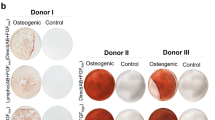
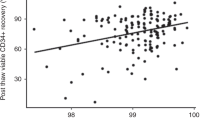
Abstract
We compared two protocols for the expansion of human mesenchymal stromal cells (hMSCs) starting from diagnostic samples of BM aspirates (2–5 ml) or using the remnants in the bag and filter at the end of the BM infusions. The protocols differed in the presence of either 10% fetal bovine serum (FBS) or 5% platelet lysate (PL). We obtained a significantly (P=0.02) better expansion with PL, obtaining a median 1010-fold compared to 198-fold with a selected batch of FBS and in fewer days (29.8 in PL versus 41.4 in FBS). Overall, we recovered a variable number from 54.8 × 106 to 365 × 106 hMSCs in PL versus a variable number from 2.7 × 106 to 31 × 106 in FBS. No difference could be found in terms of gross morphology, differentiation potential, surface markers and immunological properties (inhibition of allogeneic PHA response and mixed lymphocyte reaction) of cells expanded with PL or FBS. The preparations were found within the range of acceptability for all the quality control criteria. Due to the clinical grade nature of the PL and the reproducibility of separate preparations, we propose this method to obtain hMSCs even from minute amounts of BM cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Lurià EA et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974; 2: 83–92.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Le Blanc K, Pittenger M . Mesenchymal stem cells: progress toward promise. Cytotherapy 2005; 7: 36–45.
Korbling M, Estrov Z . Adult stem cells for tissue repair—a new therapeutic concept? N Engl J Med 2003; 349: 570–582.
Chen SL, Fang WW, Ye F, Liu YH, Quian J, Shan SJ et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004; 94: 92–95.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Lee ST, Jang JH, Cheong JW, Kim JS, Meagm HY, Hahn JS et al. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol 2002; 118: 1128–1131.
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006; 81: 1390–1397.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317.
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–395.
Introna M, Barbui AM, Golay J, Rambaldi A . Innovative cell-based therapies in onco-hematology: what are the clinical facts? Haematologica 2004; 89: 1253–1260.
Meuleman N, Tondreau T, Delforge A, Dejeneffe M, Massy M, Libertalis M et al. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol 2006; 76: 309–316.
Koller MR, Maher RJ, Manchel I, Oxender M, Smith H . Alternatives to animal sera for human bone marrow cell expansion: human serum and serum-free media. J Hematother 1998; 7: 413–423.
Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR . Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 2004; 32: 1212–1225.
Muller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 2006; 8: 437–444.
Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 2005; 205: 228–236.
Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 2007; 211: 121–130.
Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy 2005; 7: 509–519.
Acknowledgements
The work was partially supported by the Italian Association for Cancer Research (AIRC), the ‘Associazione Italiana contro le Leucemie, linfomi e mieloma (AIL) Bergamo-Sezione Paolo Belli’ and by a generous personal donation from Dr EC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
Supplementary information
Rights and permissions
About this article
Cite this article
Capelli, C., Domenghini, M., Borleri, G. et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant 40, 785–791 (2007). https://doi.org/10.1038/sj.bmt.1705798
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705798
Keywords
This article is cited by
-
Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol
Stem Cell Research & Therapy (2022)
-
Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: a systematic review
Journal of Translational Medicine (2020)
-
Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia
Biology of Sex Differences (2019)
-
Therapeutic potential of stromal cells of non-renal or renal origin in experimental chronic kidney disease
Stem Cell Research & Therapy (2018)
-
Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells
Stem Cell Research & Therapy (2018)