Abstract
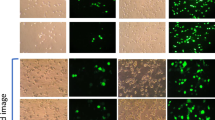
The aim of this study was to investigate the influence of cell cycle on transfection efficiency. Counterflow centrifugal elutriation was used which avoids possible side-effects from chemical treatment of cells. With this method, cell populations were fractionated by means of size and density, and fractions corresponding to discrete cell cycle phase-specific populations were transfected with various nonviral methods (Lipofectamine, TfpLys and TfPEI), adenovirus-enhanced transferrinfection (AVET system) and recombinant adenovirus. Transfection efficiency was found to be strongly dependent on the cell cycle stage at the time of transfection. Luciferase activity from cells transfected with polycation- or lipid-based transfection systems was 30- to more than 500-fold higher when transfection was performed during S or G2 phase compared with cells in G1 phase which have the lowest expression levels. In contrast, this effect was not observed with recombinant adenovirus which varied only four-fold. Our results indicate that mitotic activity enhances transfection not only by lipoplexes but also by polyplexes, but not a viral system which has an efficient nuclear entry machinery, suggesting that transfection close to M phase is facilitated perhaps by nuclear membrane breakdown. Furthermore, low transfection success into G1 cells indicates that DNA complexes deposited in G1 cells are probably not retained long enough to take advantage of mitosis effects or that passage of transfected cells through S phase is inhibitory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zabner J et al. Cellular and molecular barriers to gene transfer by a cationic lipid J Biol Chem 1995 270: 18997–19007
Mortimer I et al. Cationic lipid-mediated transfection of cells in culture requires mitotic activity Gene Therapy 1999 6: 403–411
Wilke M et al. Efficacy of a peptide-based gene delivery system depends on mitotic activity Gene Therapy 1996 3: 1133–1142
Brisson M, Huang L . Liposomes: conquering the nuclear barrier Curr Opin Mol Ther 1999 1: 140–146
Tseng WC, Haselton FR, Giorgio TD . Mitosis enhances transgene expression of plasmid delivered by cationic liposomes Biochim Biophys Acta 1999 1445: 53–64
Felgner PL et al. Nomenclature for synthetic gene delivery systems Hum Gene Ther 1997 8: 511–512
Kircheis R et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery Gene Therapy 1997 4: 409–418
Wagner E et al. Transferrin–polycation conjugates as carriers for DNA uptake into cells Proc Natl Acad Sci USA 1990 87: 3410–3414
Wagner E et al. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes Proc Natl Acad Sci USA 1992 89: 6099–6103
Shenk T . Group C adenovirus as vectors for gene therapy. In: Kaplitt MG, Loewy AD (eds) Viral Vectors Academic Press: San Diego, CA 1995 pp 43–54
Shenk T . Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM et al (eds) Virology, 3rd edn Lippincott-Raven Publishers: Philadelphia, PA 1996 pp 2111–2148
Krek W, DeCaprio JA . Cell synchronization Meth Enzymol 1995 254: 114–124
Kauffman MG, Noga SJ, Kelly TJ, Donnenberg AD . Isolation of cell cycle fractions by counterflow centrifugal elutriation Anal Biochem 1990 191: 41–46
Mikulits W et al. Dynamics of cell cycle regulators: artifact-free analysis by recultivation of cells synchronized by centrifugal elutriation DNA Cell Biol 1997 16: 849–859
Cotten M et al. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels Proc Natl Acad Sci USA 1990 87: 4033–4037
Ogris M et al. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells Gene Therapy 1998 5: 1425–1433
Plank C et al. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand Bioconjug Chem 1992 3: 533–539
Michou AI, Lehrmann H, Saltik M, Cotten M . Mutational analysis of the avian adenovirus CELO, which provides a basis for gene delivery vectors J Virol 1999 73: 1399–1410
Zauner W, Ogris M, Wagner E . Polylysine-based transfection systems utilizing receptor-mediated delivery Adv Drug Deliv Rev 1998 30: 97–113
Hagstrom JE et al. Nuclear import of DNA in digitonin-permeabilized cells J Cell Sci 1997 110: 2323–2331
Dowty ME et al. Plasmid DNA entry into postmitotic nuclei of primary rat myotubes Proc Natl Acad Sci USA 1995 92: 4572–4576
Pollard H et al. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells J Biol Chem 1998 273: 7507–7511
Zauner W et al. Differential behaviour of lipid based and polycation based gene transfer systems in transfecting primary human fibroblasts: a potential role of polylysine in nuclear transport Biochim Biophys Acta 1999 1428: 57–67
Chan CK, Jans DA . Enhancement of polylysine-mediated transferrinfection by nuclear localization sequences: polylysine does not function as a nuclear localization sequence Hum Gene Ther 1999 10: 1695–1702
Sebestyen MG et al. DNA vector chemistry: the covalent attachment of signal peptides to plasmid DNA Nat Biotechnol 1998 16: 80–85
Zanta MA, Belguise Valladier P, Behr JP . Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus Proc Natl Acad Sci USA 1999 96: 91–96
Branden LJ, Mohamed AJ, Smith CIE . A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA Nat Biotechnol 1999 17: 784–787
Langle Rouault F et al. Up to 100-fold increase of apparent gene expression in the presence of Epstein–Barr virus oriP sequences and EBNA1: implications of the nuclear import of plasmids J Virol 1998 72: 6181–6185
Dean DA . Import of plasmid DNA into the nucleus is sequence specific Exp Cell Res 1997 230: 293–302
Vacik J, Dean BS, Zimmer WE, Dean DA . Cell-specific nuclear import of plasmid DNA Gene Therapy 1999 6: 1006–1014
Raucher D, Sheetz MP . Membrane expansion increases endocytosis rate during mitosis J Cell Biol 1999 144: 497–506
Ross GF et al. Enhanced reporter gene expression in cells transfected in the presence of DMI-2, an acid nuclease inhibitor Gene Therapy 1998 5: 1244–1250
Lechardeur D et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer Gene Therapy 1999 6: 482–497
Acknowledgements
We would like to thank Josef Gotzmann and Wolfgang Mikulits (Institut für Tumorbiologie und Krebsforschung) for the stably transfected HeLa cells. We thank Ingrid Mudrak for the HeLa cells that were elutriated and Karin Paiha and Peter Steinlein for help with FACS analysis. Thanks to Bettina Grosse, Helga Vetr, Thomas Blessing and Peter Wallner for inspiring discussions and for critically reading the manuscript. This work was supported by grant S07405 from the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brunner, S., Sauer, T., Carotta, S. et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther 7, 401–407 (2000). https://doi.org/10.1038/sj.gt.3301102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301102
Keywords
This article is cited by
-
Low generational cystamine core PAMAM derivatives modified with nuclear localization signal derived from lactoferrin as a gene carrier
Korean Journal of Chemical Engineering (2023)
-
Vibropolyfection: coupling polymer-mediated gene delivery to mechanical stimulation to enhance transfection of adherent cells
Journal of Nanobiotechnology (2022)
-
Physical and mechanical cues affecting biomaterial-mediated plasmid DNA delivery: insights into non-viral delivery systems
Journal of Genetic Engineering and Biotechnology (2021)
-
Effects of the surface charge of polyamidoamine dendrimers on cellular exocytosis and the exocytosis mechanism in multidrug-resistant breast cancer cells
Journal of Nanobiotechnology (2021)
-
Targeting the Inside of Cells with Biologicals: Chemicals as a Delivery Strategy
BioDrugs (2021)