, VASW, HAM-A, HAM-D). Safety parameters were monitored daily by recording the vital signs, incidence and severity of adverse events as well as the ECG, hematological and clinical chemical analyses of blood samples at the screening and termination visits.
Statistical analysis
Data management followed the guidelines of GCP and was performed by the Coordination Centre for Clinical Trials Charité Berlin (KKS Charité). The Statistical Package for Social Sciences (Version 19 for Windows, SPSS Inc., Chicago, IL, USA) was applied to run the statistical analyses. Non-parametric tests were used, given a non-normal distribution of the data (χ2 tests, Mann–Whitney U-tests and Wilcoxon tests). Intention-to-treat analyses of the outcome measures were performed, including all randomly assigned patients who received at least one dose of the study medication.
RESULTS
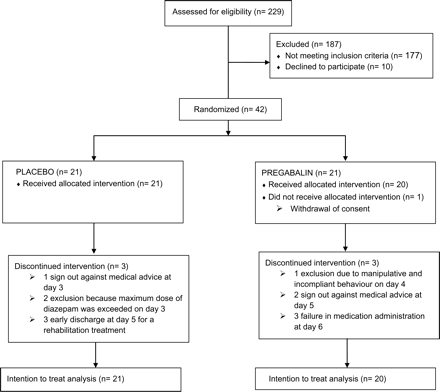
A total of 229 voluntary inpatients from the Department of Psychiatry and Psychotherapy at Campus Charité Mitte of the Charité—Universitätsmedizin Berlin were screened between July 2008 and March 2011, of whom 42 were enrolled in the study (Fig. 1). In total 83% of the patients (17 of 21 in the pregabalin and 18 of 21 in the placebo group) completed the trial per protocol. Apart from a higher age in the pregabalin group, there were no significant differences between the baseline characteristics of the groups receiving pregabalin or placebo (Table 1). Patients did not vary in terms of socio-demographic and clinical characteristics (CGI I, ADS, GAF, CCI), initial withdrawal scores (AWSS, CIWA-Ar), severity of alcohol dependence (ADS), smoking status, breathalyzer test results, years of hazardous alcohol consumption and alcohol abuse hepatic indices at baseline (ALT, AST, GGT, MCV).
Baseline characteristics
| Characteristics . | Placebo . | Pregabalin . | P†,‡,* . |
|---|---|---|---|
| Sex [n (%)] | |||
| Male | 16 (76.2) | 14 (66.7) | 0.50 |
| Female | 5 (23.8) | 7 (33.3) | |
| Age [mean ± SD (range)] | 40.8 ± 8.1 (23–51) | 47.0 ± 7.4 (30–60) | 0.01* |
| School qualifications [n (%)] | |||
| secondary modern school-leaving certificate | 4 (22.2) | 3 (15.8) | 0.89 |
| Intermediate school-leaving certificate | 11 (61.1) | 11 (57.9) | |
| General higher education entrance qualification | 1 (5.6) | 1 (5.3) | |
| Technical college diploma | 1 (5.6) | 1 (5.3) | |
| University degree | 1 (5.6) | 3 (15.8) | |
| Employment status [n (%)] | |||
| Full time occupation | 12 (66.6) | 9 (47.4) | 0.42 |
| Part time (regular occupation) | 2 (11.1) | 1 (5.3) | |
| Part time (irregular occupation) | 0 (0.0) | 2 (10.5) | |
| Retired/disabled | 1 (5.5) | 3 (15.8) | |
| Unemployed | 3 (16.6) | 4 (21.1) | |
| Smoker [n (%)] | 17 (81) | 15 (75) | 0.65 |
| Years of hazardous alcohol consumption [mean ± SD (range)] | 9.4 ± 5.8 (2–22) | 9.1 ± 7.7 (0–28) | 0.64 |
| Analyses of blood samples (mean ± SD) | |||
| ALT | 62.4 ± 42.6 | 78.8 ± 55.8 | 0.53 |
| AST | 107.4 ± 93.5 | 106.2 ± 69.5 | 0.53 |
| GGT | 379.5 ± 675.6 | 278.9 ± 446.3 | 0.71 |
| MCV | 93.1 ± 5.9 | 95.9 ± 6.0 | 0.18 |
| AWSS 1. measurement (mean ± SD) | 5.1 ± 2.0 | 5.3 ± 2.6 | 0.96 |
| CIWA-Ar 1. measurement (mean ± SD) | 8.3 ± 4.8 | 8.1 ± 5.7 | 0.58 |
| CGI I (mean ± SD) | 4.2 ± 1.3 | 4.6 ± 0.8 | 0.70 |
| Alcohol breath test [(‰ ) mean ± SD)] | 0.99 ± 1.1 | 0.78 ± 0.9 | 0.50 |
| ADS (mean ± SD) | 20.1 ± 8.0 | 19.1 ± 8.4 | 0.57 |
| GAF ( mean ± SD) | 55.7 ± 12.7 | 55.4 ± 8.7 | 0.34 |
| CCI (mean ± SD) | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.86 |
| Characteristics . | Placebo . | Pregabalin . | P†,‡,* . |
|---|---|---|---|
| Sex [n (%)] | |||
| Male | 16 (76.2) | 14 (66.7) | 0.50 |
| Female | 5 (23.8) | 7 (33.3) | |
| Age [mean ± SD (range)] | 40.8 ± 8.1 (23–51) | 47.0 ± 7.4 (30–60) | 0.01* |
| School qualifications [n (%)] | |||
| secondary modern school-leaving certificate | 4 (22.2) | 3 (15.8) | 0.89 |
| Intermediate school-leaving certificate | 11 (61.1) | 11 (57.9) | |
| General higher education entrance qualification | 1 (5.6) | 1 (5.3) | |
| Technical college diploma | 1 (5.6) | 1 (5.3) | |
| University degree | 1 (5.6) | 3 (15.8) | |
| Employment status [n (%)] | |||
| Full time occupation | 12 (66.6) | 9 (47.4) | 0.42 |
| Part time (regular occupation) | 2 (11.1) | 1 (5.3) | |
| Part time (irregular occupation) | 0 (0.0) | 2 (10.5) | |
| Retired/disabled | 1 (5.5) | 3 (15.8) | |
| Unemployed | 3 (16.6) | 4 (21.1) | |
| Smoker [n (%)] | 17 (81) | 15 (75) | 0.65 |
| Years of hazardous alcohol consumption [mean ± SD (range)] | 9.4 ± 5.8 (2–22) | 9.1 ± 7.7 (0–28) | 0.64 |
| Analyses of blood samples (mean ± SD) | |||
| ALT | 62.4 ± 42.6 | 78.8 ± 55.8 | 0.53 |
| AST | 107.4 ± 93.5 | 106.2 ± 69.5 | 0.53 |
| GGT | 379.5 ± 675.6 | 278.9 ± 446.3 | 0.71 |
| MCV | 93.1 ± 5.9 | 95.9 ± 6.0 | 0.18 |
| AWSS 1. measurement (mean ± SD) | 5.1 ± 2.0 | 5.3 ± 2.6 | 0.96 |
| CIWA-Ar 1. measurement (mean ± SD) | 8.3 ± 4.8 | 8.1 ± 5.7 | 0.58 |
| CGI I (mean ± SD) | 4.2 ± 1.3 | 4.6 ± 0.8 | 0.70 |
| Alcohol breath test [(‰ ) mean ± SD)] | 0.99 ± 1.1 | 0.78 ± 0.9 | 0.50 |
| ADS (mean ± SD) | 20.1 ± 8.0 | 19.1 ± 8.4 | 0.57 |
| GAF ( mean ± SD) | 55.7 ± 12.7 | 55.4 ± 8.7 | 0.34 |
| CCI (mean ± SD) | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.86 |
†Pearson's χ2 test.
‡Mann–Whitney U-test.
*statistically significant.
Baseline characteristics
| Characteristics . | Placebo . | Pregabalin . | P†,‡,* . |
|---|---|---|---|
| Sex [n (%)] | |||
| Male | 16 (76.2) | 14 (66.7) | 0.50 |
| Female | 5 (23.8) | 7 (33.3) | |
| Age [mean ± SD (range)] | 40.8 ± 8.1 (23–51) | 47.0 ± 7.4 (30–60) | 0.01* |
| School qualifications [n (%)] | |||
| secondary modern school-leaving certificate | 4 (22.2) | 3 (15.8) | 0.89 |
| Intermediate school-leaving certificate | 11 (61.1) | 11 (57.9) | |
| General higher education entrance qualification | 1 (5.6) | 1 (5.3) | |
| Technical college diploma | 1 (5.6) | 1 (5.3) | |
| University degree | 1 (5.6) | 3 (15.8) | |
| Employment status [n (%)] | |||
| Full time occupation | 12 (66.6) | 9 (47.4) | 0.42 |
| Part time (regular occupation) | 2 (11.1) | 1 (5.3) | |
| Part time (irregular occupation) | 0 (0.0) | 2 (10.5) | |
| Retired/disabled | 1 (5.5) | 3 (15.8) | |
| Unemployed | 3 (16.6) | 4 (21.1) | |
| Smoker [n (%)] | 17 (81) | 15 (75) | 0.65 |
| Years of hazardous alcohol consumption [mean ± SD (range)] | 9.4 ± 5.8 (2–22) | 9.1 ± 7.7 (0–28) | 0.64 |
| Analyses of blood samples (mean ± SD) | |||
| ALT | 62.4 ± 42.6 | 78.8 ± 55.8 | 0.53 |
| AST | 107.4 ± 93.5 | 106.2 ± 69.5 | 0.53 |
| GGT | 379.5 ± 675.6 | 278.9 ± 446.3 | 0.71 |
| MCV | 93.1 ± 5.9 | 95.9 ± 6.0 | 0.18 |
| AWSS 1. measurement (mean ± SD) | 5.1 ± 2.0 | 5.3 ± 2.6 | 0.96 |
| CIWA-Ar 1. measurement (mean ± SD) | 8.3 ± 4.8 | 8.1 ± 5.7 | 0.58 |
| CGI I (mean ± SD) | 4.2 ± 1.3 | 4.6 ± 0.8 | 0.70 |
| Alcohol breath test [(‰ ) mean ± SD)] | 0.99 ± 1.1 | 0.78 ± 0.9 | 0.50 |
| ADS (mean ± SD) | 20.1 ± 8.0 | 19.1 ± 8.4 | 0.57 |
| GAF ( mean ± SD) | 55.7 ± 12.7 | 55.4 ± 8.7 | 0.34 |
| CCI (mean ± SD) | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.86 |
| Characteristics . | Placebo . | Pregabalin . | P†,‡,* . |
|---|---|---|---|
| Sex [n (%)] | |||
| Male | 16 (76.2) | 14 (66.7) | 0.50 |
| Female | 5 (23.8) | 7 (33.3) | |
| Age [mean ± SD (range)] | 40.8 ± 8.1 (23–51) | 47.0 ± 7.4 (30–60) | 0.01* |
| School qualifications [n (%)] | |||
| secondary modern school-leaving certificate | 4 (22.2) | 3 (15.8) | 0.89 |
| Intermediate school-leaving certificate | 11 (61.1) | 11 (57.9) | |
| General higher education entrance qualification | 1 (5.6) | 1 (5.3) | |
| Technical college diploma | 1 (5.6) | 1 (5.3) | |
| University degree | 1 (5.6) | 3 (15.8) | |
| Employment status [n (%)] | |||
| Full time occupation | 12 (66.6) | 9 (47.4) | 0.42 |
| Part time (regular occupation) | 2 (11.1) | 1 (5.3) | |
| Part time (irregular occupation) | 0 (0.0) | 2 (10.5) | |
| Retired/disabled | 1 (5.5) | 3 (15.8) | |
| Unemployed | 3 (16.6) | 4 (21.1) | |
| Smoker [n (%)] | 17 (81) | 15 (75) | 0.65 |
| Years of hazardous alcohol consumption [mean ± SD (range)] | 9.4 ± 5.8 (2–22) | 9.1 ± 7.7 (0–28) | 0.64 |
| Analyses of blood samples (mean ± SD) | |||
| ALT | 62.4 ± 42.6 | 78.8 ± 55.8 | 0.53 |
| AST | 107.4 ± 93.5 | 106.2 ± 69.5 | 0.53 |
| GGT | 379.5 ± 675.6 | 278.9 ± 446.3 | 0.71 |
| MCV | 93.1 ± 5.9 | 95.9 ± 6.0 | 0.18 |
| AWSS 1. measurement (mean ± SD) | 5.1 ± 2.0 | 5.3 ± 2.6 | 0.96 |
| CIWA-Ar 1. measurement (mean ± SD) | 8.3 ± 4.8 | 8.1 ± 5.7 | 0.58 |
| CGI I (mean ± SD) | 4.2 ± 1.3 | 4.6 ± 0.8 | 0.70 |
| Alcohol breath test [(‰ ) mean ± SD)] | 0.99 ± 1.1 | 0.78 ± 0.9 | 0.50 |
| ADS (mean ± SD) | 20.1 ± 8.0 | 19.1 ± 8.4 | 0.57 |
| GAF ( mean ± SD) | 55.7 ± 12.7 | 55.4 ± 8.7 | 0.34 |
| CCI (mean ± SD) | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.86 |
†Pearson's χ2 test.
‡Mann–Whitney U-test.
*statistically significant.
Efficacy
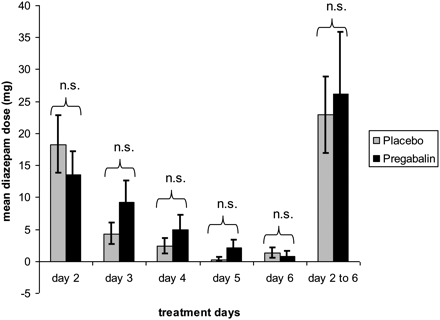
Separate analyses of the pregabalin and the placebo group showed a significant reduction in diazepam use from Day 2 to 6 (pregabalin: Z = −2.842, P = 0.004; placebo: Z = −2.916, P = 0.004). However, the comparisons of pregabalin vs. placebo could not reveal any differences regarding the amount of diazepam administered (Fig. 2). Also, the difference in the diazepam dose comparing Day 2 with Day 6 did not reach statistical significance between groups (P = 0.707).
Mean diazepam doses of the placebo and the pregabalin group from Day 2 to Day 6. I, standard error of means; n.s., not significant.
Measuring the intensity of the AWS between Day 2 and 6, all patients showed a statistically significant decrease in scores of the AWSS (pregabalin: Z = −2.629, P = 0.009; placebo: Z = −2.963, P = 0.003) and CIWA-Ar (pregabalin: Z = −2.644, P = 0.008; placebo: Z = −3.512, P = 0.000), as well as in the self-rating tool VASW between screening and termination visits (pregabalin: Z = −3.297, P = 0.001; placebo: Z = −3.623, P =0.000). Also, a significant improvement in anxiety (HAM-A, pregabalin: Z = −3.031, P = 0.002; placebo: Z = −2.537, P = 0.011), depression (HAM-D, pregabalin: Z = −3.073, P = 0.002; placebo: Z = −3.348, P = 0.001) and self-rated craving (VASC, pregabalin: Z = −2.983, P = 0.003; placebo: Z = −3.108, P = 0.002) at the beginning and end of the study was observed in all participants. However, results did not differ significantly between the two study groups at any time point (Table 2).
Mean changes between groups over time
| . | . | Placebo . | Pregabalin . | P†,‡ . |
|---|---|---|---|---|
| AWSS | Day 2 mean ± SD | 3.4 ± 2.5 | 2.6 ± 1.8 | 0.41 |
| Day 6 mean ± SD | 1.0 ± 1.2 | 0.9 ± 1.4 | 0.57 | |
| Δ beginning vs. end ± SD | 2.0 ± 2.4 | 1.4 ± 1.9 | 0.59 | |
| CIWA-Ar | Day 2 mean ± SD | 5.6 ± 4.1 | 4.5 ± 4.2 | 0.23 |
| Day 6 mean ± SD | 1.3 ± 1.6 | 1.4 ± 2.7 | 0.61 | |
| Δ beginning vs. end ± SD | 4.0 ± 3.3 | 2.2 ± 2.9 | 0.07 | |
| HAM-A | Screening mean ± SD | 13.2 ± 7.6 | 12.1 ± 6.8 | 0.55 |
| Termination mean ± SD | 6.5 ± 5.4 | 4.1 ± 3.7 | 0.16 | |
| Δ beginning vs. end ± SD | 6.8 ± 8.6 | 7.8 ± 7.9 | 0.56 | |
| HAM-D | Screening mean ± SD | 14 ± 8.3 | 13.3 ± 6.9 | 0.72 |
| Termination mean ± SD | 5.8 ± 5.0 | 4.6 ± 4.4 | 0.41 | |
| Δ beginning vs. end ± SD | 8 ± 6.9 | 8.6 ± 7.4 | 0.74 | |
| VASW | Screening mean ± SD | 53.6 ± 34.2 | 40.4 ± 25.4 | 0.12 |
| Termination mean ± SD | 3.4 ± 6.9 | 2.9 ± 4.1 | 0.88 | |
| Δ beginning vs. end ± SD | 48.7 ± 32.4 | 34.7 ± 28.9 | 0.19 | |
| VASC | Screening mean ± SD | 37.5 ± 34.9 | 30.8 ± 30.9 | 0.75 |
| Termination mean ± SD | 4.3 ± 10.8 | 2.47 ± 3.5 | 0.56 | |
| Δ beginning vs. end ± SD | 29.4 ± 29.9 | 31.3 ± 31.3 | 0.69 | |
| CGI | Screening mean ± SD | 4.2 ± 1.2 | 4.6 ± 0.8 | 0.70 |
| Termination mean ± SD | 2.3 ± 0.7 | 2.2 ± 0.8 | 0.82 |
| . | . | Placebo . | Pregabalin . | P†,‡ . |
|---|---|---|---|---|
| AWSS | Day 2 mean ± SD | 3.4 ± 2.5 | 2.6 ± 1.8 | 0.41 |
| Day 6 mean ± SD | 1.0 ± 1.2 | 0.9 ± 1.4 | 0.57 | |
| Δ beginning vs. end ± SD | 2.0 ± 2.4 | 1.4 ± 1.9 | 0.59 | |
| CIWA-Ar | Day 2 mean ± SD | 5.6 ± 4.1 | 4.5 ± 4.2 | 0.23 |
| Day 6 mean ± SD | 1.3 ± 1.6 | 1.4 ± 2.7 | 0.61 | |
| Δ beginning vs. end ± SD | 4.0 ± 3.3 | 2.2 ± 2.9 | 0.07 | |
| HAM-A | Screening mean ± SD | 13.2 ± 7.6 | 12.1 ± 6.8 | 0.55 |
| Termination mean ± SD | 6.5 ± 5.4 | 4.1 ± 3.7 | 0.16 | |
| Δ beginning vs. end ± SD | 6.8 ± 8.6 | 7.8 ± 7.9 | 0.56 | |
| HAM-D | Screening mean ± SD | 14 ± 8.3 | 13.3 ± 6.9 | 0.72 |
| Termination mean ± SD | 5.8 ± 5.0 | 4.6 ± 4.4 | 0.41 | |
| Δ beginning vs. end ± SD | 8 ± 6.9 | 8.6 ± 7.4 | 0.74 | |
| VASW | Screening mean ± SD | 53.6 ± 34.2 | 40.4 ± 25.4 | 0.12 |
| Termination mean ± SD | 3.4 ± 6.9 | 2.9 ± 4.1 | 0.88 | |
| Δ beginning vs. end ± SD | 48.7 ± 32.4 | 34.7 ± 28.9 | 0.19 | |
| VASC | Screening mean ± SD | 37.5 ± 34.9 | 30.8 ± 30.9 | 0.75 |
| Termination mean ± SD | 4.3 ± 10.8 | 2.47 ± 3.5 | 0.56 | |
| Δ beginning vs. end ± SD | 29.4 ± 29.9 | 31.3 ± 31.3 | 0.69 | |
| CGI | Screening mean ± SD | 4.2 ± 1.2 | 4.6 ± 0.8 | 0.70 |
| Termination mean ± SD | 2.3 ± 0.7 | 2.2 ± 0.8 | 0.82 |
†Mann–Whitney U-test.
‡Pearson's χ2 test, asymptotic significance.
Mean changes between groups over time
| . | . | Placebo . | Pregabalin . | P†,‡ . |
|---|---|---|---|---|
| AWSS | Day 2 mean ± SD | 3.4 ± 2.5 | 2.6 ± 1.8 | 0.41 |
| Day 6 mean ± SD | 1.0 ± 1.2 | 0.9 ± 1.4 | 0.57 | |
| Δ beginning vs. end ± SD | 2.0 ± 2.4 | 1.4 ± 1.9 | 0.59 | |
| CIWA-Ar | Day 2 mean ± SD | 5.6 ± 4.1 | 4.5 ± 4.2 | 0.23 |
| Day 6 mean ± SD | 1.3 ± 1.6 | 1.4 ± 2.7 | 0.61 | |
| Δ beginning vs. end ± SD | 4.0 ± 3.3 | 2.2 ± 2.9 | 0.07 | |
| HAM-A | Screening mean ± SD | 13.2 ± 7.6 | 12.1 ± 6.8 | 0.55 |
| Termination mean ± SD | 6.5 ± 5.4 | 4.1 ± 3.7 | 0.16 | |
| Δ beginning vs. end ± SD | 6.8 ± 8.6 | 7.8 ± 7.9 | 0.56 | |
| HAM-D | Screening mean ± SD | 14 ± 8.3 | 13.3 ± 6.9 | 0.72 |
| Termination mean ± SD | 5.8 ± 5.0 | 4.6 ± 4.4 | 0.41 | |
| Δ beginning vs. end ± SD | 8 ± 6.9 | 8.6 ± 7.4 | 0.74 | |
| VASW | Screening mean ± SD | 53.6 ± 34.2 | 40.4 ± 25.4 | 0.12 |
| Termination mean ± SD | 3.4 ± 6.9 | 2.9 ± 4.1 | 0.88 | |
| Δ beginning vs. end ± SD | 48.7 ± 32.4 | 34.7 ± 28.9 | 0.19 | |
| VASC | Screening mean ± SD | 37.5 ± 34.9 | 30.8 ± 30.9 | 0.75 |
| Termination mean ± SD | 4.3 ± 10.8 | 2.47 ± 3.5 | 0.56 | |
| Δ beginning vs. end ± SD | 29.4 ± 29.9 | 31.3 ± 31.3 | 0.69 | |
| CGI | Screening mean ± SD | 4.2 ± 1.2 | 4.6 ± 0.8 | 0.70 |
| Termination mean ± SD | 2.3 ± 0.7 | 2.2 ± 0.8 | 0.82 |
| . | . | Placebo . | Pregabalin . | P†,‡ . |
|---|---|---|---|---|
| AWSS | Day 2 mean ± SD | 3.4 ± 2.5 | 2.6 ± 1.8 | 0.41 |
| Day 6 mean ± SD | 1.0 ± 1.2 | 0.9 ± 1.4 | 0.57 | |
| Δ beginning vs. end ± SD | 2.0 ± 2.4 | 1.4 ± 1.9 | 0.59 | |
| CIWA-Ar | Day 2 mean ± SD | 5.6 ± 4.1 | 4.5 ± 4.2 | 0.23 |
| Day 6 mean ± SD | 1.3 ± 1.6 | 1.4 ± 2.7 | 0.61 | |
| Δ beginning vs. end ± SD | 4.0 ± 3.3 | 2.2 ± 2.9 | 0.07 | |
| HAM-A | Screening mean ± SD | 13.2 ± 7.6 | 12.1 ± 6.8 | 0.55 |
| Termination mean ± SD | 6.5 ± 5.4 | 4.1 ± 3.7 | 0.16 | |
| Δ beginning vs. end ± SD | 6.8 ± 8.6 | 7.8 ± 7.9 | 0.56 | |
| HAM-D | Screening mean ± SD | 14 ± 8.3 | 13.3 ± 6.9 | 0.72 |
| Termination mean ± SD | 5.8 ± 5.0 | 4.6 ± 4.4 | 0.41 | |
| Δ beginning vs. end ± SD | 8 ± 6.9 | 8.6 ± 7.4 | 0.74 | |
| VASW | Screening mean ± SD | 53.6 ± 34.2 | 40.4 ± 25.4 | 0.12 |
| Termination mean ± SD | 3.4 ± 6.9 | 2.9 ± 4.1 | 0.88 | |
| Δ beginning vs. end ± SD | 48.7 ± 32.4 | 34.7 ± 28.9 | 0.19 | |
| VASC | Screening mean ± SD | 37.5 ± 34.9 | 30.8 ± 30.9 | 0.75 |
| Termination mean ± SD | 4.3 ± 10.8 | 2.47 ± 3.5 | 0.56 | |
| Δ beginning vs. end ± SD | 29.4 ± 29.9 | 31.3 ± 31.3 | 0.69 | |
| CGI | Screening mean ± SD | 4.2 ± 1.2 | 4.6 ± 0.8 | 0.70 |
| Termination mean ± SD | 2.3 ± 0.7 | 2.2 ± 0.8 | 0.82 |
†Mann–Whitney U-test.
‡Pearson's χ2 test, asymptotic significance.
Safety and tolerability
In 5 of 21 (23.8%) cases of the placebo and 4 of 20 (20%) cases in the pregabalin group, no adverse events were reported at all. In each group, 16 patients experienced adverse events during detoxification treatment. None of these events were categorized as severe. The most common adverse events were headaches (n = 5), diarrhea (n = 4), nausea (n = 2), dizziness (n = 2) and hypertension (n = 2) in the placebo group as well as hypokalemia (n = 3), diarrhea (n = 2) and epigastralgia (n = 2) in the pregabalin group. Drop-out rates were similar in both study groups (see Fig. 1). One patient in the placebo group was excluded because the maximum dose of diazepam (60 mg/day) was exceeded on Day 3. This was the only patient who developed a severe withdrawal syndrome and did not benefit from the rescue medication.
Hematological and clinical chemical analyses of blood samples showed no relevant differences between groups at screening and termination visits.
At drug discontinuation on Day 6, no more side effects or signs of withdrawal were observed in participants of either study group.
DISCUSSION
Owing to the limitations of approved medications for the AWS, e.g. side effects of benzodiazepines or their sometimes incomplete management of severe AWS (Tsai et al., 1995; Amato et al., 2011), the detection of useful alternatives is of great clinical importance. Thus research interest in non-benzodiazepine medications has grown (Leggio et al., 2008). Pregabalin is not subject to hepatic metabolism and shows anticonvulsant, analgesic, anxiolytic and antidepressant effects, which make it a potential substance for the treatment of the AWS (see Introduction). Several research groups have delivered promising results for the use of pregabalin in the treatment of AWS (Becker et al., 2006; Di Nicola et al., 2010; Martinotti et al., 2010a). However, these preliminary findings from preclinical and first clinical studies had to be confirmed by randomized controlled trials. To the best of our knowledge this is the first prospective, randomized, double-blind, placebo-controlled trial investigating the efficacy and safety of pregabalin for the treatment of the AWS in alcohol-dependent patients in an inpatient setting. In both patient groups detoxification showed success, given the clear decrease of withdrawal symptoms without serious adverse events. Pregabalin was shown to be equally tolerable and safe as placebo. However, contrary to our main hypothesis, the use of pregabalin did not reduce the total amount of diazepam required as rescue medication during detoxification treatment more than placebo.
In a single-blind study, Martinotti et al. (2010a) reported pregabalin, tiapride and lorazepam, all to be effective and safe in treating AWS (Martinotti et al., 2010a). However, in the present randomized placebo-controlled study pregabalin was not more effective than placebo. One contributing factor to these results might be the relatively low severity of AWS in our trial, which may have led to a rather high placebo response. Furthermore, differences in medication regimes have to be taken into account: Martinotti et al. (2010a) used a symptom-triggered dose of each medication for 14 days with a maximum dose of 450 mg/day for pregabalin, whereas in the present study pregabalin was administered according to a fixed dose schedule for 6 days with altogether lower doses (Day 1, 2: 300 mg; Day 3, 4: 200 mg ; Day 5, 6 100 mg) than used by Martinotti et al. (2010a). It is possible that the dose of pregabalin was too low in our trial, since other clinical studies in different indications such as general anxiety disorders and partial seizures have reported clear dose–response effects, showing the highest efficacy of pregabalin with dosages of up to 600 mg per day (French et al., 2003; Lydiard et al., 2010).
A study with a comparable design, but evaluating the efficacy of levetiracetam for AWS obtained similar results to the present study (Richter et al., 2010). Levetiracetam was also not shown to be superior to placebo. The lack of efficacy of both levetiracetam and pregabalin for AWS may be due to the fact that both drugs have no specific effect on withdrawal symptoms or at least no benefit in the treatment of mild-to-moderate withdrawal syndromes. Some effects may not have been demonstrated because treatment was not started before participants reached a score of 4 on the AWSS. However, an early administration may be sensible to prevent kindling effects. Furthermore, a recent Cochrane review also did not provide sufficient evidence in favor of anticonvulsants for the treatment of AWS (Minozzi et al., 2010).
Considering the scales for withdrawal symptoms (AWSS, CIWA-Ar, VASW) and neuropsychological scales (HAM-A, HAM-D, VASC), significant improvements were found within both groups over time. However, these changes were also independent of the treatment received. Thus, the conclusions in other studies that pregabalin is also effective in reducing psychiatric symptoms (Di Nicola et al., 2010; Martinotti et al., 2010a) could not be confirmed by our comparison with a placebo group. In other studies regarding the treatment of anxiety symptoms outside of alcoholism, pregabalin was shown to be equally effective as benzodiazepines (Lydiard et al., 2010).
The drop-out rates and amount of adverse events did not significantly differ in the pregabalin and the placebo group. Frequent adverse events of pregabalin in other studies were dizziness and somnolence in a mild-to-moderate intensity (Feltner et al., 2003; Pande et al., 2003; Brodie, 2004), yet in our study group receiving pregabalin, the most common adverse events were hypokalemia, diarrhea and epigastralgia.
Martinotti et al. (2010a) described pregabalin as superior to lorazepam regarding the symptoms ‘headache’ and ‘orientation’. Our observation that more headaches were reported in the placebo group may confirm this result.
An abrupt or rapid discontinuation of pregabalin has been reported to cause symptoms such as insomnia, nausea, headache, anxiety, hyperhidrosis and diarrhea in some patients (Pfizer Canada Inc., 2010). Different case reports provide evidence for the development of pregabalin abuse and dependence, with vegetative withdrawal symptoms and strong craving (Filipetto et al., 2010; Grosshans et al., 2010). In the present study, no side effects or any signs of withdrawal were observed in either study group at drug discontinuation on Day 6. In other trials with longer treatment periods, patients were also described as free of rebound anxiety during drug discontinuation (Di Nicola et al., 2010) and free of significant withdrawal symptoms compared with patients discontinuing lorazepam intake (Pande et al., 2003). In contrast to pregabalin the potential for abuse and dependence has been rated as higher for benzodiazepines (Chalabianloo and Schjøtt, 2009).
It has also been pointed out that pregabalin may increase the risk for suicidal ideation in a dose-dependent manner (Mutschler et al., 2011). In our trial, none of the patients reported suicidal thoughts; however, only a maximum of half of the dose described as critical was administered.
Owing to the limitations of this study the results must be interpreted with caution; especially the small sample size of initially 42 patients is associated with rather low external validity and does not allow final conclusions. Furthermore, it may also have been more accurate to use a study protocol without a rescue medication. However, for safety and ethical reasons we had to choose this design.
Although preliminary and limited, the findings of this first randomized placebo-controlled trial suggest that pregabalin with an initial dose of 300 mg/day is safe and tolerable but lacks clear efficacy in the treatment of AWS. For definitive conclusions, future studies with larger samples, different dosing regimes, as well as different time points and ranges of administration would be necessary.
Funding
This Investigator Initiated Trial was supported by Pfizer®.
Acknowledgements
We thank our study nurse Olga Vitlif for her dedicated work and all the patients who contributed to this study.