-
PDF
- Split View
-
Views
-
Cite
Cite
J. Stephen Dumler, John E. Madigan, Nicola Pusterla, Johan S. Bakken, Ehrlichioses in Humans: Epidemiology, Clinical Presentation, Diagnosis, and Treatment, Clinical Infectious Diseases, Volume 45, Issue Supplement_1, July 2007, Pages S45–S51, https://doi.org/10.1086/518146
Close - Share Icon Share
Abstract
Human ehrlichioses are emerging tickborne infections. “Human ehrlichiosis” describes infections with at least 5 separate obligate intracellular bacteria in 3 genera in the family Anaplasmataceae. Since 1986, these agents and infections (human monocytic ehrlichiosis [HME], caused by Ehrlichia chaffeensis; human granulocytic anaplasmosis [HGA], caused by Anaplasma phagocytophilum; and human ewingii ehrlichiosis, caused by Ehrlichia ewingii) are the causes of most human ehrlichioses. Their prevalence and incidence are increasing where the appropriate tick vectors are found. The diseases generally present as undifferentiated fever, but thrombocytopenia, leukopenia, and increased serum transaminase activities are important laboratory features. Despite clinical similarities, each disease has unique features: a greater severity and a higher case-fatality rate for HME and a higher prevalence of opportunistic infections for HGA. Once an ehrlichiosis is suspected on historical and clinical grounds, doxycycline treatment should be initiated concurrently with attempts at etiologic confirmation using laboratory methods such as blood smear examination, polymerase chain reaction, culture, and serologic tests.
"Ehrlichiosis" is a generic name for infections caused by obligate intracellular bacteria in the family Anaplasmataceae, chiefly in the genera Ehrlichia and Anaplasma. Human infections with organisms in this family were first recognized in 1953, with a species now classified as Neorickettsia sennetsu; in 1986, with an Ehrlichia species; and in 1990, with an Anaplasma species. The latter 2 genera now comprise the majority of human infections, which are caused by at least 3 distinct species: Ehrlichia chaffeensis, Ehrlichia ewingii, and Anaplasma phagocytophilum. The purpose of this review is to highlight changes in nomenclature and disease terminology and to compare and contrast the epidemiology, clinical manifestations, diagnosis, and treatment of these distinctive infections.
Human Infections Caused By Anaplasmataceae Members
Although 5 Anaplasmataceae members, including A. phagocytophilum, E. chaffeensis, E. ewingii, Ehrlichia canis, and N. sennetsu, infect humans, only the former 3 species are sufficiently investigated. Circulating leukocytes are their targets, and the corresponding diseases are often named according to the infected leukocyte appended by the bacterial genus; for example, E. chaffeensis infecting monocytes causes human monocytic ehrlichiosis (HME), and A. phagocytophilum infecting granulocytes causes human granulocytic anaplasmosis (HGA) [1, 2]. HGA was formerly called “human granulocytic ehrlichiosis,” but taxonomic revisions within the Anaplasmataceae resulted in the name change [3]. The canine pathogen E. ewingii was recognized in 1998 to cause infections in humans [4]. E. ewingii is serologically similar to E. chaffeensis but, like A. phagocytophilum, propagates within neutrophils [4, 5]. To avoid confusion, it is preferable that human E. ewingii infection be called “human ewingii ehrlichiosis.” Patients in regions where these infections exist who present during tick season with fever, leukopenia and/or thrombocytopenia, and increased serum transaminase activities should have ehrlichiosis included in the differential diagnosis. Unique clinical features and pathogenetic mechanisms are associated with each pathogen [1, 6, 7], and distinguishing infections by use of laboratory diagnostics is more than academic. Although clinical features cannot distinguish the causative agents, each responds to doxycycline therapy [7, 8].
Epidemiology and Ecology
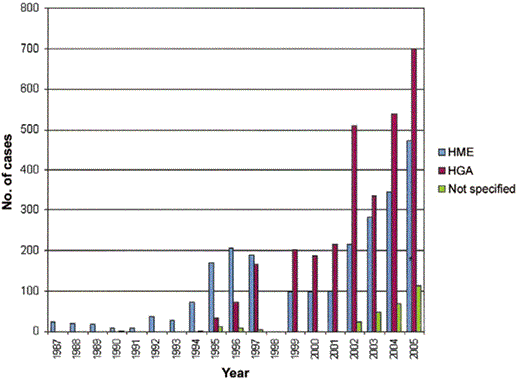
The agents of human ehrlichiosis are tickborne, and most infections occur during May through August. These infections are now reportable in the United States, and passively collected data provide some measure of their incidence and prevalence [9]. In 2005, more cases of ehrlichiosis were reported than ever before (figure 1), including 471 cases of HME (2396 cases since 1986) and 700 cases of HGA (2963 cases since 1994). However, these figures likely represent underestimates of the true incidence and prevalence, because active surveillance rates are as high as 330–414 cases per 100,000 population (0.3%–0.4%) for HME in Tennessee [10] and southeastern Missouri [11] and 24–58 cases per 100,000 population (0.02%–0.06%) for HGA in Connecticut [12] and the Upper Midwest [13]. Seroprevalence studies show rates of ∼12.5% for HME in Tennessee [14] and ∼14.9% for HGA in northwestern Wisconsin [15]. HME occurs across the south-central, southeastern, and mid-Atlantic states, corresponding to regions where white-tailed deer (Odocoileus virginianus) and lone star ticks (Amblyomma americanum) both exist [5]. Although limited to the United States, E. ewingii can also be transmitted by A. americanum ticks and may be the more prevalent species in some regions.
Cases of human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA) reported in the United States since 1986. The data reflect information available until January 2006; data for the year 1998 were unavailable.
In contrast, A. phagocytophilum infection occurs internationally, and areas of endemicity include the United States (northeastern and mid-Atlantic, Upper Midwest, and Pacific Northwest states), Europe, and Asia (China, Siberian Russia, and Korea) [6]. These regions correspond to areas where Ixodes persulcatus group ticks (I. scapularis in the eastern United States, I. pacificus in the western United States, I. ricinus in Europe, and I. persulcatus in Asia) bite humans. Small mammals, such as white-footed mice (Peromyscus leucopus); dusky-footed wood rats (Neotoma fuscipes); or others, such as Apodemus, Microtus, or Clethrionymus species are likely reservoirs [6]. A potential role for cervids as reservoirs has been demonstrated [1, 6], but their role as reservoirs must depend on parasitization by both mature and immature vector ticks, because tick transovarian transmission is inefficient. Owing to the shared tick vectors for A. phagocytophilum, Borrelia burgdorferi, Babesia microti, and tickborne encephalitis viruses, ∼10% of patients have serologic evidence of coinfection, and cases of simultaneous Lyme disease or tickborne encephalitis are well documented [6].
Clinical and Laboratory Findings
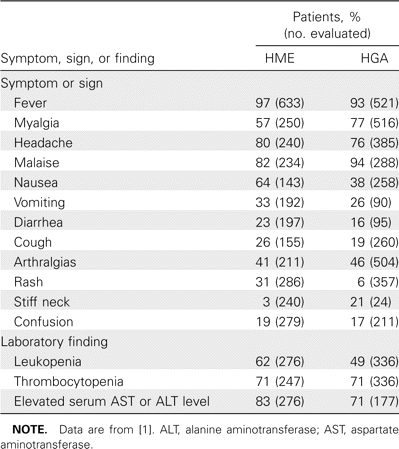
HME and HGA are caused by distinct but related bacterial species that propagate in different host cells that are then altered in different ways [1, 6]. Paradoxically, all forms of human ehrlichiosis share many clinical and laboratory manifestations, including fever, headache, myalgia and malaise, thrombocytopenia, leukopenia, and indices of hepatic injury (table 1) [6, 7, 11]. Similarly, the median age of patients with either infection is ∼50 years, and slightly more males (57%–61%) are infected than females [9]. Certain clinical features are unique to either HME or HGA, including rare CNS infections in HGA and the greater likelihood of rash in HME. Although clinically undifferentiated, identification of <100 × 109 platelets/L and <2.5 × 109 leukocytes/L is associated with a relative risk for HGA as high as 4.7–22.7 and 1.1–24.8, respectively. In contrast, leukocytosis (>10.2 × 109 cells/L) is associated with a low relative risk (0.10–0.71) for HGA [16]. The key to diagnosing HME or HGA is identification of fever (with or without evidence of other systemic findings) and thrombocytopenia, leukopenia, and elevated serum transaminase activities in a patient exposed in a tick-endemic region during times of tick activity. The latter features increase the probability of infection, because they preselect at-risk populations. However, a lack of these historical, epidemiologic, or laboratory features should not exclude HME or HGA from consideration in suspected cases.
Meta-analysis of human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA) symptoms, signs, and laboratory findings.
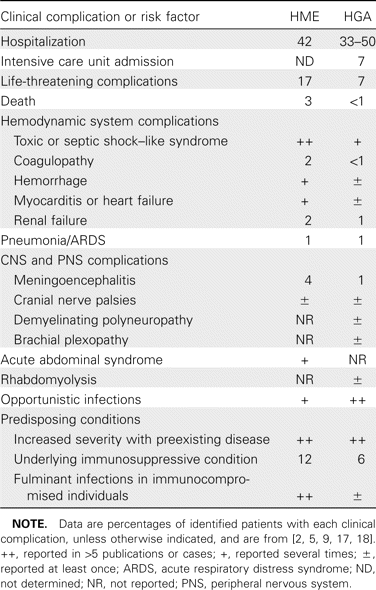
Complications of HME and HGA (table 2) are infrequent but may be evident at the time of presentation, appear within several days after onset, or, rarely, develop later and persist for long intervals in the absence of active infection [1, 6, 7, 17]. Important complications differ for HME and HGA. Patients with HME can develop a fulminant toxic or septic shock–like syndrome, particularly individuals with HIV infection or those with underlying immunocompromise (e.g., organ transplant recipients or patients undergoing immunosuppression for cancer or immune disorders) [18]. Such presentations are less frequent with HGA [9]. Similarly, ∼20% of patients with HME have evidence of CNS involvement (meningitis or meningoencephalitis), findings not easily corroborated in patients with HGA [1, 7]. In contrast, peripheral neuropathies, including brachial plexopathy, demyelinating polyneuropathy, and even isolated facial palsy, are more common with HGA and can persist for weeks or months [6]. Respiratory distress syndrome is best documented in HME, although it may also occur in HGA [1, 5]. Although persons with underlying immunosuppression—for example, as a result of kidney transplantation or HIV infection—are at higher risk for human ewingii ehrlichiosis, far fewer complications and no fatalities have been reported, in comparison with HME and HGA [18]. Fatalities due to HME occur in ∼3% of infections, most commonly in immunosuppressed persons with respiratory distress syndrome, hepatitis, or opportunistic nosocomial infections [1, 5, 9]. The case-fatality rate is lower for HGA (0.7%) and largely relates to complicating opportunistic infections, although poor outcomes are also associated with antecedent medical conditions, such as diabetes mellitus [2].
Clinical complications and risk factors for complications in human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA).
Diagnosis
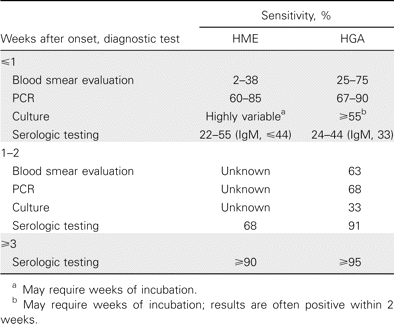
Because human ehrlichiosis can be rapidly progressive and fatal, doxycycline treatment should be initiated promptly once an empirical clinical diagnosis has been rendered, even in the absence of a confirmatory laboratory test. There are several approaches to laboratory confirmation of ehrlichiosis that should be applied at different intervals after the onset of illness (table 3).
Diagnostic tests for human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA), by time interval after onset of clinical illness.
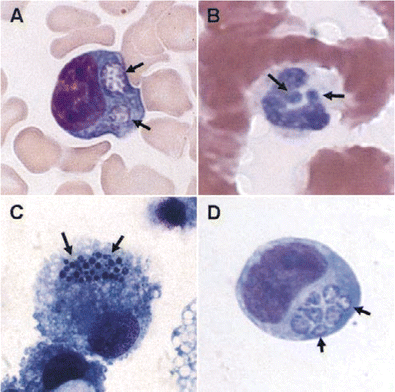
Examination of peripheral blood smears. Examination of a Wright-stained peripheral blood smear for intracytoplasmic inclusions (morulae, Latin for “mulberry”), which may be seen as stippled blue inclusions of bacteria in monocytes (HME) or neutrophils or bands (HGA), is the most rapid diagnostic method that can be used after disease onset (figure 2). This is a relatively insensitive assay for HME, in which generally <10% of infected patients will have these structures identified at presentation [10]. Blood smear evaluation is more useful for HGA diagnosis, because 25%–75% of reported cases in the United States have morulae in peripheral blood examinations, and sensitivity is highest during the first week of infection [2, 16]. Doxycycline treatment adversely affects sensitivity for detection of both E. chaffeensis and A. phagocytophilum by blood smear examination [19].
Ehrlichia chaffeensis (A and C; Wright stain) and Anaplasma phagocytophilum (B and D; Hema-3 stain) morulae (arrows) in peripheral blood monocytes (A), peripheral blood neutrophils (B), DH82 canine histiocytic cell culture (C), and human HL-60 promyelocytic cell culture (D). Original magnification, ×260. (Panel A courtesy of A. Marty.)
Molecular diagnosis by PCR. PCR performed using EDTA- or citrate-anticoagulated blood is rapidly becoming the diagnostic test of choice at or shortly after presentation. PCR obviates the need for culture, and the timeliness of results is of great value to the treating physicians. PCR detection sensitivity is relatively high; it is reported to range between 60% and 85% for E. chaffeensis infection [10, 11] and between 67% and 90% for A. phagocytophilum infection [2, 19]. PCR is the only definitive diagnostic test for E. ewingii infection, although the sensitivity and specificity of this approach are unknown [4, 18]. Recent advances in molecular methods promise even greater analytical sensitivity, and multiplex testing that could identify several agents of ehrlichiosis from a single test has been described elsewhere [20, 21]. As for blood smear microscopy, PCR sensitivity is also adversely affected by antecedent doxycycline treatment; therefore, blood samples should be obtained before or at the initiation of therapy, although treatment should not be withheld while waiting for either samples or a laboratory result.
In vitro cultivation. Another diagnostic alternative is the recovery of either E. chaffeensis or A. phagocytophilum in culture from blood or CSF (E. chaffeensis only) [10, 22]. Unfortunately, E. ewingii has not yet been cultivated in vitro. The major pitfall associated with cultivation is that there is only a small number of competent laboratories, because this technique requires the application of unique antibiotic-free cell culture methods not typically available in clinical laboratories. Although both pathogens are able to grow in several different cell lines, E. chaffeensis is most often isolated by inoculation of mononuclear leukocytes from density gradients into the DH82 canine histiocytic cell line [10, 18]. Cultures are monitored 2–3 times weekly for several weeks by sampling cells in the supernatant and staining with a rapid Romanowsky method, such as Diff-Quik. Small clusters of tiny bacteria aggregate inside of intracytoplasmic vacuoles to form morulae (figure 2). Individual cells can contain multiple morulae in culture, although this is infrequent in vivo. Typically, cultures require 2–6 weeks of incubation before infected cells are detected.
Similarly, A. phagocytophilum can be recovered by culture of leukocyte fractions or whole EDTA-treated blood on human promyelocytic HL-60 cells [22]. The sensitivity of culture for detection of A. phagocytophilum can be equivalent to that of PCR and blood smear examination [2, 16, 19]; however, positive culture results are usually not obtained before ∼1 week and can remain negative for ⩾2 weeks. Cultures are monitored similarly to the way in which those of E. chaffeensis are monitored, and the morulae have a very similar appearance (figure 2). Prior doxycycline treatment diminishes the sensitivity of culture to a greater degree than it does for PCR or blood smear examination.
Serodiagnosis. The most sensitive method of diagnostic confirmation of either HME or HGA is the detection of a seroconversion or 4-fold change in antibody titer during the convalescent phase [2, 11, 13, 17, 23]. The most frequently applied serologic test is based on fluorescent detection of antibodies reactive with whole E. chaffeensis– or A. phagocytophilum–infected tissue culture cells or purified bacteria fixed to glass slides. Polyvalent antibody detection provides sensitivity rates of 88%–90% for HME [11, 24] and 82%–100% for HGA [25]; for IgM, sensitivity ranges from 44% for E. chaffeensis [11] to 27%–37% for A. phagocytophilum [25]. Specificity studies have been conducted only for A. phagocytophilum, for which specificity has been found to range from 83% to 100% [25]. Most nonspecificity results from cross-reactivity among E. chaffeensis and A. phagocytophilum antibodies, such that testing for both pathogens is warranted for suspected cases [25].
There are several potential problems with serologic diagnosis that can obfuscate the interpretation of a positive test result. Factors to consider include the following: (1) IgG antibodies may persist for months to years after infection in the absence of relapse or persistent clinical manifestations [23, 24]; (2) high seroprevalence rates exist in some regions, even among individuals with no clinical evidence of infection [15, 26, 27]; and (3) analysis of a single acute-phase serum sample may result in the detection of as few as 3% of patients with HGA and HME [11, 23]. These shortcomings can be minimized by emphasizing the use of antibody tests for patients with characteristic clinical manifestations, to improve predictive value, and by comparing acute- and convalescent-phase titers against both E. chaffeensis and A. phagocytophilum [25]. Thus, a serologic reaction in a patient lacking typical clinical findings should not be interpreted as representing active, persistent, chronic, or even dormant infection [23]. On the basis of the published performance characteristics of the fluorescent antibody test for HGA using convalescent-phase serum (mean sensitivity, 89.9%; mean specificity, 99.2%) [25], the posttest positive predictive value under conditions of low (4%) prevalence or pretest probability is still 82%; this value increases to >96% when prevalence or pretest probability increases to 25%. Likewise, the negative predictive value of the HGA immunofluorescent assay is 91% and 97% when the pretest probability of infection is 50% and 25%, respectively. Regardless, a number of conditions are associated with false-positive serologic test results, including Rocky Mountain spotted fever, typhus, Q fever, brucellosis, Lyme disease, Epstein-Barr virus infection, and autoimmune conditions that yield autoantibodies (rheumatoid factor, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and antiplatelet antibodies).
Treatment
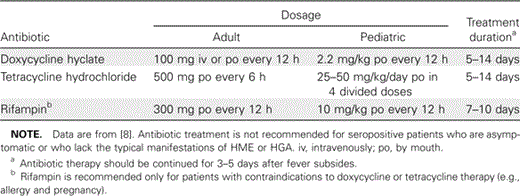
Although no clinical trials have been conducted, empirical data show that all forms of ehrlichiosis respond to tetracyclines [8]. Currently recommended regimens are shown in table 4. Response is generally very rapid, with improvement evident within 24–48 h, although this can be prolonged in those for whom a significant treatment delay has occurred. Doxycycline is preferred over tetracycline because of its twice-daily oral dosing, better patient tolerance, and the relatively lower risk of adverse drug effects for children <8 years of age. There is excellent in vitro susceptibility data to support the clinical impression of tetracycline efficacy for both E. chaffeensis and A. phagocytophilum infection [8, 28, 29]. Doxycycline is also the drug of choice for children <8 years of age who are seriously ill [8]. For situations in which doxycycline is contraindicated (e.g., allergy and pregnancy), few data support alternative regimens. β-Lactams, cephalosporins, macrolides, and aminoglycosides are inactive against E. chaffeensis and A. phagocytophilum in vitro, and there is no evidence for in vivo efficacy of these drugs. Despite some reports of clinical response to chloramphenicol, in vitro MICs discourage its use for treatment of HME or HGA [8]. Similarly, levofloxacin and other fluoroquinolones have variably acceptable MICs for A. phagocytophilum in vitro, but limited clinical applications suggest that they are not effective for HME and may not be effective for HGA [8]. Some empiric clinical success has also been reported for rifampin [30], the usefulness of which is supported by low MICs in vitro [8, 28].
Currently recommended therapeutic regimens for human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA).
Doxycycline therapy is highly efficacious, and posttherapy relapse has never been reported [8]. When there is objective evidence of concurrent HGA and Lyme disease, doxycycline can be used to treat both infections in adults. For children <8 years of age who are not seriously ill, the recommended approach is doxycycline treatment for 3 days after the patient's fever has abated, followed by treatment with another antibiotic (e.g., amoxicillin) that is active against B. burgdorferi to complete a full 14-day course of therapy. Individuals treated in this manner should be followed closely to ascertain that HGA has completely resolved.
Acknowledgments
Personal note from J.S.D.: This article is dedicated to the memory of Dr. Theodore E. Woodward, who was Professor Emeritus of Medicine during my tenure as a medical student at the University of Maryland School of Medicine, Baltimore. My interactions with him at this time were instrumental in focusing me toward an academic career in medicine and rickettsiology. I will always be grateful for his teaching, kind encouragement, wit, and generosity, and for his recollections of interesting infectious diseases cases and pioneer investigators, as well as his humorous anecdotes.
Financial support. National Institute of Allergy and Infectious Diseases (grants R01 AI41213, R01 AI44102, and R21 NS050711).
Supplement sponsorship. This article was published as part of a supplement entitled “Tribute to Ted Woodward,” sponsored by an unrestricted grant from Cubist Pharmaceuticals and a donation from John G. McCormick of McCormick & Company, Hunt Valley, Maryland.
Potential conflicts of interest. All authors: no conflicts.
References
- polymerase chain reaction
- doxycycline
- epidemiology
- fever
- human monocytic ehrlichiosis
- anaplasmataceae
- ehrlichia chaffeensis
- ehrlichiosis
- leukopenia
- opportunistic infections
- serologic tests
- thrombocytopenia
- transaminases
- infections
- bacteria
- diagnosis
- ticks
- human ehrlichiosis
- ehrlichia ewingii
- case fatality rate
- anaplasma phagocytophilum
- human granulocytic anaplasmosis
- blood smear





Comments