Abstract
Objective:
To determine whether Methenamine Hippurate (MH) or cranberry tablets prevent urinary tract infections (UTI) in people with neuropathic bladder following spinal cord injury (SCI).
Study design:
Double-blind factorial-design randomized controlled trial (RCT) with 2 year recruitment period from November 2000 and 6 month follow-up.
Setting:
In total, 543 eligible predominantly community dwelling patients were invited to participate in the study, of whom 305 (56%) agreed.
Methods:
Eligible participants were people with SCI with neurogenic bladder and stable bladder management. All regimens were indistinguishable in appearance and taste. The dose of MH used was 1 g twice-daily. The dose of cranberry used was 800 mg twice-daily. The main outcome measure was the time to occurrence of a symptomatic UTI.
Results:
Multivariate analysis revealed that patients randomized to MH did not have a significantly longer UTI-free period compared to placebo (HR 0.96, 95% CI: 0.68–1.35, P=0.75). Patients randomized to cranberry likewise did not have significantly longer UTI-free period compared to placebo (HR 0.93, 95% CI: 0.67–1.31, P=0.70).
Conclusion:
There is no benefit in the prevention of UTI from the addition of MH or cranberry tablets to the usual regimen of patients with neuropathic bladder following SCI.
Similar content being viewed by others
Introduction
People with spinal cord injury (SCI) and neurogenic bladders are at significant risk of morbidity from urinary tract infection (UTI). Approximately, 2 UTI episodes per year are experienced by an average person.1 Urinary continence in this population is most commonly managed by intermittent, indwelling or suprapubic urethral catheterization, or reflex voiding with external collection devices.
Current practice for UTI management is that asymptomatic bacteriuria is not treated because it is not associated with adverse urological outcomes in the spinal injured population.2, 3 Treatment is generally indicated for symptomatic UTIs. Recurrent antimicrobial usage is a recognized factor in the development of multiresistant microorganisms.4
The urinary antiseptics, Methenamine Hippurate (MH) and Cranberry preparations are in widespread use to prevent UTIs in persons with SCI.5 A review of urinary antiseptic use at a spinal injuries rehabilitation center in Sydney, Australia in 121 patients during 1996–1998 revealed that 50% of patients were using Cranberry and 23% MH. A recent Cochrane systematic review on the use of MH to prevent UTIs in susceptible populations found no reliable evidence of efficacy.5, 6 Another Cochrane review found that there may be some evidence for efficacy of Cranberry in women (without SCI), but there was insufficient information for other population groups.6 Both reviews emphasize the need for well designed trials to answer this study question.
MH (marketed as Hiprex) acts via the production from hexamine of formaldehyde, which acts as a bacteriostatic agent.7, 8 It is uncertain whether urinary acidification and the direct bacteriostatic effect of hippuric acid contribute significantly to its action.9 No convincing evidence supporting concomitant acidification was found in a recent Cochrane meta-analysis.5 It has been described in the literature that for MH, the amount of time that the active metabolite (formaldehyde) remains in the bladder in a permanently catheterized patient is probably not sufficient for it to be clinically effective.10 Despite this, MH is routinely used in permanently catheterized patients – usually without clamping regimens that have the aim of retaining urine in the bladder for sufficient time for MH to be effective.
Cranberry products reportedly act by reducing bacterial adherence to the bladder wall.11, 12, 13, 14 A possible bacteriostatic effect from acidification of the urine and the formation of hippuric acid is thought to be less significant.15
This paper reports the results of the large-scale clinical trial necessary to determine whether MH and Cranberry are effective in preventing UTI in people with SCI. The primary hypothesis investigated was that the time to occurrence of a symptomatic UTI is not increased by either MH or Cranberry tablets.
Methods
Before commencement, the study was approved by the Ethics Committees of the hospitals participating in the trial.
Eligibility criteria
The eligibility criteria for the trial were: SCI with neurogenic bladder; stable bladder management with either indwelling urethral or suprapubic catheter, intermittent catheterization, or reflex voiding with or without a condom drainage device; absence of complex urological or serious renal or hepatic pathology; not being prescribed antibiotics at the time of enrolment and absence of symptoms of a UTI at the time of enrolment. Patients had to be willing to stop any intercurrent urinary antiseptics before entering the trial. Patients were ineligible if they had a previous allergy to any of the tested interventions.
Participants
Subjects were sampled from the New South Wales (State) Spinal Cord Injuries Database16 and related database records of the two hospitals in New South Wales, Australia, which receive admissions for acute spinal services (Royal North Shore and Prince of Wales Hospitals). Between November 2000 and August 2002, 543 eligible predominantly community dwelling patients were invited to participate in the study, of whom 305 (56%) agreed.
Randomization and interventions
Patients were randomly assigned to one of four groups using a factorial design. These groups were: MH (2 g) with Cranberry (1600 mg), MH (2 g) with Cranberry placebo, Cranberry (1600 mg) with MH placebo and MH placebo with Cranberry placebo. All four regimens were indistinguishable in appearance (size and shape) and taste (iron oxide coating), and all patients received the same number of tablets, split into a twice-daily regimen. Centralized randomization was performed by telephone by a Clinical Trials Center. A unique randomization number and an allocation code were communicated to a centralized pharmacy (with no clinically involved personnel involved) for direct or courier distribution of medication to the participant. Randomization was intended to be dynamically balanced17 by patient location (inpatient or outpatient) and bladder management type. All investigators and participants were blinded to patient allocation. Clinicians and patients were blinded to allocation as well as the results of baseline bacteriological assessment results. All staff were blinded to allocation in the assessment of symptomatic and microbiological outcomes.
Outcome measures
The primary end point (Box 1) was an occurrence of a symptomatic UTI, as this is the current criterion for treating patients in the spinal injured population.18 All participants were contacted biweekly by the research team to confirm that a patient remained asymptomatic or that a symptomatic UTI had occurred. Patients had to have fulfilled the symptomatic criteria for a UTI before being categorized as such. Additionally, all patients were given a laminated business card outlining the study protocol for diagnosing a UTI for any treating doctor as well as contact numbers for the researchers. This card also included an aid for diagnosing autonomic dysreflexia (a medical emergency occurring in spinal injured patients injured at or above the T6 neurological level), which is one of the symptomatic criteria, as this is a condition not always recognized in the general medical community. The primary outcome measure was time from randomization to the first symptomatic UTI, or 6 months if no primary endpoint occurred. Secondary outcome measures were bacteriological urinary analysis at time of primary end point and adverse events. Patients were followed up via biweekly telephone calls until either the primary end point was reached or 6 months elapsed.
Sample size
A study by Waites, Canupp and Devivo1 demonstrated that the incidence of symptomatic UTI was 1.82 episodes/year in people with SCI using the bladder techniques of external urinary collection, and intermittent catheterization. Of the 64 patients studied, 60 (94%) had at least one symptomatic UTI in a 12-month follow-up period (personal communication). This is equivalent to 75% of participants having a symptomatic UTI in 6 months. Our study was designed to have 80% power and to detect a decrease from 75 to 60% at the two-sided 5% significance level. This is equivalent to a 50% increase in the median time to the first UTI, or a 10% decrease (from 94 to 84%) in patients having a UTI in 12 months. A total of 280 participants were required, or 350 participants to allow for a 20% drop out rate.19
Statistical analysis
To assess the generalizability of the results, those included in the trial were compared with those who were excluded or opted not to participate. The χ2-test was used to compare categorical variables and the two-sample t-test was used for continuous variables.
Analysis was by intention to treat. Survival analysis was used to examine the effects of MH and Cranberry on the time to the primary end point (UTI). The logrank test statistic was used to test the significance of the unadjusted effect of a variable on UTI-free survival time. The logrank test for trend was used for ordered categorical variables. Kaplan–Meier life tables were used to calculate the quartiles of survival rates across the strata of the covariates. Cox proportional hazards models were used to calculate unadjusted hazard ratios (HR) and HRs adjusted for important covariates. To determine which variables were considered a priori to be associated with UTI in the SCI population, two content expert investigators (JM, SR) were blinded from all baseline information and independently rated all covariates collected for the trial (strong, moderate or weak association). Any variable rated as weak by either content expert was not used in the multivariate analysis. Urinary bacterial count was also included to adjust for the clinically important difference between groups at study baseline.
Analysis was performed using SAS v8 and Minitab.
Results
Participants had a mean age of 43.5 years (SD 13.5, range 16–82 years) and were predominantly male (83%). Fifty-five percent of patients had tetraplegia and 49% had a complete spinal injury. The median time since SCI was 12 years (range 1 month to 61 years).
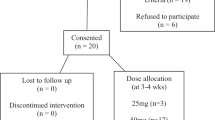
In total, 543 people were initially approached to participate. Of them, 111 did not meet the study criteria (Figure 1). Of these, 14 had a stated contraindication or allergy to MH or Cranberry, four had a current symptomatic UTI, 26 had unstable bladder management, 27 were on long-term antibiotics, 14 had significant renal or hepatic pathology, 26 were taking an intervention therapy and did not want to stop. A further 127 people refused to participate, leaving 305 participants.
The included patients had a longer mean time since SCI by 2.6 years (P=0.01) compared with those excluded. There were no other significant differences between the included and excluded groups with regard to gender, level or completeness of spinal injury (ASIA definition20) or time since spinal injury.
There was no clinically important difference between the treatment groups at baseline in any of the characteristics measured with the exception of bladder management type and urine bacterial count in the MH compared to placebo group (Table 1).
Randomization was intended to be dynamically balanced17 by patient location (inpatient or outpatient) and bladder management type. Table 1 reveals that the balancing was successful with regard to patient location, but not bladder management type for the MH versus placebo comparison. This imbalance was detected by the external randomization center too late to fully correct it. Bladder management type was therefore adjusted for in the multivariate analysis.
The Kaplan–Meier curves for MH (Figure 2) and Cranberry (Figure 3) compared to placebo are almost indistinguishable, with no evidence of a treatment effect. This indicates no UTI-free survival benefit for either intervention. The unadjusted analysis (Table 2) confirms that there is no statistically significant effect of MH tablets (HR 0.94, 95% confidence interval (CI): 0.68–1.32) or for Cranberry tablets (HR 0.93, 95% CI: 0.66–1.29).
Having no general practitioner visits for UTI in the previous 6 months was associated with a significantly longer UTI-free period. There was a significant increase in UTI-free survival at 6 months as time since injury increased (log rank trend χ2=4.37, df=1; P=0.04).
There was no statistically significant relationship between UTI-free survival and inpatient/outpatient status, gender, age or education at time of recruitment. The numbers of participants who suffered an intercurrent renal tract stone was low (fifteen), which makes interpretation of the effect of this variable problematic.
Multivariate analysis using Cox proportional hazards regression (Table 3) showed that there remained no significant effect of MH compared to placebo after adjusting for the number of general practitioner visits for UTI in the previous 6 months, duration of SCI, bladder management type, completeness of injury and baseline urinary organisms (HR 0.96, 95% CI: 0.68–1.35; P=0.75) or for Cranberry (HR 0.93, 95% CI: 0.67–1.31; P=0.70) compared to placebo. The only significant predictor of a future UTI was the number of UTIs in the preceding 6 months.
Repeating the multivariate analysis for combined treatment versus placebo subgroup using Cox proportional hazards regression (post hoc analysis) showed that there remained no significant effect of combined therapy with MH and Cranberry compared with placebo after adjusting for the same covariates (HR 0.93, 95% CI: 0.56–1.55; P=0.91).
Comparisons of the symptomatic UTI outcome measure with bacteriological and microbiological outcome measures (Table 4) revealed that a white cell count of greater than 100 was accompanied by symptoms suggestive of a UTI on 61% of the occasions. Similarly, a bacterial count of greater than 108 organisms per liter was accompanied by symptoms suggestive of a UTI only 54% of the time. 16% of participants categorized as fulfilling the symptomatic criteria for a UTI did not have microbiological or bacteriological markers suggestive of the diagnosis.
Adverse reactions experienced by trial participants were mild in nature and occurred in only 14 of the 305 participants. Diarrhea or constipation were the most commonly experienced adverse reactions (11 participants) while nausea (two) and rash (one) were less common. There was no difference in adverse event rates between the groups.
Discussion
This randomized clinical trial demonstrates that neither MH nor Cranberry tablets prevent UTI in people with SCI. It is the largest reported study to examine this important issue and provides an adequate sample size to clarify this question for this population.
Our results confirm the relationship between previous urinary infections and increased likelihood of reoccurrences. In our spinal injured population having one UTI in the preceding 6 months increases the risk of a subsequent UTI by 25% in the following 6 month period. Having two or more UTI in the same time period increases the risk by 89% (Table 3). The initial unadjusted relationship between duration of spinal injury and days free from UTI was no longer statistically significant in the multivariate model. Combining the treatment groups in a subgroup analysis (MH and cranberry versus placebo) did not lead to a statistically significant improvement in UTI prevention. Unfortunately, we are not able to comment about the effect of the presence of renal tract calculi on UTI occurrence due to low numbers of participants with this comorbidity.
The value of microbiological and bacteriological outcome measures in predicting a symptomatic UTI is poor (61 and 54% respectively; Table 4). This outlines the significant problem of relying solely on microbacteriological outcome measures to diagnose UTI in this population where many people have asymptomatic colonization of their urinary tract and asymptomatic raised urinary leukocyte counts. Our study results support the clinical practice of not routinely treating asymptomatic bacteruria in people with SCI and neurogenic bladders.
Of the approximately 3500 people with SCI living in New South Wales Australia, approximately 1 million Australian dollars per annum ($US 0.75 m) is spent on urinary antiseptics at current usage levels. Urinary antiseptics such as MH and Cranberry are widely used due to the high incidence of UTIs experienced by this population group and concerns about antibiotic resistance. Given a demonstrated lack of effectiveness of this prophylactic intervention, the current widespread use of these urinary antiseptics in the spinal injury population cannot be justified.
Side effects from either intervention were mild and infrequent but the trial exclusion criteria prevented participation by those with a history of adverse events to either of the active substances. This may have resulted in an underestimation of the true adverse event rate in this population.
Limitations of this study include the failure to balance adequately for bladder management type (with potential bias towards the null) and failure to recruit the targeted 350 participants. Neither issue is likely to alter the study findings significantly, however. Firstly the multivariate analysis revealed that there was no effect of bladder management type on UTI incidence. Likewise, the clearly null result for both MH and Cranberry compared to placebo suggests that the addition of a further 45 participants to the study would not have altered the study findings. This study remains the largest available RCT on this subject.
A further limitation of our study is that 16% of patients who indicated symptoms suggestive of a UTI did not have microbiological or bacteriological criteria supportive of this diagnosis when formal microbiological results became available. For clinical and ethical reasons, based on symptoms these participants were treated with antibiotics with a presumptive diagnosis of UTI, because it was felt that delay in allowing local practitioners to treat this (predominantly outpatient) population was an unwarranted health risk.
We do not believe that the continued use of these agents for prophylaxis is justified in people with neurogenic bladders following SCI. They do not appear to reduce the occurrence of UTI but they do impose a significant cumulative cost to the health system. In addition, it may be possible to generalize these results beyond that of neuropathic bladder secondary to SCI. The bladder problems most commonly seen in the studied population are due to supra or infrasacral pathophysiology.21 These results probably apply to people with other types of spinal pathology including multiple sclerosis. Rarely are pure suprapontine bladder types encountered in our studied population, suggesting caution in extrapolating the results to this type of bladder dysfunction, including bladder dysfunction in older people. It is still not known whether different methods of delivery such as rotating schedules of different urinary antiseptics or other combinations or compounds with presumed urinary antisepsis may lead to an effective nonantibiotic based preventive regimen for this population group. However, the results from this study should lead to questioning of these management practices.
MH may not be as effective in permanently catheterized patients.10 The purpose of this trial, however, was to test the efficacy of these urinary antiseptics as they are currently administered in the community. Including a bladder clamping regimen to address this possibility was considered impractical when designing this trial due to the risk of autonomic dysreflexia should the clamp be accidentally left in place, in patients with absent or insufficient hand function. As there was no evidence of a significant effect of intermittent versus permanent catheterization found on the adjusted analysis, the practice of clamping catheters in order to putatively increase the efficacy of MH is not supported by our data.
Conclusion
MH and Cranberry tablets are not effective in prolonging the UTI-free period in people with SCI. These results could potentially be extrapolated to other population groups with supra or infrasacral neuropathic bladder dysfunction. It is important that the lack of prophylactic effect of our current urinary antiseptic medications is brought to the attention of clinicians facilitating the care of people with SCI.
References
Waites KB, Canupp KC, Devivo MJ . Epidemiology and risk factors for urinary tract infection following spinal cord injury. Arch Phys Med Rehab 1993; 74: 691–695.
Kuhlemeier KV, Stover SL, Lloyd LK . Prophylactic antibacterial therapy for preventing urinary tract infection in spinal cord injury patients. J Urol 1985; 134: 514–517.
Cardenas DD, Hooton TM . Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehab 1995; 76: 272–279.
Anonymous. The prevention and management of urinary tract infections among people with spinal cord injuries. National institute on disability and rehabilitation research consensus statement. J Am Paraplegia Soc 1992; 15: 194–204.
Lee BB, Bhuta T, Craig J, Simpson J . Methenamine hippurate for preventing urinary tract infections. The Cochrane Database of Systematic Reviews 2002 Electronic Document.
Jepson RG, Mihaljevic L, Craig J . Cranberries for the prevention of urinary tract infections. The Cochrane Database of Systematic Reviews 2004 Electronic Document.
3M Pharmaceuticals Pty Ltd. MIMS Full prescribing information for Hiprex. Mims Online. MIMS Australia 2006.
Devenport JK, Swenson JR, Dukes Jr GE, Sonsalla PK . Formaldehyde generation from methenamine salts in spinal cord injury. Arch Phys Med Rehab 1984; 65: 257–259.
Bodel PT, Cotran R, Kass EH . Cranberry juice and the antibacterial action of hippuric acid. J Lab Clin Med 1959; 54: 881–887.
Saint S, Lipsky BA . Preventing catheter-related bacteriuria: should we? can we? how? Arch Int Med 1999; 159: 800–808.
Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N . Anti-escherichia coli adhesin activity of cranberry and blueberry juices. NEJM 1991; 324: 1599.
Sobota AE . Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol 1984; 131: 1013–1016.
Soloway MS, Smith RA . Cranberry juice as a urinary acidifier. JAMA 1988; 260: 1465.
Zafriri D, Ofek I, Adar R, Pocino M, Sharon N . Inhibitory activity of cranberry juice on adherence of type I & type P fimbriated escherichia coli to eucaryotic cells. Antimicrob Agents Chemother 1989; 33: 92–98.
Avorn J, Monane M, Gurwitz J . Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 1994; 271: 751–756.
Lee BB, Middleton JW, Rutkowski SB, Tagney B . The introduction of the NSW spinal injuries database system. Paralymp Sci Conf 2000.
Signorini DF, Leung O, Simes RJ, Beller E, Gebski VJ, Callaghan T . Dynamic balanced randomization for clinical trials. Stat Med 1993; 12: 2343–2350.
National Institute on Disability and Rehabilitation Research Statement. The prevention and management of urinary tract infections amongst people with spinal cord injuries. J Am Paraplegia Soc 1992; 15: 194–204.
Freedman LS . Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1982; 1: 121–129.
Maynard Jr FM et al. International standards for neurological and functional classification of spinal cord injury. American spinal injury association. Spinal Cord 1997; 35: 266–274.
Biering-Sorensen F, Nielans HM, Dorflinger T, Sorensen B . Urological situation five years after spinal cord injury. Scand Jf Urol Nephrol 1999; 33: 157–161.
Author information
Authors and Affiliations
Additional information
Sponsorship: (1) Motor Accidents Authority of New South Wales (2) Brucia Pharmaceuticals (non-financial)
Rights and permissions
About this article
Cite this article
Lee, B., Haran, M., Hunt, L. et al. Spinal-injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord 45, 542–550 (2007). https://doi.org/10.1038/sj.sc.3101974
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101974
Keywords
This article is cited by
-
Mapping of Dietary Interventions Beneficial in the Prevention of Secondary Health Conditions in Spinal Cord Injured Population: A Systematic Review
The Journal of nutrition, health and aging (2023)
-
Behandlung rezidivierender Harnwegsinfekte bei PatientInnen mit neurogener Blasenfunktionsstörung
Journal für Urologie und Urogynäkologie/Österreich (2022)
-
Behandlung rezidivierender Harnwegsinfekte bei PatientInnen mit neurogener Blasenfunktionsstörung
Urologie in der Praxis (2021)
-
Natural therapeutics for urinary tract infections—a review
Future Journal of Pharmaceutical Sciences (2020)
-
Cranberry Products for the Prevention of Catheter-Associated Urinary Tract Infections
Current Bladder Dysfunction Reports (2020)