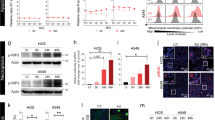
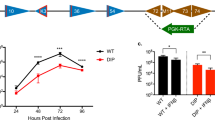
Abstract
Direct infection of tumor cells with viruses transferring protective or therapeutic genes, a frequently used procedure for production of tumor vaccines in human gene therapy, is an approach which is often limited by the number of tumor cells that can reliably be infected as well as by issues of selectivity and safety. We report an efficient, selective and safe way of infecting human tumor cells with a natural virus with interesting pleiotropic immune stimulatory properties, the avian paramyxovirus Newcastle disease virus (NDV). Two of the six viral genes (HN and F) modify the tumor cell surface by introduction of new adhesion molecules for lymphocyte interactions and other viral genes stimulate host cell genes and local production of cytokines and chemokines which can recruit a broad antitumor response in vivo. A large variety of human tumor cells is shown to be efficiently infected by NDV with viral replication being independent of tumor cell proliferation. Such properties make NDV a suitable agent for modification of noncultured freshly isolated and γ-irradiated patient-derived tumor cells. For the apathogenic non-lytic strain NDV-Ulster which is used in our clinical vaccine trials, we demonstrate selective replication in tumor cells as compared with corresponding normal cells. Furthermore, we present evidence that new virions produced by infected tumor cells are non-infectious using three different quantitative test methods. Our results demonstrate feasibility and broad applicability of this strategy of human tumor vaccine modification. Post-operative vaccination with the autologous virus-modified vaccine ATV-NDV thus provides a reasonable potential for pleiotropic modifications of the immune response of cancer patients against their own tumor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Alexander DJ, Allan HW . Newcastle disease virus pathotypes Avian Pathol 1974 3: 269–278
Cassel WA, Garret RE . Newcastle disease virus as an antineoplastic agent Cancer 1965 7: 863–868
Ito Y, Nagai Y, Maeno K . Interferon induction in mice spleen cells and mouse fibroblasts (L cells) stimulated by various strains of Newcastle disease virus J Gen Virol 1982 62: 349–352
Lorence RM, Rood PA, Kelly KW . Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-α and augmentation of its cytotoxicity J Natl Cancer Inst 1988 80: 1305–1312
Reichard KW et al. Newcastle disease virus selectively kills human cancer cells J Surg Res 1991 52: 448–453
Zorn U et al. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus Cancer Biother 1994 9: 225–234
Kenney S, Pagano JS . Editorial Viruses as oncolytic agents: a new age of ‘therapeutic’ viruses? J Natl Cancer Inst 1994 86: 1185–1186
Svet-Moldavsky GJ . Tumor-induced skin heterogenization. II. Virus causing the phenomenon J Natl Cancer Inst 1970 45: 475–484
Austin FC, Boone CW . Virus augmentation of the antigenicity of cell extracts Adv Cancer Res 1979 30: 301–345
Cassel WA, Murray DR, Phillips HS . A phase 2 study on the postsurgical management of stage 2 malignant melanoma with a Newcastle disease virus oncolysate Cancer 1983 52: 856–860
Cassel WA, Murray DR . A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate Med Oncol Tumor Pharmacother 1992 9: 169–171
Sinkovics JG, Papadopoulos NE, Plager C . Viral oncolysate for the immunotherapy of human tumors In: Yohn DS, Blakeslee JR (eds) . Advances in Comparative Leukemia Research: International Symposium for Comparative Research on Leukemia and Related Diseases, 10th edn Elsevier: North Holland, New York 1982 pp 613–615
Heicappell R et al. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells Int J Cancer 1986 37: 569–577
Schirrmacher V et al. Successful application of non-oncogenic viruses for antimetastatic cancer immunotherapy Cancer Rev 1986 5: 19–49
Liebrich W et al. In vitro and clinical characterization of a Newcastle disease virus-modified autologous tumor cell vaccine for treatment of colorectal cancer patients Eur J Cancer 1991 27: 703–710
Schirrmacher V, von Hoegen P . Importance of tumor cell membrane integrity and viability for CTL activation by cancer vaccines Vaccine Res 1993 2: 183–196
Kobayashi H . Viral xenogenization of intact tumor cells Adv Cancer Res 1997 30: 279–299
Peters LC, Hanna MG Jr . Active specific immunotherapy of established micro-metastases: effect of cryopreservation procedures on tumor cell immunogenicity in guinea pigs J Natl Cancer Inst 1980 64: 1521–1525
Schirrmacher V et al. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. IV. Antigenic differences between the parental tumor line and its metastasizing variant Int J Cancer 1979 23: 245–252
Bosslet K, Schirrmacher V, Shantz G . Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. VI. Similar specificity patterns of protective anti-tumor immunity in vivo and of cytolytic T cells in vitro Int J Cancer 1979 24: 303–313
Schirrmacher V, Schild HJ, Gückel B, von Hoegen P . Tumor specific CTL response requiring interactions of four different cell types and dual recognition of MHC class I and class II restricted tumor antigens Immunol Cell Biol 1992 71: 311–326
Schirrmacher V, Heicappell R . Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. II. Establishment of specific systemic anti-tumor immunity Clin Exp Met 1987 5: 147–156
Schirrmacher V, Haas C, Bonifer R, Ertel C . Virus potentiation of tumor vaccine T-cell stimulatory capacity requires cell surface binding but not infection Clin Cancer Res 1997 3: 1135–1148
Kingsbury DW . Paramyxoviridae and their replication In: Fields BN, Knipe DM (eds) . Virology 2nd edn. Raven Press: New York 1990 pp 945–962
Garten W et al. Mutational changes of the protease susceptibility of glycoprotein F of Newcastle disease virus: effects on pathogenicity J Gen Virol 1980 50: 135–147
Lorence RM et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy J Natl Cancer Inst 1994 86: 1228–1233
Csatary LK et al. Attenuated veterinary virus vaccine for the treatment of cancer Cancer Detect Prev 1993 17: 619–627
Von Hoegen P, Weber E, Schirrmacher V . Modification of tumor cells by a low dose of Newcastle disease virus: augmentation of the tumor-specific T cell response in the absence of an anti-viral response Eur J Immunol 1988 18: 1159–1166
Von Hoegen P, Zawatzky R, Schirrmacher V . Modification of tumor cells by a low dose of Newcastle disease virus. III. Potentiation of tumor specific cytolytic T cell activity via induction of interferon-α, β Cell Immunol 1990 126: 80–90
Ertel C et al. Viral hemagglutinin augments peptide specific cytotoxic T-cell responses Eur J Immunol 1993 23: 2592–2596
Jurianz K et al. Adhesive function of Newcastle disease virus hemagglutinin in tumor–host interaction Int J Oncol 1995 7: 539–545
Ahlert T, Schirrmacher V . Isolation of a human melanoma adapted Newcastle disease virus mutant with highly selective replication patterns Cancer Res 1990 50: 5962–5968
Schirrmacher V et al. Active specific immunotherapy with autologous tumor cell vaccines modified by Newcastle disease virus: experimental and clinical studies In: Schirrmacher V, Schwartz-Albiez R (eds) . Cancer Metastasis Springer Verlag: Berlin, Heidelberg, New York 1989 pp 157–170
Pomer S et al. Tumor response and 4 year survival data of patients with advanced renal cell carcinoma treated with autologous tumor vaccine and subcutaneous r-IL-2 and IFN-alpha2b Int J Oncol 1995 6: 947–954
Bohle W et al. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor cell vaccine: first clinical results with tumor cell vaccines modified with live but avirulent Newcastle disease virus Cancer 1990 66: 1517–1523
Lokuta MA et al. Mechanisms of murine RANTES chemokine gene induction by Newcastle disease virus J Biol Chem 1996 271: 13731–13738
Schirrmacher V, Griesbach A, Zangemeister U . γ-Irradiated viable tumor cells as whole cell vaccines can stimulate in situ syngeneic anti-tumor CTL and DTH reactivity while tumor cell lysates elicit only DTH reactivity Vaccine Res 1994 3: 31–48
Ockert D et al. Newcastle disease virus infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma Clin Cancer Res 1996 2: 21–28
Schlag P et al. Active specific immunotherapy with NDV modified autologous tumor cells following liver metastases resection in colorectal cancer: first evaluation of clinical response of a phase II trial Cancer Immunol Immunother 1992 35: 325–330
Kirchner HH, Anton P, Atzpodien J . Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines World J Urol 1995 13: 171–173
Ahlert T et al. Tumor cell number and viability as quality and efficacy parameters of autologous virus modified cancer vaccines J Clin Oncol 1997 15: 1354–1366
Hirst KG . The agglutination of rat cells by allantoic fluid of chick embryos infected with influenca virus Science 1941 94: 22–25
Long L et al. Monoclonal antibodies to hemagglutinin-neuraminidase and fusion glycoproteins of Newcastle disease virus: relationship between glycosylation and reactivity J Virol 1986 57: 1198–1202
Iorio RM, Bratt MA . Monoclonal antibodies as functional probes of the HN glycoprotein of Newcastle disease virus: antigenic separation of the hemagglutinin and neuraminidase sites J Immunol 1984 133: 2215–2219
Haas C et al. Bispecific antibodies increase T cell stimulatory capacity in vitro of human autologous virus modified tumor vaccine Clin Cancer Res 1998 4: 721–730
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schirrmacher, V., Haas, C., Bonifer, R. et al. Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Ther 6, 63–73 (1999). https://doi.org/10.1038/sj.gt.3300787
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3300787
Keywords
This article is cited by
-
Combined therapy of oncolytic Newcastle disease virus and rhizomes extract of Rheum ribes enhances cancer virotherapy in vitro and in vivo
Molecular Biology Reports (2020)
-
Newcastle disease virus mediated apoptosis and migration inhibition of human oral cancer cells: A probable role of β-catenin and matrix metalloproteinase-7
Scientific Reports (2019)
-
Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity
Nature Communications (2017)
-
Recombinant Newcastle disease virus expressing P53 demonstrates promising antitumor efficiency in hepatoma model
Journal of Biomedical Science (2016)
-
Oncolysis by paramyxoviruses: preclinical and clinical studies
Molecular Therapy - Oncolytics (2015)