Abstract
The quality of mesorectal resection is crucial for resection in rectal cancer, which should be performed by laparoscopy for better outcome. The use of indocyanine green (ICG) fluorescence is now routinely used in some centers to evaluate bowel perfusion. Previous studies have demonstrated in animal models that selective intra-arterial ICG staining can be used to define and visualize resection margins in rectal cancer. In this animal study, we investigate if laparoscopic intra-arterial catheterization is feasible and the staining of resection margins when performing total mesorectal excision with a laparoscopic medial to lateral approach is possible. In 4 pigs, laparoscopic catheterization of the inferior mesenteric artery (IMA) is performed using a seldinger technique. After a bolus injection of 10 ml ICG with a concentration of 0.25 mg/ml, a continuous intra-arterial perfusion was established at a rate of 2 ml/min. The quality of the staining was evaluated qualitatively. Laparoscopic catheterization was possible in all cases, and the average time for this was 30.25 ± 3.54 min. We observed a significant fluorescent signal in all areas of the IMA supplied, but not in other parts of the abdominal cavity or organs. In addition, the mesorectum showed a sharp border between stained and unstained tissue. Intraoperative isolated fluorescence augmentation of the rectum, including the mesorectum by laparoscopic catheterization, is feasible. Inferior mesenteric artery catheterization and ICG perfusion can provide a fluorescence-guided roadmap to identify the correct plane in total mesorectal excision, which should be investigated in further studies.
Similar content being viewed by others
Introduction
Intraarterial Indocyanine Green (ICG) perfusion into the main tumor supplying artery may help to identify the correct resection borders in oncological surgery. As described before, a continuous ICG-perfusion via IMA (inferior mesenteric artery) may visualize the correct plane for total mesorectal excision (TME) in the case of rectal cancer1,2,3. Forgione et al. were able to confirm our concept3. This technique may improve the quality and safety of mesorectal resection, a faster learning curve and improve overall neurological outcome.
It is known that the quality of mesorectal resection is crucial for predicting the local recurrence risk4. Patients benefit from minimal-invasive rectal excision due to short-term recovery, fewer surgical site infections, and less overall blood loss5 while the longterm oncological outcome seems to be comparable in the open versus laparoscopic approach4,6. This suggests that the laparoscopic approach for lower rectal excision and TME might be the gold standard for surgery of rectal cancer. However, Creavin et al. showed that laparoscopic resections were associated with a higher rate of incomplete mesorectal excisions4. As mentioned before, this implicates a higher risk for local recurrence. The reason might belong learning curves for the technique involved, depending on the individual skill of the surgeon and also higher conversion rates in less trained surgeons7,8.
In the last few years, fluorescence-guided surgery (FGS) with ICG has become widespread in several medical and surgical specialties9. There are also few studies regarding laparoscopic liver surgery, examining if intraarterial ICG-injection can help identify resection borders. In these studies, a hybrid operative suite with a transfemoral approach is used to catheterize the arteries10,11. Forgione et al. were able to show that staining of the mesocolon via a transfemoral approach is also possible3.
One limitation occurring in our previous study was the need for laparotomy for puncturing the IMA1. As described above, interventional catheterization would be an option, but just a few hospitals have access to a hybrid operative suite and the risk of complications is described between 0.05 and 2%12,13.
Another option would be laparoscopic artery catheterization. This is described in the literature, mainly for hepatic artery catheterization for regional chemotherapy14,15,16.
In this animal study, we want to examine if a laparoscopic approach with laparoscopic catheterization and ICG-perfusion of the IMA and image-guided identification of the resection areas of the mesorectum with near-infrared angiographic enhancement is possible and feasible.
Material and methods
Animals
A total of two swine (weight: 40–45 kg, age: 3–4 months old) were used in this non-survival study. All procedures were carried out in strict accordance with recommendations and guidance for the care and use of laboratory animals of the National Institutes of Health, which received full approval by the local Ethical Committee on Animal Experimentation (FIM-18105) by Fördergemeinschaft für Innovative Medizin in Beichlingen, Germany and Ludwig-Maximilians-University (LMU). The study was carried out in compliance with the ARRIVE guideline. Induction was achieved using Azaperon (Stresnil), i.m. (2 mg/kg) combined with Atropine s.c. (0.02–0.1 mg/kg) and Ketamin 10% i.m. (20 mg/kg). Anesthesia was maintained with 2% Isoflurane, Pancuronium, Meloxicam, Metamizole, Ketamine, and, if necessary, Fentanyl. At the end of the procedure, animals were humanely killed with an intravenous injection of a lethal dose of T61.
Surgical procedure and laparoscopic ICG video angiography
The surgical approach was achieved in the following fashion: The IMA was prepared with a medial-to-lateral approach after standard laparoscopy. For temporary closure, we used a small bulldog clamp, which was put into the abdomen through a 12 mm trocar, and applied it to the distal IMA. The first step during the medial approach for rectum resection is ligating the vessel of the inferior mesenteric artery. We did this by a proximal clipping technique followed by the final cutting of the IMA (see Supplementary Video17 [00:06–01:12]).
Subsequently, we inserted an introducer system (Arrow, Two-Lumen Central Venous Catheterization Set, Teleflex Medical GmbH Willy-Rüsch-Str. 4–10 D-71394 Kernen) in Seldinger technique and applied the catheter (Pajunk, SonoLong Echo NanoLine, Pajunk GmbH Medizintechnologie Karl-Hall-Strasse 1 78187 Geisingen Germany). After the arteriae section, we placed the catheter into the IMA and fixed it with two ligatures preventing retrograde flow. After the bulldog clamp was removed, we checked for bleeding (see Supplementary Video17 [01:15–02:01]).
As soon as locating the rectum and mesorectum was achieved, ICG was applied to the IMA. Therefore, we established continuous intra-arterial perfusion via a syringe pump of ICG. ICG (Pulsion Medical Systems SE, Hans-Riedl-Str. 21, 85622 Feldkirchen, Germany) concentration was 0.25 mg/ml mixed with aqua ad iniectabilia with a perfusion rate of 2 ml/min. We started with a bolus of 10 ml. Fluorescence-imaging was observed with a NIR camera by real-time overlapping to the real camera image (Maxer Viron X, Maxer Medizintechnik GmbH, 78549 Spaichingen, Germany). Finally, we started mesorectal excision in standard surgical fashion (see Supplementary Video17 [02:08–05:20]).
The time for laproscopic catheterization was defined from identification of the IMA to completion of the last ligation. All data are presented as mean ± standard deviation (SD).
Results
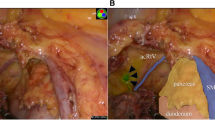
After an initial bolus of 10 ml of ICG (this is a total of 2.5 mg ICG), we directly observed an infrared signal. The overlay video-image showed a promising ICG-perfusion of the rectum and mesorectum without an infrared signal of other parts of the situs. We observed a sharp border between colored and non-colored tissue, and this border seemed to match the visceral fasciae (Figs. 1, 2, 3). We stopped continuous perfusion after 8 min (total of 6.5 mg ICG in 26 ml) because of an optically sufficient infrared signal of the rectum and mesorectum. Furthermore, we wanted to prevent further systemic ICG application as ICG accumulation in the liver was already noticeable. The time for laparoscopic catheterization was 30.25 ± 3.54 min.
During mesorectal resection, which we mainly performed with a Ligasure Device (Ligasure, Medtronic GmbH, Earl-Bakken-Platz 1, 40670 Meerbusch), you can see how visceral and parietal fasciae even under blunt dissection separate and how infrared signals match (Fig. 2). While sealing and cutting adhesions some ICG remains on the instrument, so we cleaned the instrument a few times during surgery to prevent ICG contamination of other parts of the situs. The ICG signal was observed until the very end of the rectum at the bottom of the wall of the pelvis (Fig. 3, see Supplementary Video17 [02:08–05:20]). After 40 min without further ICGperfusion, the ICG signal did not change subjectively.
Discussion
The quality of mesorectal excision is mostly related to complete resection of the fascial envelope, and as mentioned before, the quality of mesorectal resection is crucial for predicting local recurrence risk4. This image-guided approach may help the surgeon identify the correct plane faster and safer. In our experience, most surgeons favor a laparoscopic approach for rectal resection, if possible. For a medial-to-lateral approach, laparoscopic intraarterial catheterization would not even change operative workflow.
Minimally invasive catheter placement for regional chemotherapy into the hepatic artery using conventional laparoscopic techniques has been described in literature15,16,18. Franklin et al. reported a series of 20 catheterizations without intraoperative complications16. Van Nieuwenhove et al. reported a series of 29 catheterizations without major perioperative complications15. Cheng et al. reported a case series of 38 patients with laparoscopic hepatic artery infusion pump (LHAIP) implantations without major intraoperative complications18. All authors describe laparoscopic catheterization as a challenging but safe and feasible procedure15,16,18, which accords with our experience. In our approach the catheterized vessel.
(IMA) will be resected. Postoperative complications should not occur relating to catheterization. Furthermore, the dissection of the IMA is way more straightforward than the dissection of hepatic vessels. There are also case reports in literature regarding catheterization of arteries with robotic systems such as the DaVinci, which may decrease the time for catheterization and improve safeness19.
With a mean time of 30.25 ± 3.54 min for catheterization, our approach is comparable to interventional catheterization described by Forgione et al. but without the risks of additional intervention3.
Nevertheless, even though dissecting the artery and the catheterization technique might be timeconsuming in the beginning, the new technique holds great potential to be time-saving procedure-time during mesorectal excision because of better and faster identification of the correct resection plane.
In previous experiments1,2, a rapid ICG washout was observed with intra-arterial administration, so the experiments were performed with continuous administration. Forgione et al. have investigated this both quantitatively and qualitatively and have come to the results here of an optimal infusion rate of 20 ml/h with a dosage of 0.01 mg/kg3. This is roughly in line with our dosage of 6.5 mg in total. Continuous perfusion appears to be an important factor for adequate visualization of the mesorectum, presumably this will also be a decisive factor in humans, although the exact length of the catheter and appropriate catheter openings may also play a major role here.
Another possibility that is created by arterial catheterization is regional chemotherapy. Even if controversial, some studies suggest that regional chemotherapy may improve outcomes in some patient groups20. Also, new surgical methods like TaTME could improve from this technique. Identification of the embryonal planes during transanal preparation maybe improve correct dissection along embryonal planes.
In our opinion, this technique may offer a new approach in oncological surgery and may, especially in rectal surgery, improve the outcome. At the end: further studies must deal with some crucial questions:
Which application method works best and offers the best integration into the standard operating procedures: Laparoscopic or robotic catheterization in comparison to interventional catheterization with a hybrid operating room or preoperative catheterization by interventional radiologists.
Which Dose, Volume, and perfusion-rate of ICG offer the best results?
Does the ICG-perfused area correspond to the real fascia conditions in humans?
Is the additional effort worth it? Does this new approach improve circumferential resection margin, locoregional recurrence-free survival, urogenital dysfunction, the learning curve, and duration of the surgery?
Conclusion
Intraoperative isolated fluorescence augmentation of the rectum, including the mesorectum by laparoscopic catheterization, is feasible. By catheterization of the inferior mesenteric artery and ICG-perfusion, you may achieve a fluorescence-guided roadmap to identify the right plane during total mesorectal excision, which should be examined in further studies.
References
Heiliger, C. et al. Intraarterial indocyanine green (ICG) fluorescence augmentation by marking embryonal resection areas in colorectal surgery: A feasibility study in a porcine model. Minim. Invasive Ther. Allied Technol. https://doi.org/10.1080/13645706.2018.1544568 (2018).
Frank, A. H. R., Heiliger, C., Andrade, D., Werner, J. & Karcz, K. Intra-arterial versus negative-staining of embryonal resection borders with indocyanine green (ICG) fluorescence for total mesorectal excision in colorectal cancer: An experimental feasibility study in a porcine model. Minim. Invasive Ther. Allied Technol. https://doi.org/10.1080/13645706.2020.1762655 (2020).
Forgione, A. et al. Precision image-guided colonic surgery: Proof of concept for enhanced preoperative and intraoperative vascular imaging. Surg. Endosc. https://doi.org/10.1007/s00464-020-08000-w (2020).
Creavin, B., Kelly, M. E., Ryan, E. & Winter, D. C. Meta-analysis of the impact of surgical approach on the grade of mesorectal excision in rectal cancer. Br. J. Surg. 104, 1609–1619 (2017).
Małczak, P. et al. Is the laparoscopic approach for rectal cancer superior to open surgery? A systematic review and meta-analysis on short-term surgical outcomes. Wideochirurgia i inne Tech. maloinwazyjne = Videosurgery other miniinvasive Tech. 13, 129–140 (2018).
Pędziwiatr, M. et al. There is no difference in outcome between laparoscopic and open surgery for rectal cancer: A systematic review and meta-analysis on short- and long-term oncologic outcomes. Tech. Coloproctol. 21, 595–604 (2017).
Małczak, P. et al. Is the laparoscopic approach for rectal cancer superior to open surgery? A systematic review and meta-analysis on short-term surgical outcomes. Videosurg. Other Miniinvas. Tech. 13, 129–140 (2018).
Francis, N. K. et al. Does the number of operating specialists influence the conversion rate and outcomes after laparoscopic colorectal cancer surgery?. Surg. Endosc. 32, 3652–3658 (2018).
Baiocchi, G. L., Diana, M. & Boni, L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J. Gastroenterol. 24, 2921–2930 (2018).
Diana, M. et al. Superselective intra-arterial hepatic injection of indocyanine green (ICG) for fluorescence image-guided segmental positive staining: Experimental proof of the concept. Surg. Endosc. 31, 1451–1460 (2017).
Ueno, M. et al. Indocyanine green fluorescence imaging techniques and interventional radiology during laparoscopic anatomical liver resection (with video). Surg. Endosc. 32, 1051–1055 (2018).
Lee, M. S. et al. Minimizing femoral artery access complications during percutaneous coronary intervention: A comprehensive review. Catheter. Cardiovasc. Interv. 84, 62–69 (2014).
Fruhwirth, J., Pascher, O., Hauser, H. & Amann, W. Local vascular complications after iatrogenic femoral artery puncture. Wien. Klin. Wochenschr. 108, 196–200 (1996).
Franklin, M., Trevino, J., Hernandez-Oaknin, H., Fisher, T. & Berghoff, K. Laparoscopic hepatic artery catheterization for regional chemotherapy: Is this the best current option for liver metastatic disease?. Surg. Endosc. 20, 554–558 (2006).
Van Nieuwenhove, Y., Aerts, M., Neyns, B. & Delvaux, G. Techniques for the placement of hepatic artery catheters for regional chemotherapy in unresectable liver metastases. Eur. J. Surg. Oncol. 33, 336–340 (2007).
Franklin, M. E. & Gonzalez, J. J. Laparoscopic placement of hepatic artery catheter for regional chemotherapy infusion: Technique, benefits, and complications. Surg. Laparosc. Endosc. Percutan. Tech. 12, 398–407 (2002).
Heiliger, C., Piecuch, J., Frank, A., Andrade, D., Ehrlich-Treuenstätt, V., Evtimova, D., Kühn, F., Werner, J., Karcz, K. Laparoscopic intraarterial catheterization with selective ICG fluorescence imaging in colorectal surgery. https://doi.org/10.6084/m9.figshare.11955165.v1 (2021).
Cheng, J., Hong, D., Zhu, G., Swanstrom, L. L. & Hansen, P. D. Laparoscopic placement of hepatic artery infusion pumps: Technical considerations and early results. Ann. Surg. Oncol. 11, 589–597 (2004).
Hellan, M. & Pigazzi, A. Robotic-assisted placement of a hepatic artery infusion catheter for regional chemotherapy. Surg. Endosc. 22, 548–551 (2008).
Xu, J. et al. Preoperative hepatic and regional arterial chemotherapy in the prevention of liver metastasis after colorectal cancer surgery. Ann. Surg. 245, 583–590 (2007).
Acknowledgements
Many thanks to Dr. Frank Pölzing and his team from Fördergemeinschaft für Innovative Medizin in Beichlingen, Germany, for the laboratory, competent help, and their hospitality. Also, many thanks to Maxer Endoscopy GmbH, Wurmlingen, Germany for technical supply and resourcing of the ICG-endoscope.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.H., A.F., D.A., D.E., K.K have carried out the animal experiments. J.P., F.K., J.W. have accompanied the design of the experiments. V.vE-T. helped with the translation and coorection of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heiliger, C., Piecuch, J., Frank, A. et al. Laparoscopic intraarterial catheterization with selective ICG fluorescence imaging in colorectal surgery. Sci Rep 11, 14753 (2021). https://doi.org/10.1038/s41598-021-94244-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94244-y
This article is cited by
-
„Cognition-Guided Surgery“ – computergestützte intelligente Assistenzsysteme für die onkologische Chirurgie
Wiener klinisches Magazin (2022)
-
„Cognition-Guided Surgery“ – computergestützte intelligente Assistenzsysteme für die onkologische Chirurgie
Forum (2022)
-
Technische Innovationen und Blick in die Zukunft
Wiener klinisches Magazin (2022)
-
Technische Innovationen und Blick in die Zukunft
Der Chirurg (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.