Abstract
This study demonstrated that the vitamin D3 decomposition product VDP1 exerts an antibacterial action against Helicobacter pylori but not against other bacteria. Treatment with VDP1 induced a collapse of cell membrane structures of H. pylori and ultimately lysed the bacterial cells. A unique dimyristoyl phosphatidylethanolamine in the membrane lipid compositions contributed to the interaction of VDP1 with H. pylori cells. In separate experiments, VDP1 had no influence on the viability of the human cancer cell lines MKN45 and T47D and lacked any vitamin D3-like hormonal action against the latter. In both 1H and 13C NMR analyses, the spectra patterns of VDP1 corresponded with those of Grundmann's ketone. These results suggest that VDP1 (or Grundmann's ketone-type indene compound) may become a fundamental structure for the development of new antibacterial substances with selective bactericidal action against H. pylori.
Similar content being viewed by others
Introduction
Helicobacter pylori colonizes the human gastric epithelium in approximately half of the world's population. The bacterium takes the form of a curved Gram-negative rod equipped with flagella for motility and can only grow in microaerophilic environments1. As a pathogen, H. pylori causes chronic gastritis and peptic ulcers in infected individuals and persons who carry the bacteria for many years face an elevated risk of gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma2,3. H. pylori-infected patients are medicated with antibacterial substances such as amoxicillin, clarithromycin, or metronidazole. Year by year, however, H. pylori strains resistant to these medicines have been increasing4,5. If the outbreak of resistant strains is to be prevented, it will be essential to develop new antibacterial agents that act selectively on unique biological features of H. pylori. It will also be essential, in developing new anti-H. pylori agents, to ensure that the agents confer no effects on the survival or drug-resistant expression of other bacterial species, especially human intestinal bacteria.
Recent studies by our group have revealed that phosphatidylethanolamine (PE) in H. pylori lipid compositions carries a myristic acid molecule, has a high binding affinity to non-esterified steroidal compounds such as free cholesterol, pregnenolone and progesterone at the carbon-3 position in its frames and plays an important role in the interaction between the bacterial cells and these steroidal compounds6,7. We have also found that dimyristoyl PE (DMPE) is one of the most prevalent PE molecular species in H. pylori cell membrane lipid compositions. Of the steroidal compounds, progesterone and its analogues induce the cell lysis of H. pylori by binding to the myristoyl PE molecular species of the bacterial cells7,8. These investigations by our group suggest that chemical compounds with high binding affinity to the H. pylori PE may confer selective antibacterial action against this bacterium.
Vitamin D3 is a steroidal compound in which the carbon-3 position in the seco-steroid frame is non-esterified with a side chain such as a fatty acid molecule. The carbon position-3 of two vitamin D3 metabolites, namely, the circulating form of vitamin D3 (25-hydroxyvitamin D3) and activated form of vitamin D3 (1α,25-dihydroxyvitamin D3), also lacks an acyl group in the seco-steroid frame. No earlier investigations, however, have elucidated the interaction of the three vitamin D3 species with H. pylori cells. We therefore analyzed the interaction of vitamin D3 species with H. pylori cells.
Results
Antibacterial activities of three vitamin D3 species against H. pylori
When H. pylori without free cholesterol (FC) was incubated for 24 h in the presence of the vitamin D3 species at the concentrations ranging from 1 to 10 μM, every vitamin D3 species at the 5 μM concentration suppressed the CFU count to below the limits of detection (Fig. 1b). In sum, all three vitamin D3 species tested exhibited the antibacterial action against H. pylori.
Antibacterial activities of the three vitamin D3 species against H. pylori.
(a) The chemical structures of vitamin D3 (D3), 25-hydroxyvitamin D3 (25D3) and 1α,25-dihydoxyvitamin D3 (1,25D3). (b) FC-free H. pylori strain NCTC 11638 was incubated for 24 h in the presence of each D3 species in a broth and the CFUs were counted. The gray bars in the graphs represent the baseline CFU count measured immediately after the incubation was started. Three independent experiments with the respective D3 species were performed to calculate the mean CFU ± SD per ml.
Alteration of the anti-H. pylori activities of the vitamin D3 species via non-biological degradations
Seco-steroids are known to be structurally unstable compounds. Vitamin D3 is biologically metabolized or degraded via the catalytic action of hydroxylases of cytochrome P450 families such as CYP11A1, CYP27A1, CYP27B1 and CYP24A19. In addition, vitamin D group has been shown to non-biologically decompose via the exposure to high humidity and high temperature10. This study adopted the non-biological degradation of vitamin D3 species and investigated the antibacterial activity of the degraded vitamin D3 species against H. pylori. We dispersed the three vitamin D3 species for 24 to 48 h into distilled water warmed at 70°C in order to decompose the compounds and analyze the decomposition by TLC. The TLC analysis detected conspicuous reductions in the spot densities of all three vitamin D3 species examined in both 24-h and 48-h incubations (Fig. 2a). Each of vitamin D3 species decomposed via the 48-h incubation in distilled water (70°C) was added into a bacterial suspension of FC-free H. pylori and shaken for 2 h. The antibacterial actions of 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 against H. pylori were conspicuously attenuated by the heat degradation of the compounds. As a consequence, the CFU counts of H. pylori were reduced more in the presence of intact 25-hydroxyvitamin D3 or intact 1α,25-dihydroxyvitamin D3 than in the presence of decomposed 25-hydroxyvitamin D3 or decomposed 1α,25-dihydroxyvitamin D3 (Fig. 2b). On this basis, 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 were found to directly act on H. pylori cells and ultimately eradicate them. Unlike 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3, the heat-degraded vitamin D3 conferred a remarkably augmented antibacterial action against H. pylori. Hence, the CFU counts of H. pylori were reduced more in the presence of decomposed vitamin D3 than in the presence of intact vitamin D3. These results indicate that the antibacterial action of vitamin D3 against H. pylori depended on a certain decomposition product resulting from the warming of the vitamin D3 dispersed into the distilled water.
Antibacterial activities of decomposed vitamin D3 species against H. pylori.
(a) Three vitamin D3 species dispersed into distilled water were incubated for 24 to 48 h at 70°C, recovered via the organic solvent distribution method and analyzed by TLC. D3, vitamin D3; 25D3, 25-hydroxyvitamin D3; 1,25D3, 1α,25-dihydroxyvitamin D3. (b) The decomposition products obtained from the 48-h incubation of the experiments described in panel (a) were incubated for 2 h in the bacterial suspensions of FC-free H. pylori and the CFUs were then counted. The minus and plus symbols in the graph are the CFU levels of H. pylori incubated in the presence of the intact D3 species and decomposed D3 species, respectively. The control in the graph is the CFU level of H. pylori incubated without any D3 specimens. The dashed line bar in the graph represents the baseline CFU measured immediately after the incubation was started. Three independent experiments with the respective D3 species were performed to calculate the mean CFU ± SD per ml.
Antibacterial activities of vitamin D3 decomposition products against H. pylori
Next, we dispersed vitamin D3 for 1 week into distilled water warmed at 70°C to obtain vitamin D3 decomposition products (VDPs) in abundant amounts, divided the VDPs into four fractions by column chromatography and investigated the anti-H. pylori activities in the divided fractions. When the FC-free H. pylori was incubated for 24 h in a broth containing a 0.5 μg/ml concentration of each VDP fraction, the most conspicuous decrease in CFU counts was observed in H. pylori incubated with VDP fraction-1. Next, we further divided the VDP fraction-1 into nine aliquots by column chromatography and investigated the anti-H. pylori activities in each of the aliquots. When FC-free H. pylori was incubated for 24 h in a broth containing a 0.5 μg/ml concentration of each aliquot, the most conspicuous reduction in CFU counts was observed in H. pylori incubated with aliquoted fraction 1–5 or 1–6 (Fig. 3a).
Anti-H. pylori activities in the aliquots of VDP fraction-1 and TLC analysis of VDP1 and VDP2.
(a) FC-free H. pylori was incubated for 24 h in a broth containing aliquoted fraction-1 to measure the CFUs. The dashed line bar in the graph represents the baseline CFU count. Three independent experiments were performed to calculate the mean CFU ± SD per ml. (b) The collected fractions 1–5 and 1–6 (200 μg) were analyzed by 2-dimensional TLC. (c) The purified VDP1 (50 μg) and VDP2 (50 μg) were analyzed by TLC.
Purification of VDP1 and VDP2 from the VDP fraction-1
We next performed a 2-dimensional TLC analysis of the aliquots collected from the VDP fractions 1–5 and 1–6. The TLC analysis detected three high-density spots of VDP (Fig. 3b). We then tried to purify the three VDPs via HPLC and were successful in purifying two, VDP1 and VDP2 (Fig. 3c). VDP3, the third VDP, seemed to be structurally unstable or to volatilize easily, as it disappeared during the purification.
Antibacterial activities of VDP1 and VDP2 against H. pylori
When FC-free H. pylori was incubated for 24 h in the presence of either VDP1 or VDP2 at concentrations ranging from 0.5 to 2 μg/ml, VDP1 at the 1 μg/ml concentration suppressed the CFU count of FC-free H. pylori to below the limits of detection (Fig. 4a). VDP2 exerted a weaker antibacterial action against the FC-free H. pylori than VDP1. The CFUs were thus maintained at the baseline level even in the presence of VDP2 at the highest concentration, 2 μg/ml. Earlier studies by our group have demonstrated that H. pylori acquires resistance to the antimicrobial actions of phosphatidylcholines, antibiotics and bile salts via the incorporation of FC into the cell membranes11,12,13. To investigate further in the present study, we examined the antibacterial activities of VDP1 and VDP2 against FC-retaining H. pylori. When the same experiments performed on FC-free H. pylori were performed on FC-retaining H. pylori in a broth containing 30 μM FC, VDP1 at the 2 μg/ml concentration suppressed the CFU count to below the limit of detection (Fig. 4a). Meanwhile, the antibacterial effect of VDP2 against the FC-retaining H. pylori was completely abolished. Hence, the CFU count of this phenotypic H. pylori in the presence of VDP2 at the 2 μg/ml concentration was comparable to the CFU count in the absence of VDP2.
Anti-H. pylori action of the purified VDP1 and VDP2 and interaction of the VDPs with the PE vesicles.
(a) FC-free H. pylori or FC-retaining H. pylori was incubated for 24 h in the presence of either VDP1 or VDP2 in a broth with or without FC (30 μM) and the CFUs were counted. The gray bars in the graphs represent the baseline CFU counts. The mean CFU ± SD per ml was obtained from three independent experiments. (b) PE vesicle suspensions containing CBB were shaken for 2 h in the presence of either VDP1 or VDP2 and A590 nm was measured in the supernatant. CBB elution was denoted by the relative A590 nm to the A590 nm measured as a value of 1 in the supernatant of the PE vesicles incubated without VDPs. The statistical significance of differences between the CBB elutions from the DMPE vesicles and DPPE vesicles was evaluated by the t-test based on data obtained from three independent pair-experiments. N.S. stands for “no statistical significance”.
Binding affinity of VDP1 and VDP2 to PE vesicles
Recent studies by our group have revealed that H. pylori PE carrying a myristic acid molecule plays an important role in the selective binding of non-esterified steroidal compounds at the carbon-3 position in its frame and that H. pylori cell membranes interact with those steroidal compounds via the mediation of the PE molecular species6,7. We next examined whether VDP1 and VDP2 derived from a steroidal compound (vitamin D3) have a higher binding affinity to PE molecular species carrying two myristic acid molecules (DMPE) than to PE molecular species carrying two palmitic acid molecules (DPPE). A suspension of PE vesicles containing Coomassie brilliant blue (CBB) prepared with either DMPE or DPPE was shaken for 2 h in the presence of the 10 μg/ml concentration of VDP1 or VDP2 and the absorbance of CBB eluted from the PE vesicles was measured in the supernatants at a wavelength of 590 nm. The level of CBB elution from DMPE vesicles incubated with VDP1 was statistically higher than the level of CBB elution from DPPE vesicles incubated with VDP1 (Fig. 4b). This tell us that DMPE vesicles interact more strongly with VDP1 than DPPE vesicles and that VDP1 destabilizes the vesicle structure of DMPE and induces CBB elution from the vesicles. In contrast, the levels of CBB elution from the DMPE and DPPE vesicles incubated with VDP2 did not statistically differ. Hence, VDP2 has a low binding affinity to both DPPE vesicles and DMPE vesicles. Given that PE is the most predominant glycerophospholipid in the cell membrane lipid compositions of H. pylori14 and given that DMPE is one of the most prevalent PE molecular species of this bacterium6, we can assume that VDP1 at least binds to the PE carrying two myristic acid molecules on the H. pylori cells. Based on the results shown in Fig. 4, we paid attention to VDP1 and performed the detailed experiments for clarifying the anti-H. pylori activity of VDP1.
Bactericidal mechanism of VDP1 against H. pylori
H. pylori transforms morphologically from a helical shape to a coccoid shape when exposed to an anaerobic atmosphere14. When H. pylori altered to the coccoid shape is returned to a microaerophilic condition, the bacterium is incapable of recovering its helical shape or of forming colonies on an agar plate by a conventional culture procedure. To analyze the antibacterial mechanism of VDP1 against H. pylori, we performed another set of experiments under anaerobic conditions. When FC-retaining H. pylori was incubated for 48 h in the presence or absence of VDP1 (10 μg/ml) in PBS under anaerobic conditions, the CFUs were below the limits of detection regardless of the presence of VDP1 (Fig. 5a). Meanwhile, the OD660 nm of the bacterial suspension was statistically lower in the presence of VDP1 than in its absence (Fig. 5b). To investigate whether the bacterial cells had lysed via the action of VDP1, as this result implied, we observed the cellular shapes in the bacterial suspensions under a microscope. The bacterial cells before the incubation under anaerobic conditions were rod-like in shape (Fig. 5c). Yet in the bacterial suspension incubated without VDP1, the H. pylori cells changed to a coccoid shape after 48 h of incubation under anaerobic conditions. Meanwhile, the H. pylori cells in the bacterial suspension incubated with VDP1 for 48 h under the same conditions degraded into cellular debris. We next measured the amounts of FC in the cell supernatants of the FC-retaining H. pylori incubated for 48 h in the presence or absence of VDP1. The FC in the cell supernatants incubated with VDP1 was statistically more abundant than the FC in the cell supernatants incubated without VDP1 (Fig. 5d). In sum, VDP1 induced a leakage of FC from the cell membranes of H. pylori. These results, together with the result of Fig. 4b, indicate that VDP1 binds to the DMPE of H. pylori membrane lipid compositions, destabilizes its membrane structures and ultimately lyses the bacterial cells.
Cell lysis of H. pylori induced by the action of VDP1.
(a) FC-retaining H. pylori was incubated for 48 h in the presence or absence of VDP1 at a 10 μg/ml concentration in PBS containing dMβCD (30 μM) under an anaerobic condition and the CFUs were counted. Three independent experiments were carried out to calculate the mean CFU ± SD per ml. (b) The OD660 nm of bacterial suspensions was measured after performing the same experiment described in panel (a). The statistical significance of differences in OD660 nm between the bacterial suspensions in the presence and absence of VDP1 was evaluated by the t-test based on data obtained from three independent pair-experiments. (c) H. pylori cells obtained from the experiment described in panel (a) were microscopically observed. (d) The cell membrane lipids of FC-retaining H. pylori were extracted from the bacterial cell supernatant obtained from the same experiment described in panel (a), in order to quantify the FC amounts via the ferrous chloride-sulfuric acid method. The statistical significance of differences in FC amounts detected in the bacterial cell supernatants in the presence and absence of VDP1 was evaluated by the t-test based on data obtained from three independent pair-experiments. The “0 h” in panels (a), (b), (c) and (d) indicates the CFU, OD660 nm, bacterial shape and FC amount analyzed immediately before exposure to the anaerobic atmosphere, respectively.
Chemical structure of VDP1
The analysis of both 1H-NMR and 13C-NMR identified VDP1 with Grundmann's ketone, (1R,3aR,7aR)-1-[(1R)-1,5-dimethylhexyl]octahydro-7a-methyl-4H-inden-4-one, which was obtained via the oxidative reaction of vitamin D3 catalyzed by ruthenium (Fig. 6a). The NMR spectra of the purified VDP1 were detected as follows: 1H NMR (500 MHz, CDCl3) δ 2.44 (dd, J = 11.8, 7 Hz, 1H), 2.26 (ddd, J = 14.2, 6.0, 2.0 Hz, 1H), 2.22 (m, 1H), 2.12 (ddd, J = 13.1, 9.6, 2.8 Hz, 1H), 2.00 (m, 1H), 1.90 (m, 2H), 1.72 (m, 1H), 1.52 (m, 3H), 1.42 (m, 2H), 1.34 (m, 2H), 1.30 (m, 1H), 1.14 (m, 3H), 1.04 (m, 1H), 0.95 (d, J = 6.3 Hz, 3H), 0.87 (d, J = 2.3 Hz, 3H), 0.87 (d, J = 2.3 Hz, 3H), 0.64 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 212.5, 62.2, 56.9, 50.1, 41.2, 39.6, 39.1, 36.2, 35.7, 28.1, 27.7, 24.2, 24.0, 23.0, 22.7, 19.2, 18.9, 12.6. Meanwhile, the NMR spectra of the synthesized VDP1 were detected as follows: 1H NMR (500 MHz, CDCl3) δ 2.45 (dd, J = 11.8, 7 Hz, 1H), 2.27 (ddd, J = 14.2, 6.0, 2.0 Hz, 1H), 2.22 (m, 1H), 2.12 (ddd, J = 13.1, 9.6, 2.8 Hz, 1H), 2.00 (m, 1H), 1.90 (m, 2H), 1.72 (m, 1H), 1.57 (m, 1H), 1.52 (m, 2H), 1.42 (m, 1H), 1.39 (m, 1H), 1.34 (m, 2H), 1.30 (m, 1H), 1.14 (m, 3H), 1.04 (m, 1H), 0.95 (d, J = 6.3 Hz, 3H), 0.87 (d, J = 2.3 Hz, 3H), 0.87 (d, J = 2.3 Hz, 3H), 0.64 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 212.4, 62.2, 56.9, 50.1, 41.1, 39.6, 39.1, 36.1, 35.7, 28.1, 27.7, 24.2, 24.0, 23.0, 22.7, 19.2, 18.9, 12.6 (see Supplementary Figs. S1 and S2).
Anti-H. pylori activity of synthetic VDP1.
(a) Chemical structure of VDP1. (b) The minimum inhibitory concentrations (MICs) of VDP1 and 1α,25-dihydroxyvitamin D3 (1,25D3) for the FC-retaining H. pylori strain NCTC 11638. (c) Eight bacterial species (107 CFU/ml), including five strains of H. pylori, were incubated for 24 h in a broth (1.5 ml) containing various concentrations of VDP1 in addition to 30 μM FC. After the incubation, the CFUs were counted to determine the minimum bactericidal concentrations (MBCs) that completely eradicate the 106 CFU of bacteria. Seven bacterial species were used in the experiment: S. aureus, E. coli, Salmonella, K. pneumoniae, P. mirabilis, S. marcescens and P. aeruginosa. (d) The FC-retaining H. pylori strain NCTC 11638 was incubated for 0.5 to 2 h in the presence of a 2 μg/ml concentration of VDP1, amoxicillin (AX) or kanamycin (KM) in a broth (1.5 ml) containing 30 μM FC and the CFUs were counted. The mean CFU ± SD per ml was calculated from three independent experiments.
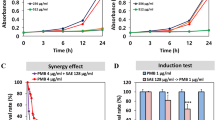
Antibacterial activity of synthetic VDP1 against H. pylori and other bacteria
We next confirmed whether the VDP1 itself was the source of the bactericidal action against H. pylori, using a chemical synthetic VDP1 generated from the oxidative reaction of vitamin D3. We first examined the minimum inhibitory concentration (MIC) of chemical synthetic VDP1 for H. pylori by the agar plate dilution method. The MIC of 1α,25-dihydroxyvitamin D3 for the FC-retaining H. pylori was 1.25 μg/ml (3 μM). Yet, the MIC of chemical synthetic VDP1 for the same phenotypic H. pylori required larger concentration than the 5 μg/ml concentration (>19 μM) (Fig. 6b). In sum, the conventional agar plate dilution method was unsuitable for the determination of MIC of VDP1 for H. pylori. Therefore, we adopted the broth dilution method for determination of the minimum bactericidal concentration (MBC) of VDP1 that completely kills the 106 CFU of H. pylori. As observed with the purified VDP1, the chemical synthetic VDP1 also exerted antimicrobial action against FC-retaining H. pylori. The CFUs of five H. pylori strains incubated for 24 h in the presence of synthetic VDP1 at concentrations from 1.5 to 2.5 μg/ml were therefore below the limits of detection (Fig. 6c). Meanwhile, another seven bacteria, namely E. coli, S. aureus, P. aeruginosa, K. pneumoniae, P. mirabilis, S. marcescens and S. enterica subsp. enterica serovar Typhimurium were entirely unaffected by any antibacterial action of the synthetic VDP1. Even in the presence of VDP1 at a 50 μg/ml concentration, the CFU counts of these bacterial species were comparable to the counts in the absence of VDP1. Thus, H. pylori turned out to be tremendously more susceptible to VDP1 than the other bacterial species. This is likely to be explained by the difference in the predominant fatty acid molecules attached to the PE of the respective bacteria: myristic acid is the predominant molecule attached to H. pylori PE, while palmitic acid is the predominant molecule attached to the PE of all of the other bacteria except for S. aureus that does not possess PE15,16,17,18,19,20,21.
Bactericidal capability of synthetic VDP1 against H. pylori
We next assessed the ability of VDP1 to eradicate H. pylori compared to amoxicillin and kanamycin, antibiotics that disturb bacterial metabolism. According to measurements of the CFUs of the FC-retaining H. pylori along the time axis of incubation in the presence of a 2 μg/ml concentration of VDP1, amoxicillin, or kanamycin, VDP1 conferred a more rapid bactericidal action against H. pylori than the other two antibiotics (Fig. 6d). This result demonstrates that VDP1 directly injures the cell membranes of H. pylori without necessarily inhibiting the cellular metabolism.
Influence of synthetic VDP1 on the viability of MKN45 cells and T47D cells
We next examined whether the synthetic VDP1 has toxic effects against cell lines of human gastric cancer (MKN45) and breast cancer (T47D). MKN45 cells or T47D cells were incubated for 72 h in the presence of VDP1 at concentrations ranging from 2 to 10 μM, for estimation of the cell viability by MTT assay. VDP1 had no influence on the viability of either MKN45 cells or T47D cells: the cells incubated in the presence of VDP1 at every concentration proliferated to a degree comparable to the same cells incubated without VDP1 (Fig. 7a). This finding confirms that VDP1 is incapable of eliciting toxicity against human cancer cell lines and it furthermore suggests that any cytotoxic side effects from VDP1 are likely to be extremely weak in human cells.
Lack of toxicity and vitamin D3-like activity of VDP1 against human cells.
(a) MKN45 cells or T47D cells were incubated for 72 h in the presence of VDP1 at the concentration ranges bactericidal to H. pylori in order to estimate the cell viability by MTT assay. The cell viabilities obtained from experiments performed in triplicate to calculate the mean value ± SD are denoted by the relative A540 nm in comparison with the value of 1 at the A540 nm of the viability of the cells incubated without VDP1. (b) Caspase-cleaved keratin 18 (ccK18) was detected in the culture supernatant of T47D cells stimulated for 72 h with either VDP1 or 1α,25-dihydroxyvitamin D3 (1,25D3). The statistical significance of ccK18 amounts between the cells with or without stimulation was evaluated via the t-test based on data obtained from three independent pair-experiments. N.S. stands for “no statistical significance”. (c) Transient receptor potential vanilloid type 6 (TRPV6) protein was detected in the lysates of T47D cells stimulated with either VDP1 (1 μM) or 1,25D3 (1 μM). The left-hand panel shows the SDS-PAGE profiles of proteins stained by CBB. The right-hand panel shows the TRPV6 protein profiles detected with anti-TRPV6 antibody. The same amounts of cell lysates were applied to SDS-PAGE in the two panels. Lanes 1, 2 and 3 in the two panels are the protein profiles of un-stimulated cells, 1,25D3-stimulated cells and VDP1-stimulated cells, respectively.
Inability of VDP1 to induce a vitamin D3-like activation of T47D cells
An 1α,25-dihydroxyvitamin D3 induces the formation of a heterodimer consisting of vitamin D receptor (VDR) and retinoid X receptor (RXR) inside the nucleus of the mammalian cell. A recent study has demonstrated that 1α,25-dihydroxyvitamin D3 is capable of binding to retinoic acid-related orphan receptors α and γ (RORα and RORγ)22. The VDR-RXR coupled with 1α,25-dihydroxyvitamin D3 binds to the vitamin D3 response elements (VDREs) located 5′-upstream from the target genes, acts as a transcriptional factor and up-regulates the gene expression of various proteins such as caspases and TRPV623,24,25,26,27,28,29,30. Caspases increase the expression levels of caspase-cleaved keratin 18 (ccK18), a cytokeratin associated with cell apoptosis31,32. In sum, 1α,25-dihydroxyvitamin D3 is capable of eliciting increases in the production of ccK18 and TRPV6 proteins in human cells. Incidentally, ROR monomer coupled with 1α,25-dihydroxyvitamin D3 interacts with the gene sequences of ROR responsive element (RORE) and inhibits the expression of the target genes22. To examine whether synthetic VDP1 exhibits a vitamin D3-like action, we stimulated T47D cells for 72 h with either VDP1 or 1α,25-dihydroxyvitamin D3 and analyzed the expression levels of ccK18 and TRPV6. The level of ccK18 protein in T47D cells stimulated with 1α,25-dihydroxyvitamin D3 was higher than that in cells stimulated with VDP1, while the level of cck18 in T47D cells stimulated with VDP1 was comparable to the level in un-stimulated cells (Fig. 7b). As seen in the experiments with ccK18, the protein band of TRPV6 was denser in T47D cells stimulated with 1α,25-dihydroxyvitamin D3 than in cells without stimulation, but the level of TRPV6 protein detected in T47D cells stimulated with VDP1 was comparable to the level of its protein detected in un-stimulated cells (Fig. 7c). These results suggest the possibility that VDP1 is incapable of activating human cells, as was observed with 1α,25-dihydroxyvitamin D3.
Discussion
A recent study by another group has reported that a relatively large amount of the Grundmann's ketone derivative dotted into a paper disk shows the antimicrobial action especially against fungi and Gram-positive bacteria on the agar plate33. The above-mentioned study, however, examined neither the antibacterial activity of its Grundmann's ketone derivative against H. pylori nor the antimicrobial activity of Grundmann's ketone (VDP1) itself. This study demonstrated the anti-H. pylori activity of VDP1 itself, although the MIC of VDP1 for H. pylori was incapable of measuring by the conventional agar plate dilution method. The Grundmann's ketone derivative investigated by another group has a positive charge due to the amine in its detergent-like structure and is therefore guessed to diffuse even into the agar plate from the paper disk. Meanwhile, VDP1 is a high non-polar ketone compound. On this basis, VDP1 may be difficult to extensively diffuse into the agar plate. Therefore, the agar plate dilution method seems to be unsuitable for the decision of MICs of VDP1 for the bacteria. Incidentally, given that the polarity of 1α,25-dihydroxyvitamin D3 is higher than that of VDP1 due to the three hydroxyl groups in its seco-steroid frame, we can assume that 1α,25-dihydroxyvitamin D3 diffuses more extensively than VDP1 into the agar plate. We will need to synthesize such new VDP1 derivatives as reported by another group33 to compare the agar diffusion of the VDP1 derivatives with that of VDP1 itself.
An earlier study by another group has shown that elderly women who had been supplied a vitamin D3 analogue had a lower rate of H. pylori infection than elderly women without the analogue, though it was unclear whether the analogue had exerted a direct antibacterial action against the H. pylori34. The present study demonstrated that intact compounds of both 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 exerted antibacterial actions against H. pylori without FC. Our findings also confirmed that the two vitamin D3 metabolites are capable of eradicating the FC-retaining H. pylori (data not shown). Hence, we have discovered that 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 confer a novel biological action that is clearly distinct from the hormonal action on mammals35,36,37,38,39,40,41,42,43. The colonization of the human stomach by H. pylori is accomplished in childhood. We know this because the gut immunities of children are still incomplete44,45. Serum 25-hydroxyvitamin D3 levels in childhood vary between the pediatric populations investigated and children with extremely low 25-hydroxyvitamin D3 levels in serum are commonly observed46,47. This may suggest that low 25-hydroxyvitamin D3 levels in serum are correlated with the incidence of H. pylori-infection in childhood. The goal for future studies will be to elucidate the detailed antibacterial mechanism of 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 against H. pylori and to analyze the epidemiological relationship between serum 25-hydroxyvitamin D3 levels and H. pylori-infection in children.
This study elucidated the bactericidal effect of VDP1 (Grundmann's ketone) against H. pylori in vitro. In the next stage, experiments using animal models will be essential to estimate the treatment effect of VDP1 on H. pylori-infected hosts and to investigate the side effects of the vitamin D3 decomposition product on mammals. We will also need to examine whether other bacterial species from the genus Helicobacter are susceptible to the bactericidal action of VDP1.
Methods
Bacterial culture
Bacteria were cultured in a pleuropneumonia-like organisms (PPLO) broth (Difco Laboratories, Detroit MI) at 37°C in a Concept 400 microaerophilic chamber (10% CO2, 5% O2 and 85% N2) (Ruskinm Technology, Leeds, UK).
Colony-forming units (CFUs)
Bacterial suspensions (100 μl) prepared in 10-fold serial dilutions using a PPLO broth were spread on brain-heart infusion agar (Difco Laboratories) plates containing 5% horse serum (Gibco, Auckland, NZ) and cultured for 1 week in the microaerophilic chamber. The bacterial colonies grown were counted on the agar plates spread with appropriate bacterial cell diluents to calculate the log10 CFU/ml.
Bacterial cell density (OD660 nm)
Bacterial suspensions (200 μl) were measured at a wavelength of 660 nm. PBS was used as the medium of bacterial suspension.
Microscopic observation
Bacterial cells suspended in PBS (10 μl) were applied on a glass slide, dried at room temperature and stained for 10 min with Coomassie brilliant blue (CBB) solution (0.05% CBB, 9% acetate, 45.5% methanol).
Analysis of the hydrophobic compounds
Solvents of chloroform-methanol (2:1) were added at 5-fold volume to an aqueous solution in a glass bottle and intensely shaken. The mixed medium of water and organic solvents was re-separated into two liquid phases via incubation for 24 h at 4°C. After the upper liquid phase was removed, the solvents of the lower liquid phase were vaporized under nitrogen airflow or via use of a rotary evaporator to obtain the hydrophobic compounds. To analyze the hydrophobic compounds via thin-layer chromatography (TLC), the specimens dissolved in a chloroform-methanol (2:1) solution (40 μl) were dotted onto a Silica Gel 60 plate (Merck, Darmstadt, Germany) and fractionated on the plate surface with the developing solvents. After the TLC, the plate was sprayed with a 60% sulfuric acid solution and heated at 120 to 180°C to visualize the spots of the components on the TLC plate surface.
Quantification of free cholesterol (FC)
Lipid specimens dissolved in acetic acid solution (600 μl) were added to a ferrous chloride-sulfuric acid reagent [phosphoric acid-sulfuric acid (2:25) solution containing 0.2% FeCl2-6H2O] solution (400 μl), thoroughly stirred and incubated for 15 min at room temperature. After color reaction and cooling, the absorbance (A550 nm) of the mixed solution (200 μl) was measured at a wavelength of 550 nm. The amounts of FC in the specimens were quantified based on an FC standard curve calculated from the A550 nm (y axis) and known FC amounts (x axis).
Preparation of a phosphatidylethanolamine (PE) vesicle suspension containing CBB
A powder (15 mg) of dimyristoyl PE (DMPE: Sigma-Aldrich Inc., MO) or dipalmitoyl PE (DPPE: Sigma-Aldrich Inc.) dispersed into a 50 mM Tris (pH 7.5) buffer (4 ml) containing 150 mM sucrose was sonicated for 6 to 8 h in a cold sonicator of the bucket type. After confirmation of the PE vesicle formation via microscopic observation, the PE vesicles were washed three times with a 50 mM Tris (pH 7.5) buffer (1 ml) via centrifugation (10000 × g, 5 min), suspended in the same buffer (2 ml) containing 0.1% CBB, sonicated for 1 h and shaken overnight at 4°C. After the PE vesicle suspension containing CBB was washed three times with a 50 mM Tris (pH 7.5) buffer via centrifugation (10000 × g, 5 min), a PE vesicle suspension of the same buffer (100 μl) was adjusted to a value of 2 in the OD660 nm and stored at −20°C until use in experiments.
Vitamin D3 species and others
Vitamin D3 from Wako Pure Chemical Industries Ltd. (Tokyo, Japan), circulating-form of vitamin D3 (25-hydroxyvitamin D3) from Enzo Life Sciences Inc. (NY), or activated form of vitamin D3 (1α,25-dihydroxyvitamin D3) from Cayman Chemical Co. (MI) was dissolved in ethanol at a 5 mM concentration and stored as stock solution at −80°C. The 0.2% concentration of ethanol had no influence on the survival of either bacteria or human cells. Two antibiotics, namely, kanamycin sulfate from EMD Biosciences Inc. (CA) and amoxicillin from Sigma-Aldrich Inc., were respectively dissolved in distilled water at 1 mg/ml concentrations and stored at 4 or −30°C as stock solutions.
Assay of bacterial growth
Bacteria were shaken in the presence of various concentrations of antibacterial substances in either PPLO broth alone (1.5 ml) or PPLO broth (1.5 ml) containing a 30 μM concentration of FC in the microaerophilic chamber and the CFUs were measured. Five H. pylori strains were investigated: NCTC 11638, ATCC 43504, 26695 and clinical isolates A-13 and A-19. Two phenotypes of H. pylori were used: FC-free cells and FC-retaining cells. The FC-free H. pylori were prepared by the culture procedure without FC. The FC-retaining H. pylori were prepared by culturing for three generations in the presence of FC (Wako Pure Chemical Industries Ltd.) at a 30 μM concentration. Seven typical bacterial strains maintained for many years in our laboratory were also used in this assay: Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Serratia marcescens and Salmonella enterica subsp. enterica serovar Typhimurium.
Non-biological degradation of vitamin D3 species
Each of the vitamin D3 species (150 nmol) was stirred for 24 to 48 h in distilled water (1.5 ml) at 70°C, recovered by the organic solvent distribution method described in the section on the analysis of hydrophobic compounds, subjected to TLC with a chloroform-acetone-methanol (9:1:1) solvent system to analyze its degradation. Next, the decomposition products obtained from each vitamin D3 species were dispersed into the bacterial suspensions (1.5 ml) of the H. pylori strain NCTC 11638 and were shaken for 2 h in the microaerophilic chamber to count the CFUs.
Purification of vitamin D3 decomposition products 1 and 2 (VDP1 and VDP2)
Vitamin D3 (100 mg) was dispersed into distilled water (100 ml) and incubated for 1 week at 70°C to obtain its decomposition products (VDPs) and the VDPs were then recovered via the organic solvent distribution method and dissolved in a chloroform solvent (1 ml). After the VDP solution was applied onto an Iatrobead 6RS-8060 (Mitsubishi Kagaku Iatron Inc., Tokyo, Japan) column (1-cm diameter, 5-cm height) activated with a chloroform solvent, the VDPs were divided into four fractions respectively eluted with a chloroform solvent (10 ml), chloroform-acetone (9:1) solvents (10 ml), chloroform-acetone (7:3) solvents (10 ml) and chloroform-acetone (4:6) solvents (10 ml) and dried using a rotary evaporator.
Next, the VDP fraction eluted with the chloroform solvent was further divided into nine aliquots (500 μl/tube) eluted with diethylether-chloroform (7:3) solvents using the same Iatrobead 6RS-8060-column and dried using a rotary evaporator. Two-dimensional TLC was carried out to confirm VDP1 and VDP2 using hexane-diethylether (6:4) solvents and then hexane-ethylacetate (10:1) solvents.
Next, the aliquots containing VDP1 and VDP2 were collected and applied onto a Purif Pack SI30 Flash column (size 20, 2-cm diameter, 6-cm height) (Shoko Scientific, Kanagawa, Japan). The VDP1 and VDP2 were eluted from the column at a flow velocity of 15 ml/min using a hexane solvent containing ethylacetate with concentration gradient and the chromatograms of eluates were monitored by measuring the absorbance (A254 nm) at a wavelength of 254 nm (ChemGenesis Inc., Tokyo, Japan).
Finally, VDP1 and VDP2 were purified by high-performance liquid chromatography (HPLC) using a GL Science Inertsil SIL 100A column (2-cm diameter, 25-cm height; GL Sciences Inc., Tokyo, Japan). The HPLC was carried out at a flow velocity of 20 ml/min using a hexane solvent containing 3% ethylacetate by monitoring the chromatograms of eluates measured with A254 nm (ChemGenesis Inc.). The purity of VDP1 and VDP2 after the HPLC application was confirmed by TLC analysis with a hexane-ethylacetate (10:1) solvent system. The purified VDP1 and VDP2 were dissolved in ethanol solution and stored at −80°C until use in experiments.
Analysis of the interaction of VDP1 and VDP2 with the PE vesicles
A PE vesicle suspension (50 μl) prepared by the method described in the section on preparing the phosphatidylethanolamine (PE) vesicle suspension containing CBB was added into a 50 mM Tris (pH 7.5) buffer (1.45 ml) containing 15 μg of either VDP1 or VDP2 and shaken for 2 h at 37°C. After the PE vesicles were removed via centrifugation (10000 × g, 5 min), the absorbance of the supernatant (200 μl) was measured at a wavelength of 590 nm to detect the elution of CBB from the PE vesicles.
Analysis of the anti-H. pylori mechanism of VDP1
A bacterial suspension (200 μl) of H. pylori strain NCTC 11638 was cultured for 24 h with shaking in a PPLO broth (10 ml) containing a 30 μM concentration of FC and 10 μM concentration of 2,6-di-O-methyl-β-cyclodextrin (dMβCD: Sigma-Aldrich Inc.) in the microaerophilic chamber. This culture procedure was repeated three times to prepare the FC-retaining H. pylori cells. After three washes with PBS by centrifugation (10000 × g, 5 min), the bacterial cells (109 CFU) were suspended in PBS (5 ml) containing dMβCD (30 μM) and shaken for 48 h in the presence or absence of VDP1 (10 μg/ml) at 37°C in a box filled with anaerobic gas using an AnaeroPack (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). The CFUs were counted, the OD660 nm of the bacterial suspension (200 μl) was measured and the bacterial cells stained with CBB were observed. After filtrating the bacterial suspension (4 ml) through a GDXS 25 syringe filter (Whatman, Buckinghamshire, UK), the lipids from the H. pylori cells were extracted from the filtrates by the organic solvent distribution method to measure the FC amounts released from the cell membranes.
Analysis of the chemical structure of VDP1
Nuclear magnetic resonance (NMR) spectra were recorded on 500 MHz for 1H and 125 MHz for 13C in the indicated solvent using a Model ECA-500 (JEOL Ltd., Tokyo, Japan). Chemical shifts were reported in parts per million (ppm) relative to the signal (0 ppm) for internal tetramethylsilane in CDCl3. 1H-NMR spectral data were reported as follows: CDCl3 (7.26 ppm). 13C-NMR spectral data were reported as follows: CDCl3 (77.0 ppm).
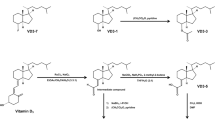
Chemical synthesis of VDP1
To the stir solution of a catalytic amount of RuCl3 and NaIO4 (1.22 mmol) in ethylacetate-acetonitrile-water (3:3:1) at 0°C, vitamin D3 (0.78 mmol) from Wako Pure Chemical Industries Ltd. was added. After stirred for 5 h at room temperature, the reaction mixture was diluted with hexane, quenched with 10% aq. Na2S2O3 and the aqueous layer was extracted with two portions of hexane. The combined extract was washed with brine and evaporated in vacuo. The residue was chromatographed on a silica gel (Merck) with hexane-ethylacetate (4:1) to give VDP1 (Grundmann's ketone) from the aqueous layer (see Supplementary Fig. S3)48,49,50. The mass spectrum of the synthetic VDP1 was detected via the gas chromatography-mass spectrometry (GC-MS) using a JMS-Q1000GCMkII system (JEOL Ltd.) in order to be compared to the mass spectrum of the VDP1 described in the section on purifying the vitamin D3 decomposition products 1 and 2 (VDP1 and VDP2) (see Supplementary Fig. S4).
Measurement of minimum inhibitory concentrations (MICs)
Various concentrations of specimens were added to the 30 μM FC-dispersed PPLO agar plates. The bacterial suspension (10 μl) of FC-retaining H. pylori (108 CFU/ml) was dropped onto the agar plates and incubated for 1 week under microaerobic conditions at 37°C. After incubation, the MICs of the specimens for H. pylori were measured by confirming the growth of colonies on the agar plates. Two independent experiments were performed to determine the MICs.
Analysis of cytotoxicity of VDP1
Human breast cancer T47D cells from DS Pharma Biomedical Co., Ltd. (Osaka, Japan) and human gastric cancer MKN45 cells from GeneticLab Co., Ltd. (Sapporo, Japan) were maintained in an RPMI 1640 (10% FCS-RPMI) medium (Sigma-Aldrich Inc.) supplemented with 10% heat-inactivated fetal calf serum (FCS: Gibco), 10 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.2% NaHCO3 in a humidified chamber at 37°C filled with a 5% CO2 gas. After the cells were washed once with PBS and adjusted to 105.3 cell/ml using an RPMI 1640 medium supplemented with 2.5% FCS in place of 10% FCS, the cell suspension (100 μl) was inoculated into the same medium (100 μl) containing VDP1 at various concentrations and incubated for 72 h at 37°C in the 5% CO2 incubator. A 20 μl volume of MTT (Sigma-Aldrich Inc.) reagent (5 mg/ml in PBS) was added into the cell cultures in the final 4 h of the 72-h incubation. After the cell supernatant was removed, the cells were lysed in an isopropanol solution (200 μl) containing 5% formic acid in order to solubilize the formazan blue crystals produced from the viable cells and the absorbance of formazan blue solution (150 μl) was measured at a wavelength of 540 nm.
Detection of caspase-cleaved keratin 18 (ccK18)
T47D cells adjusted to 105.5 cell/ml using 10% FCS-RPMI medium (1 ml) were incubated for 72 h in the presence of various concentrations of either VDP1 or 1α,25-dihydroxyvitamin D3 at 37°C in the 5% CO2 incubator and the culture supernatants were then recovered. The ccK18 in the culture supernatants was detected using a M30 CytoDeath ELISA kit (Peviva AB, Stockholm, Sweden) according to the manufacturer's instructions.
Detection of transient receptor potential vanilloid type 6 (TRPV6) protein
After T47D cells were cultured by the same method described in the section on detecting caspase-cleaved keratin 18 (ccK18), the culture supernatant was removed and the cells were lysed in a 50 mM Tris (pH 6.8) buffer (100 μl) containing 5% glycerol, 0.002% bromophenol blue (BPB), 2% sodium dodecyl sulfate (SDS) and 40 mM dithiothreitol (DTT). Cell lysates (5 μl) were electrophoresed in a 0.1% SDS-12.5% polyacrylamide gel to separate the proteins (SDS-PAGE). The proteins were then stained with CBB solution or transferred onto a Clear Blot P-membrane (p-membrane: ATTO Co., Tokyo, Japan) activated with a Tris-glycine buffer (100 mM Tris, 192 mM glycine, 5% methanol) using a blotting device (ATTO Co.). The p-membrane was shaken overnight in 20 mM Tris (pH 7.6) buffered saline (TBS) containing 1% skim milk at 25°C and shaken for 2 h in the presence of anti-TRPV6 rabbit IgG polyclonal antibody (Santa Cruz Biotechnology Inc., CA) in a reaction buffer (0.1% skim milk, 0.1% Tween 20 in TBS) at 25°C. The p-membrane was then washed three times with Tween 20-TBS (0.1% Tween 20 in TBS) and shaken for 2 h at 25°C in the presence of horseradish peroxidase (HRP) conjugate anti-rabbit IgG polyclonal antibody (Cell Signaling Technology Inc., MA) in the reaction buffer. After further washes of the p-membrane in Tween 20-TBS (three tines), TBS (twice) and distilled water (once), the enzymatic substrate reaction of HRP was developed on the p-membrane in a 100 mM Tris (pH 7.5) buffer containing 0.04% H2O2 and 0.027% DAB (3,3′-diaminobenzidine-tetra-hydrochloride-dihydrate) and the reaction was stopped by a wash in water.
References
Marshall, B. & Warren, J. R. Unidentified carved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1274 (1983).
Fukase, K. et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label randomized controlled trial. Lancet 372, 392–397 (2008).
Stolte, M. et al. Helicobacter and gastric MALT lymphoma. Gut 50, III19-III24 (2002).
Kobayashi, I. et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J. Clin. Microbiol. 45, 4006–4010 (2007).
Francesco, V. De. et al. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointestin. Liv. Dis. 19, 409–414 (2010).
Shimomura, H., Hosoda, K., Hayashi, S., Yokota, K. & Hirai, Y. Phosphatidylethanolamine of Helicobacter pylori functions as a steroid-binding lipid in the assimilation of free cholesterol and 3β-hydroxyl steroids into the bacterial cell membrane. J. Bacteriol. 194, 2658–2667 (2012).
Amgalanbaatar, A. et al. Antibacterial activity of a novel synthetic progesterone species carrying a linoleic acid molecule against Helicobacter pylori and the hormonal effect of its steroid on a murine macrophage-like cell line. J. Steroid Biochem. Mol. Biol. 140, 17–25 (2014).
Hosoda, K., Shimomura, H., Hayashi, S., Yokota, K. & Hirai, Y. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol. Lett. 318, 68–75 (2011).
Slominski, A. T. et al. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 144, 28–39 (2014).
Grady, L. T. & Thakker, K. D. Stability of solid drugs: degradation of ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) at high humidities and elevated temperatures. J. Pharm. Sci. 69, 1099–1102 (1980).
Shimomura, H. et al. Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori. FEMS Microbiol. Lett. 301, 84–94 (2009).
McGee, D. J. et al. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL37. Antimicrob. Agents Chemother. 55, 2897–2904 (2011).
Trainor, E. A., Horton, K. E., Savage, P. E., Testerman, T. L. & McGee, D. J. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect. Immun. 79, 88–97 (2011).
Shimomura, H., Hayashi, S., Yokota, K., Oguma, K. & Hirai, Y. Alteration in the composition of cholesteryl glucosides and other lipids in Helicobacter pylori undergoing morphological change from spiral to coccoid form. FEMS Microbiol. Lett. 237, 407–413 (2004).
Shokri, A. & Larsson, G. Characterisation of the Escherichia coli membrane structure and function during fed batch cultivation. Microb. Cell Fact. 3, 9 (2004).
Dunnick, J. K. & O'Leary, W. M. Correlation of bacterial lipid composition with antibiotic resistance. J. Bacteriol. 101, 892–900 (1970).
Amine, A., Saloua, K. S., Mouadh, M., Alya, E. I. M. & Ahmed, M. [The absence of the “GATC-binding protein SeqA” affects DNA replication in Salmonella enterica serovar Typhimurium]. DNA replication and related cellular processes [ Kusic-Tisma, J. (ed.)] [283–300] (Rijeka, Croatia, 2011).
Gmeiner, J. & Martin, H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Eur. J. Biochem. 67, 487–494 (1976).
Zalkin, H., Law, J. H. & Goldfine, H. Enzymatic synthesis of cyclopropane fatty acids catalyzed by bacterial extracts. J. Biol. Chem. 238, 1242–1248 (1963).
Kearns, D. B., Robinson, J. & Shimkets, L. J. Pseudomonas aeruginosa exhibits directed twitching motility up phosphatidylethanolamine gradients. J. Bacteriol. 183, 763–767 (2001).
Beining, P. R., Huff, E., Prescott, B. & Theodore, T. S. Characterization of the lipids of mesosomal vesicles and plasma membranes from Staphylococcus aureus. J. Bacteriol. 121, 137–143 (1975).
Slominski, A. T. et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. Res. Commun. 28, 2775–2789 (2014).
Mathiasen, I. S., Lademann, U. & Jäättelä, M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 59, 4848–4856 (1999).
Bolanz, K. A., Hediger, M. A. & Landowski, C. P. The role of TRPV6 in breast carcinogenesis. Mol. Cancer Ther. 7, 271–279 (2008).
Bolanz, K. A., Kovacs, G. G., Landowski, C. P. & Hediger, M. A. Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol. Cancer Res. 7, 2000–2010 (2009).
Baker, A. R. et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA 85, 3294–3298 (1988).
Haussler, M. R. et al. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J. Endocrinol. 154, 557–573 (1997).
Thompson, P. D., Jurutka, P. W., Haussler, C. A., Whitfield, G. K. & Haussler, M. R. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. J. Biol. Chem. 273, 8483–8491 (1998).
Carlberg, C. Mechanisms of nuclear signaling by vitamin D3 interplay with retinoid and thyroid hormone signaling. Eur. J. Biochem. 231, 517–527 (1995).
Schräder, M., Nayeri, S., Kahlen, J. P., Müller, K. M. & Carlberg, C. Natural vitamin D3 response elements formed by inverted palindromes: polarity-directed ligand sensitivity of vitamin D3 receptor-retinoid X receptor heterodimer-mediated transactivation. Mol. Cell Biol. 15, 1154–1161 (1995).
Leers, M. P. et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope expressed during early apoptosis. J. Pathol. 187, 567–572 (1999).
Schutte, B. et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp. Cell Res. 297, 11–26 (2004).
Krojer, M., Keller, M. & Bracher, F. 7-Aza-des-A-steroids with antimicrobial and cytotoxic activity. Sci. Pharm. 81, 329–338 (2013).
Kawaura, A. et al. Inhibitory effect of long term 1α-hydroxyvitamin D3 administration on Helicobacter pylori infection. J. Clin. Biochem. Nutr. 38, 103–106 (2006).
Gombart, A. F., Borregaard, N. & Koeffler, H. P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3 . FASEB J. Res. Commun. 19, 1067–1077 (2005).
Meyer, M. B., Watanuki, M., Kim, S., Shevde, N. K. & Pike, J. W. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol. Endocrinol. 20, 1447–1461 (2006).
Song, Y. et al. Calcium transpoter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25-dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinol. 144, 3885–3894 (2003).
Christakos, S., Gabrielides, C. & Rhoten, W. B. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional consideration and molecular biology. Endocrine Rev. 10, 3–26 (1989).
Demay, M. B., Kierman, M. S., DeLuca, H. F. & Kronenberg, H. M. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3 . Proc. Natl. Acad. Sci. USA 89, 8097–8101 (1992).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Mineral Res. 19, 429–435 (2004).
Kolek, O. I. et al. 1α,25-dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liv. Physiol. 289, G1036–G1042 (2005).
Yamamoto, R. et al. 1α,25-dihydroxyvitamin D3 acts predominantly in mature osteoblasts under conditions of high extracellular phosphate to increase fibroblast growth factor 23 production in vitro. J. Endocrinol. 206, 279–286 (2010).
Noda, M., Vogel, R. L., Craig, A. M., Prahl, J. & DeLuca, H. Identification of a DNA sequence responsible for binding of 1,25-hydroxyvitamin D3 receptor and 1,25-hydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (Spp-1 or osteopontin) gene expression. Proc. Natl. Acad. Sci. USA 87, 9995–9999 (1990).
Asaka, M. et al. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter 6, 294–299 (2001).
Kobayashi, T. et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gas. Cancer 7, 233–239 (2004).
Brehm, J. M. et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 179, 765–771 (2009).
Mansbach, J. M., Ginde, A. A. & Camargo, Jr, C. A. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124, 1404–1410 (2009).
Plietker, B. & Niggemann, M. RuCl3/CeCl3/NaIO4: a new bimetallic oxidation system for the mild and efficient dihydroxylation of unreactive olefins. J. Org. Chem. 70, 2402–2405 (2005).
Windaus, A. & Grundmann, W. Über die konstitution des vitamins D2 . II. Justus Liebigs Annalen der Chemie. 524, 295–299 (1936).
Sicinski, R. R. & DeLuca, H. F. Ruthenium tetroxide oxidation of Grundmann's ketone derivative from vitamin D3 . Bioor. Med. Chem. Lett. 5, 159–162 (1995).
Acknowledgements
This work was supported by a Research Award to JMU Graduate Students and in part by JKA Promotion Funds from Keirin Race.
Author information
Authors and Affiliations
Contributions
H.S., S.H. and Y.H. conceived the study and H.S. constructed the experiment methods and wrote a large part of the manuscript. K.H. and A.A. carried out the bioassay of chemical compounds together with H.S. and K.W. and H.M. synthesized and purified the chemical compound and analyzed it together with T.T. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hosoda, K., Shimomura, H., Wanibuchi, K. et al. Identification and characterization of a vitamin D3 decomposition product bactericidal against Helicobacter pylori. Sci Rep 5, 8860 (2015). https://doi.org/10.1038/srep08860
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08860
This article is cited by
-
Association of serum vitamin D levels on Helicobacter pylori infection: a retrospective study with real-world data
BMC Gastroenterology (2023)
-
Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients
Scientific Reports (2020)
-
Effect of 25-hydroxyvitamin D on Helicobacter pylori eradication in patients with type 2 diabetes
Wiener klinische Wochenschrift (2019)
-
Dietary habits and Helicobacter pylori infection: a cross sectional study at a Lebanese hospital
BMC Gastroenterology (2018)
-
Identification of low oxygen-tolerating bacteria in prostate secretions of cancer patients and discussion of possible aetiological significance
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.