Abstract
The pathogenesis and complications of type 2 diabetes (T2DM) are closely linked with defective glucose metabolism, obesity, cardiovascular disease and an inability to mount an effective immune response to certain pathogenic organisms. Perturbations in key innate immune receptors known as Toll-like receptors (TLRs) and inflammatory mediators such as IL-6, TNFα and IL-1β have been linked with T2DM. Herein, we sought to establish whether patients with T2DM and underlying complications exhibit perturbations in cytokine and TLR expression. Serum cytokine and mRNA levels of cytokines/TLRs in monocytes (M) and neutrophils (N) were measured in a cohort of 112 diabetic patients: good glycaemic control without complications (GC), good glycaemic control with complications (GCC), poor glycaemic control without complications (PC) and poor glycaemic control with complications (PCC) and compared them with 34 non-diabetic volunteers (NGT). Serum cytokine levels were normal in all study participants. In the GC group, cytokine and TLR gene expression were enhanced compared to NGT. In contrast, suppressed cytokine and TLR gene expression were evident in PC, GCC & PCC groups when compared to the GC. In conclusion, whereas serum pro-inflammatory cytokine levels are unaltered in T2DM patients, differences in inflammatory gene profiles exist among the T2DM patient groups.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2DM) is the most prevalent human metabolic disease and its effects are now the leading cause of human morbidity and mortality1. T2DM, characterized by hyperglycaemia, is strongly associated with both macrovascular (such as coronary artery disease, peripheral arterial disease, and stroke) and microvascular complications (such as diabetic retinopathy, nephropathy and neuropathy)2. Studies have suggested that low-grade inflammation, characterized by pro-inflammatory cytokine production, is involved in the pathogenic processes causing T2DM3,4. Further, excessive production of these cytokines in T2DM has been associated with the development of microvascular and macrovascular complications and may predict the development of diabetes3. It has been shown that inflammatory processes in the pancreatic islets, adipose tissue (AT), liver and muscle provoke insulin resistance (IR) and β-cell dysfunction and may therefore antedate the diagnosis of diabetes5. The AT is a major source of cytokines such as TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1)6,7,8. Further, blood monocytes and neutrophils are sentinel cells that drive inflammation and concomitant pro-inflammatory cytokine production in vivo3,9. Many studies have also shown that the gene expression and phenotypic profiles of monocytes and neutrophils are altered in response to glucose and in a T2DM milieu9,10,11,12,13. Given this, we opted to study whether alterations in inflammatory gene expression occurred in both monocytes and neutrophils in the context of T2DM.
Toll-like receptors (TLRs) are a variety of pathogen pattern recognition receptors that serve to detect pathogen associated molecular patterns (PAMPs) present on pathogens including bacteria and viruses and to detect danger-associated molecular patterns (DAMPs)14,15. Studies have also shown that TLR2 and TLR4 expression levels are elevated in patients with T2DM16,17. In fact, TLR4 has been shown to play an important role in the pathogenesis of atherosclerosis, diet-induced obesity, and the related insulin resistance18. In the present study, we opted to explore the expression levels of key cytokines, chemokines and all TLRs in T2DM subjects and to explore whether alterations are linked with micro- and macrovascular complications, and duration of diabetes.
Results
Participant characteristics
The role of various risk factors for the development and progression of diabetes have been demonstrated by several epidemiologic studies19,20. These factors include type and duration of diabetes, age, gender, glycaemic control, hypertension, body mass index, smoking, serum lipids and presence of microalbuminuria; all these risk factors were examined in the current study (Table 1). Females were 67.6% in NGT and 42.9% in T2DM group. The median age was higher in T2DM subjects compared to NGT controls (p < 0.001) (Table 1). The duration of diabetes was calculated for all groups and as it reflects the course of disease, the groups with complications and poor glycaemic control group had longer duration of diabetes compared to good glycaemic control group (p < 0.001). The profile for smoking was similar in both NGT and T2DM groups: 20.5% were smokers in NGT compared to 20.5% in T2DM groups, and 30.7% were ex-smoker in NGT as compared to 43.5% in T2DM. There were significant differences in the BMI and WHR among the groups (p < 0.01) wherein the T2DM subjects had higher BMI and WHR compared to NGT controls. The median systolic BP (but not diastolic) was significantly raised in GC, GCC and PCC (p < 0.05). The fasting glucose was higher in all T2DM subjects compared to NGT controls (p < 0.05). All T2DM subjects had raised total cholesterol and triglycerides, and low HDL compared to NGT controls (p < 0.05). LDL levels were lower in T2DM subjects compared to NGT; this may be attributed to lipid lowering agents.
Previous epidemiological studies have demonstrated increased concentrations of CRP in patients with T2DM21. In the present study, we aimed to evaluate patients with ‘steady state’ T2DM. Therefore, individuals with acute and chronic illness and a concomitant significant elevation in CRP levels (raised CRP levels multiples higher than baseline associated with conditions such as common cold, skin infections, urinary tract infections, joint inflammation, active autoimmune conditions such as Crohn’s disease, conditions with arterial inflammation such as polymyalgia rheumatism and malignancy) were excluded from the study. The aforementioned exclusion criteria was adopted since very high CRP (indicative of illness in both controls and diabetic patients) may act as a confounder in evaluating the baseline ‘steady state’ of pro- and anti- inflammatory forces. Notably, the inclusion and exclusion criteria that were adopted in the study were thorough and strictly adhered to; patients that met with our inclusion and exclusion criteria with mildly raised CRP were not excluded in this study. In the present study, a significant difference in CRP levels was not observed between different groups (Table 1).
A detailed drug history was obtained for all patients and it was observed that a higher percentage of patients in PC (52.4%) and PCC (71.9%) were administered insulin compared to GC (7.4%) and GCC (28.1%) groups (Table 2). In all groups, most T2DM subjects were administered lipid lowering agents: GC (85.2%), GCC (93.8%), PC (61.9%) and PCC (87.5%) and also administered angiotensin converting enzyme (ACE) inhibitor and angiotensin receptor blocker (ARB) in view of HTN or micro/macro albuminuria: GC (74.1%), GCC (87.5%), PC (76.2%), and PCC (81.3%) (Table 2).
All PCC patients had one or more microvascular complications (retinopathy, neuropathy and nephropathy). Additionally, 53.1% (17 patients) of these had one or more macrovascular complications. In GCC group, 56.3% (18 patients) had one or more microvascular complications, 34.4% (11 patients) had both macro & microvascular complications and 9.4% (3 patients) had only macrovascular complications. Among microvascular complications, retinopathy was higher in PCC patients (40.6%) compared to GCC patients (18.6%). No significant difference was observed in neuropathy in between two subgroups. 15.6% of PCC patients had diabetic foot disease compared to 6.3% GCC patients. Of the PCC patients, 68.8% had nephropathy compared to 56.3% in GCC subgroup. The proportion of patients having CKD was higher in PCC subgroup than GCC subgroup (CKD stage 3; 45.5% vs. 27.7%, CKD stage 4; 9% vs. 0%) (Table 3).
Levels of cytokines in sera from NGT and T2DM patients
Following the analysis of cytokines/chemokine levels in serum, there was no statistical significant difference in serum TNFα, IL-6, and IL-1β levels among the different groups (Fig. 1A–C). The median values of serum TNFα and IL-6 were comparable in GC, GCC and PCC patients compared to NGT. While comparable levels of serum IL-1β were observed in GC and GCC, levels were decreased in PC and PCC, when compared with NGT. Serum IFN-β was significantly increased in PC and comparable in GC group, when compared to NGT control (Fig. 1D) and decreased in the GCC and PCC group (p < 0.05). However, following adjustment for covariates, serum IFN-β levels became statistically insignificant. Following the analysis of serum IL-10, RANTES, IL-8, IL-12 p70 and IFNγ, there was no statistical significant difference in serum levels among the different groups (Fig. 1E–I). Whereas a trend towards increased serum IL-10 was observed in GC and PC patients, a marginal trend towards suppressed IL-10 was observed in GCC and PCC patients, compared with NGT (Fig. 1E). Following assessment of RANTES, whereas a trend towards increased levels in PC and PCC patients was observed when compared with NGT control group, levels were comparable in GC, GCC and NGT (Fig. 1F). Whereas a trend towards decreased IL-8 was observed in GCC, PC and PCC, when compared with NGT, levels were comparable in GC and NGT (Fig. 1G). A trend towards decreased levels of both IL-12 and IFNγ was detected in the serum taken from T2DM subjects when compared to the NGT control group.
Next, multifactorial ANOVA analysis of the serum cytokines measured in the present study was undertaken after adjustment for age, sex, WHR, BMI, duration of diabetes, creatinine and medications (insulin, sulfonylurea, metformin, GLP-1 analogues, DPP IV inhibitors, aspirin and statins). No significant difference in cytokine levels among the study groups was shown and there was no correlation between serum cytokine levels and BMI in T2DM subjects.
Gene expression of TNFα, IL-6, IL-1β, IFN-β and Rantes in monocytes and neutrophils from NGT and T2DM patients
The transcriptional regulation of cytokines involved in regulating the inflammatory response were analysed using a quantitative PCR approach wherein the 2−(ΔΔCt) method was used to analyse the relative changes in gene expression22. As expected, monocytes expressed mRNA encoding TNFα, IL-6, IL-1β, IFN-β and RANTES. A significant increase in TNFα mRNA expression was observed in the monocytes obtained from PC and PCC subjects when compared to NGT (Fig. 2A). A significant increase in IL-6 mRNA expression was observed in the monocytes obtained from GC and PC subjects when compared to NGT (Fig. 2B). A significant decrease in IL-1β mRNA expression was observed in the monocytes obtained from all T2D subjects when compared to NGT (Fig. 2C). Interestingly, IFN-β mRNA expression was increased in monocytes from GC, PC and PCC subjects when compared to NGT (Fig. 2D). Similarly, the highest levels of RANTES mRNA was observed in monocytes obtained from PC and PCC subjects (Fig. 2E). Levels of IL-6 (p < 0.05), IL-1β (p < 0.05), IFN-β (p < 0.001) and RANTES (p < 0.001) mRNA expression in monocytes remained significant among different groups following multifactorial ANOVA after adjustment for age, sex, WHR, BMI, duration of diabetes, creatinine and medications (insulin, sulfonylurea, metformin, GLP-1 analogues, DPP IV inhibitors, aspirin and statins). Additionally, a statistically significant correlation was found between IL-1β mRNA in monocytes and BMI in T2DM subjects (Spearman’s r = 0.41, p < 0.05). In summary, the highest level of IL-6 mRNA was observed in GC group, whereas highest level of IFNβ mRNA and Rantes mRNA were observed in the PC group, when compared to NGT. In contrast, IL-1β mRNA remained suppressed in the T2DM groups, compared to NGT.
Gene expression of TNFα, IL-6, IL-1β, IFN-β and Rantes in monocytes and neutrophils from NGT and T2DM patients. Analysis of TNFα (A,F), IL-6 (B,G), IL-1β (C,H), IFN-β (D,I) and RANTES (E,J) gene expression in monocytes (A–E) and neutrophils (F–J) from NGT and T2DM patients (n = 8 per group). Top and bottom horizontal lines of the boxplots indicate 25th and 75th percentiles respectively; lines within the box indicate median values. *p < 0.05, **p < 0.01, ***p < 0.001 vs NGT.
In neutrophils, a significant increase in TNFα mRNA expression was observed in GC subjects when compared to NGT (Fig. 2F). A significant increase in IL-6 mRNA expression was observed in neutrophils obtained from GC and PC subjects when compared to NGT (Fig. 2G). A significant decrease in IL-1β mRNA expression was observed in the neutrophils obtained from PC subjects when compared to NGT (Fig. 2H). IFN-β and RANTES mRNA was increased in neutrophils from GC and PC subjects when compared to NGT (Fig. 2I,J). TNFα (p < 0.001), IL-6 (p < 0.001), IFN-β (p < 0.001) and RANTES (p < 0.001) mRNA expression levels remained significant among different groups following multifactorial ANOVA after adjustment for age, sex, WHR, BMI, duration of diabetes, creatinine and medications (insulin, sulfonylurea, metformin, GLP-1 analogues, DPP IV inhibitors, aspirin and statins). A correlation between cytokine mRNA levels and BMI was not evident in neutrophils. Collectively, our data suggests that whereas comparable TNFα, IL-6, IFN-β and RANTES mRNA expression levels were evident among the GCC, PCC and NGT groups, TNFα, IL-6, IFN-β and RANTES mRNA expression levels were highest in neutrophils obtained from GC subjects, when compared to NGT controls. In contrast, IL-1β mRNA expression levels were not elevated among the T2DM subject groups.
Gene expression of TLR1-10 in monocytes and neutrophils from NGT and T2DM patients
In monocytes, TLR1, 3, 5 and 7–10 mRNA levels were elevated in GC subjects when compared to NGT; levels of TLR1, 3, 5, 7, 9, and 10 remained statistically significant following adjustment for age, sex, WHR, BMI, duration of diabetes, creatinine and medications (insulin, sulfonylurea, metformin, GLP-1 analogues, DPP IV inhibitors, aspirin and statins) using multifactorial ANOVA (Fig. 3A–J). In contrast, TLR1, 5, 7 & 10 levels were elevated in monocytes obtained from GCC subjects compared to NGT (p < 0.05) (Fig. 3A–J). In monocytes obtained from PC subjects, whereas TLR7 expression was enhanced, expression of all other TLRs was normal when compared to NGT. Interestingly, in PCC monocytes, expression of all TLRs examined was comparable to NGT.
Gene expression of TLR1-10 in monocytes and neutrophils from NGT and T2DM patients. Analysis of TLR1 (A,K), TLR2 (B,L), TLR3 (C,M), TLR4 (D,N), TLR5 (E,O), TLR6 (F,P), TLR7 (G,Q), TLR8 (H,R), TLR9 (I,S), TLR10 (J,T) gene expression in monocytes (A–J) and neutrophils (K–T) from NGT and T2DM patients (n = 8 per group). Top and bottom horizontal lines of the boxplots indicate 25th and 75th percentiles respectively; lines within the box indicate median values. *p < 0.05, **p < 0.01, ***p < 0.001 vs NGT.
In neutrophils, TLRs1, 3–10 mRNA levels were elevated in GC subjects when compared to NGT; levels remained statistically significant following adjustment for covariates as mentioned previously (Fig. 3K–T). Whereas TLR2, 4–5 levels were elevated in GCC when compared to NGT, only TLR4 mRNA expression was elevated in the PCC subjects (Fig. 3K–T). TLR5, 7 and 9, but not other TLRs, were elevated in neutrophils obtained from PC subjects. A correlation between TLRs mRNA levels in either neutrophils or monocytes, and BMI was not evident in the T2DM subjects.
Correlation between fasting glucose levels, insulin and inflammation
In the GC group, there was a significant correlation between fasting glucose and monocyte IL-1β (r = 0.738; p = 0.04) and TLR8 (r = 0.94, p = 0.001), and neutrophil RANTES (r = −0.742, p = 0.04) mRNAs. In the GCC group, there was a significant correlation between fasting glucose and monocyte RANTES (r = 0.747; p = 0.03) and neutrophil TLR5 (r = −0.730, p = 0.04) mRNAs. In the PCC group, there was a significant correlation between fasting glucose and serum RANTES (r = 0.403; p = 0.02) and monocyte IL-1β mRNA (r = 0.857, p = 0.02).
In the GC group, there was a significant correlation between duration of diabetes and monocyte RANTES (r = −0.715, p = 0.05) and TLR8 (r = 0.741, p = 0.04) mRNA, and neutrophil TLR2 mRNA (r = −0.838, p = 0.01). In the GCC, there was no correlation. In the PC group, there was a correlation between neutrophil TLR8 (r = 0.771, p = 0.03) and TLR9 (r = 0.747, p = 0.04) mRNAs. In the PCC group, there was a correlation between duration of diabetes and neutrophil TLR1 (r = −0.730, p = 0.04), TLR3 (r = 0.766, p = 0.03) and TLR5 (r = 0.843, p = 0.01) mRNAs.
Discussion
The main objective of this study was to investigate the role of inflammatory biomarkers in the pathophysiology of T2DM. To the best of our knowledge, this is the first study that has sought to evaluate serum cytokine levels and cytokines/TLRs gene expression in both monocytes and neutrophils among T2DM cohorts.
In this study, we showed that in neutrophils, the highest levels of the proinflammatory cytokines, TNFα, IL-6 and IFN-β mRNA were present in the GC subjects when compared to NGT control. Similarly, in monocytes, IL-6 and IFN-β mRNA levels were elevated in GC subjects, when compared to NGT. Our results correlate with previous studies which showed that TNFα mRNA and IL-6 levels were elevated in T2DM subjects23,24. Chemokines, such as IL-8 and RANTES play a critical role in driving and inflammatory milieu since they mediate the arrival of inflammatory cells to the site of both acute and chronic inflammation25. Herein, our study demonstrated that RANTES mRNA expression was elevated in neutrophils (but not monocytes) obtained from the GC subjects. Our study correlates, in part, with previous studies, which demonstrate that serum RANTES levels are increased in T2DM subjects25,26. Intriguingly, our study showed that while RANTES mRNA levels were increased in some of the T2DM subjects, levels of RANTES in serum were comparable among the different groups.
Our study also demonstrated that TNFα, IL-6, IFN-β and RANTES mRNA and serum levels were not significantly elevated in GCC subjects when compared to NGT. In the PC subjects, while levels of IL-6, IFN-β and RANTES mRNA were raised in both monocytes and neutrophils, serum protein levels were not significantly different among the groups.
In the PCC subjects, while levels of IFN-β and Rantes mRNA were raised in monocytes, levels remained unchanged in neutrophils, when compared to NGT. Despite an increase in cytokine mRNA level, serum protein levels were not significantly different among the groups. Further TNF-α mRNA was significantly overexpressed in PC and PCC monocytes, but after adjustment for covariates, it became statistically insignificant.
Regarding IL-12, we observed low serum levels of IL-12 and IFN-γ in T2DM subjects, which was statistically insignificant. Our findings are in contrast to that previously reported by Tsiavou et al., who demonstrated low serum levels of IFN- γ and IL-12 in T2DM subjects27. In contrast, Wegner et al., demonstrated elevated IL-12 serum levels in T2DM subjects28. Regarding IL-10, an anti-inflammatory cytokine, our study demonstrated that IL-10 levels were comparable in T2DM and NGT subjects, however it remained statistically insignificant. This is in contrast to Yaghini et al., who previously showed that IL-10 levels were decreased in T2DM subjects29.
Contrasting with TLR2 and TLR4, there is a paucity of data exploring the role of other TLRs in T2DM. However, upregulated expression of TLR1–9 and TLR11–13 was shown in two murine models of obesity30. Given that TLRs are key enablers of the inflammatory response, serving to drive the production of inflammatory cytokines, we sought to explore the expression levels of TLR1-10 in monocytes and neutrophils obtained from T2DM and healthy participants. In monocytes, our study demonstrated that, in general, levels of TLR mRNA expression were enhanced in the monocytes obtained from GC subjects. Similarly, in neutrophils, expression of most TLR mRNAs was increased in GC subjects when compared to NGT. Correlating with our data generated from T2DM patients with good glycaemic control (GC), previous studies have demonstrated that the expression of TLR2 and TLR4 was increased in monocytes derived from patients with T2DM, mean duration 29 months17. Further, other studies demonstrated that TLR2 and TLR4 mRNA levels were elevated in PBMCs taken from T2DM patients31, though mean disease duration was not indicated32.
Interestingly, in our study, T2DM patients with mean disease duration >8 years, namely T2DM patients with poor glucose control, and subjects with complications showed a trend towards suppressed TLR mRNA expression when compared to GC subjects. In monocytes, whereas TLR7 levels were elevated in PC, levels of all TLRs were comparable to NGT among PCC subjects. Similarly, in neutrophils, GCC subjects moderately overexpress TLR4 & 5, whereas PC overexpressed TLR5, 7 & 9 compared to NGT. PCC subjects overexpressed only TLR4 in neutrophils, and no significant change in the expression of TLR3, 6, 8, 9 & 10 was noted in PCC compared to NGT. Further, after adjustment for age, sex, WHR, BMI, duration of diabetes, creatinine and medications using lognormal data with multifactorial ANOVA, TLR1, 3, 5, 6, 7, 9 and 10 mRNA levels remained statistically significant among the groups.
Taken together, these data suggest that TLR gene expression is elevated in T2DM patients with good glucose control when compared to the general population. However, in T2DM patients with good glucose control with complications, levels of TLRs are decreased when compared to individuals with similar glucose control without complications. Further, in T2DM patients with poor glucose control in the absence and presence of complications, levels of TLR gene expression are decreased when compared to T2DM patients with good glucose control. In fact, levels of TLR expression among patients with poor glucose control are more comparable to that observed in the general population. Given the apparent differences in the expression of TLRs among the T2DM groups, namely the enhanced TLR expression in patients with good glucose control versus the downregulation of TLRs in patients with poor glucose control and in patients with complications, it is plausible to speculate that decreased TLR expression in the latter groups may have occurred to curtail sustained TLR activation and concomitant inflammatory processes. Whether decreased TLR expression leads to compromised innate immune signalling mechanisms and concomitant predisposition to infections requires further investigation.
Our study has shown that IL-6 and IFN-β mRNA levels are elevated in monocytes and neutrophils from GC and PC and that TNFα mRNA levels are elevated in neutrophils from GC group, compared with NGT. Intriguingly, our study failed to show elevated serum TNFα, IL-6, IFNβ, IL-8, IL-10, IL-12 and IFNγ levels in T2DM subjects despite perturbations in cytokine/TLR mRNAs levels being evident in the monocytes and neutrophils taken from T2DM patients. In terms of TLR expression, studies have shown that metformin33, statins, PPAR-γ agonists34 and angiotensin receptor blockers35 lower the expression of TLR2 and TLR4 in T2DM patients. Further, previous studies have shown that insulin infusion significantly suppressed TLRs 1, 2, 4, 7, and 9 mRNA expressions in mononuclear cells obtained from T2DM individuals18. Notably, in the current study, a greater number of T2DM patients from the GCC, PC and PCC were prescribed insulin compared to the GC patient group. Given that our T2DM subjects were prescribed and were administering these medications, it is plausible to speculate that long term administration of these drugs, for example in the GCC, PC and PCC groups, may contribute to the impaired TLR expression among the cohort when compared to the GC group.
In conclusion, our study shows that while perturbations in the gene expression of cytokines are evident in patients with T2DM, serum levels of the cytokines examined remain comparable between individuals with and without T2DM. Further, our study also suggests that levels of TLRs, receptors intimately linked with the inflammatory process, are perturbed in patients with good glucose control in the absence of complications. However, levels of TLR mRNA expression decrease as the disease duration progresses over time. Whether this perturbation is the result of disease process itself, the prescribed medications, or whether patients with perturbed inflammatory markers are more susceptible to developing diabetes and associated microvascular and macrovascular complications, remains to be investigated. However, the consistent and significantly suppressed cytokine and TLR gene expression in patients with poor glycaemic control and with complications suggests a “burnt out” disease state. Landmark clinical trials (UKPDS, Steno 2, ADVANCE) have already shown that intensive glycaemic control leads to a reduction in morbidity and mortality over time36,37,38,39,40. This study raises the potential role of early aggressive glucose lowering and cardiovascular risk factor management in reversing the peak inflammation associated with the early stages of T2DM and thus reducing the risk of cardiovascular and microvascular complications later in life. Further investigation is required to examine this hypothesis.
Research Design and Methods
Study subjects and design
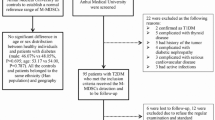
Healthy control participants were screened for diabetes using the standard oral glucose tolerance test (American Diabetes Association (ADA)). Healthy control participants with abnormal blood results (diabetes or pre-diabetes) and individuals with a history of chronic illness and/or individuals taking medication were excluded from the NGT group. T2DM participants had established diagnosis according to the ADA criteria. All participants were >18 years old and were not pregnant. Exclusion criteria included the following: Evidence of current infection (white cell count >11 or CRP >normal range), current treatment with antibiotics, neutropenia (a leucocyte count of less than 2000 per cubic millimetre), pregnancy or breast-feeding, non diabetic renal disease or liver disease (aspartate aminotransferase or alanine aminotransferase of more than three times the upper limit of the normal range), ongoing or previous cancer and the use of oral/inhaled glucocorticoids, immunosuppressive treatment or immunodeficiency.
Based on these criteria, 146 participants were recruited from a regional hospital. Healthy volunteers without diabetes acted as the control group (normal glucose tolerance, NGT; n = 34) and 112 T2DM patients (mean duration 95 months) with four different profiles were recruited: T2DM with good glycemic control (HbA1c <7.5%) and no macrovascular complications (heart disease, transient ischaemic stroke (TIA)/stroke or peripheral arterial disease (PAD)) and microvascular complications (retinopathy, neuropathy or nephropathy) GC, n = 27), T2DM with good glycemic control and complications (GCC, n = 32), T2DM with poor glycemic control (HbA1c >7.5%) without complications (PC, n = 21), and T2DM with poor glycaemic control and complications (PCC, n = 32). In case of the healthy volunteers, each participant met the inclusion and exclusion criteria for the study. Each healthy control participant was screened for diabetes and prediabetes using the standard oral glucose tolerance test41. Participants with abnormal results were excluded from the study. Personal and medical data was obtained by patient interview, by using hospital medical notes, and from using hospital laboratory test results. The demographic information was obtained from research participants and their weight, height, blood pressure & waist and hip circumferences were determined. BMI was calculated as body weight (in kilograms) divided by body height (in meters) squared. Waist-to-hip ratio (WHR) was calculated as waist divided by hip circumference. Informed consent was obtained from all participants. The protocol was approved by the Midlands Research Ethics Committee, Health Service Executive, Ireland, and by the Ethical Review Board, Maynooth University, Maynooth, Ireland and all experiments were performed in accordance with relevant guidelines and regulations.
Biochemical Analysis
Routinely, fasting blood samples were collected from consenting T2DM patients and healthy control subjects in the morning between 8–10 am to minimise the impact of diurnal variation on the study. More specifically, about 40 ml peripheral blood was collected from each participant including healthy volunteers and was used to measure various biochemical parameters and cytokines. A haemoglobin analyser HA-8160 (Menarini Pharmaceuticals, Ireland) was used to measure HbA1C (HPLC Chromatography Method) and Advia analysers were used to measure the whole blood counts. Lipid profile, urea, creatinine, sodium, potassium, asparate aminotransferase, alanine aminotransferase, bilirubin, alkaline phosphatase, gamma glutamyl transferase, CRP, ferritin, coagulation screen, thyroid profile and plasma glucose were measured using a Roche Modular 1800 analyser. An early morning urine sample for ACR measurement was also collected at the same time.
Cytokine measurements
For cytokine analysis, the blood was placed on ice and then centrifuged (3000 rpm, at 4 °C) within 1 hour of venesection. Serum was collected and stored at −80 °C. Serum was used for measuring TNFα, IL-6, IL-1β, IL-10, IL-8, IL-12p70 and IFN-γ using a 96-Well MULTI-ARRAY 7-Multiplex Assay (Meso Scale Discovery). Serum was used for measuring IFN-β and Rantes using single-plex 96-well plates (Meso Scale Discovery).
Mononuclear cell isolation and TLR mRNA quantification
Peripheral Blood Mononuclear Cells (PBMC) were isolated from heparinized peripheral blood by density gradient centrifugation. Human CD14 positive monocytes were isolated from PBMCs using the EasySep™ Human CD14 Positive Selection Cocktail, and neutrophils were isolated using the EasySep™ Human Neutrophil Enrichment Kit by negative selection. Initially, to ensure the robustness of the isolation procedure, a number of samples were analysed by flow cytometry to establish and confirm the purity of the monocyte and neutrophil population post selection. Next, total RNA was extracted from monocytes and neutrophils using the RNeasy isolation kit (Qiagen). RNA (1 μg per 25 μl reaction) was converted to first strand cDNA and stored at −20 °C42. Real-time PCR quantification was performed with DyNAmoHS SYBR Green kit (Finnzymes) using the OPTICON® system (MJ Research). For the amplification of TLRs & cytokines, in house-designed primers were used (Supplemental Table S1) using the housekeeping gene hypoxanthine phosphoribosyl transferase (HPRT) as a reference point42.
Statistical analysis
Data are expressed as median (interquartile range) and represented as box-and-whiskers plots. The dark midline in the box represents the distribution’s median value. The top and bottom edges of the box respectively represent the 75th and 25th percentile values. The top and bottom of the vertical lines, the whisker respectively represent the upper and lower maximum value. χ2 test or Fisher exact test (as appropriate) were used to compare proportions. The Mann–Whitney test was used in case of non-normally distributed parameters to compare median between two groups. Kruskal-Wallis test was used for multiple inter-group comparisons in case of parameters that did not show normal distribution. If a significant difference was found in inter-group comparisons, post hoc multiple comparison analysis with the Dunns multiple comparison test was performed. Data were transformed to log normal format and multifactorial ANOVA was applied to evaluate the effect of variables (age, sex, BMI, WHR and duration of diabetes) on different inflammatory markers. Correlations between values were examined by calculating Spearman correlation coefficients. All the statistical analysis was performed using the Prism 6.0 computer program (GraphPad, La Jolla, CA) and p value less than 0.05 was considered significant.
Change history
05 April 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Guest, C. B., Park, M. J., Johnson, D. R. & Freund, G. G. The implication of proinflammatory cytokines in type 2 diabetes. Front Biosci 13, 5187–5194 (2008).
Chawla, A., Chawla, R. & Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian journal of endocrinology and metabolism 20, 546–551 (2016).
Festa, A. et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102, 42–47 (2000).
Navarro, J. F. & Mora, C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. TheScientificWorldJournal 6, 908–917 (2006).
Goldberg, R. B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. The Journal of clinical endocrinology and metabolism 94, 3171–3182 (2009).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science New York, N.Y 259, 87–91 (1993).
Fried, S. K., Bunkin, D. A. & Greenberg, A. S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. The Journal of clinical endocrinology and metabolism 83, 847–850 (1998).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Min, D. et al. Alterations in monocyte CD16 in association with diabetes complications. Mediators of inflammation 2012, 649083 (2012).
Dasu, M. R. & Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. American journal of physiology 300, E145–154 (2011).
Horvath, P. et al. Fasting glucose level modulates cell surface expression of CD11b and CD66b in granulocytes and monocytes of patients with type 2 diabetes. Journal of investigative medicine: the official publication of the American Federation for Clinical Research 61, 972–977 (2013).
Alba-Loureiro, T. C. et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 40, 1037–1044 (2007).
Hou, H. W. et al. Rapid and label-free microfluidic neutrophil purification and phenotyping in diabetes mellitus. Scientific reports 6, 29410 (2016).
Miggin, S. M. & O’Neill, L. A. New insights into the regulation of TLR signaling. Journal of leukocyte biology 80, 220–226 (2006).
Piccinini, A. M. & Midwood, K. S. DAMPening inflammation by modulating TLR signalling. Mediators of inflammation 2010 (2010).
Reyna, S. M. et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57, 2595–2602 (2008).
Dasu, M. R., Devaraj, S., Park, S. & Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes care 33, 861–868 (2010).
Ghanim, H. et al. Acute modulation of toll-like receptors by insulin. Diabetes care 31, 1827–1831 (2008).
Klein, R. et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology 109, 1225–1234 (2002).
Cai, X. L., Wang, F. & Ji, L. N. Risk factors of diabetic retinopathy in type 2 diabetic patients. Chinese medical journal 119, 822–826 (2006).
Liu, C., Feng, X., Li, Q., Wang, Y. & Hua, M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 86, 100–109 (2016).
Siednienko, J., Halle, A., Nagpal, K., Golenbock, D. T. & Miggin, S. M. TLR3-mediated IFN-beta gene induction is negatively regulated by the TLR adaptor MyD88 adaptor-like. European journal of immunology 40, 3150–3160 (2010).
Tsiotra, P. C. et al. Visfatin, TNF-alpha and IL-6 mRNA expression is increased in mononuclear cells from type 2 diabetic women. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 39, 758–763 (2007).
Gonzalez, Y. et al. High glucose concentrations induce TNF-alpha production through the down-regulation of CD33 in primary human monocytes. BMC immunology 13, 19 (2012).
Maier, R. et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Molecular vision 14, 637–643 (2008).
Herder, C. et al. RANTES/CCL5 gene polymorphisms, serum concentrations, and incident type 2 diabetes: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. European journal of endocrinology/European Federation of Endocrine Societies 158, R1–5 (2008).
Tsiavou, A., Degiannis, D., Hatziagelaki, E., Koniavitou, K. & Raptis, S. A. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res 24, 381–387 (2004).
Wegner, M., Winiarska, H., Bobkiewicz-Kozlowska, T. & Dworacka, M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine 42, 312–316 (2008).
Yaghini, N. et al. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iranian Red Crescent medical journal 13, 752 (2011).
Kim, S. J., Choi, Y., Choi, Y. H. & Park, T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. The Journal of nutritional biochemistry 23, 113–122 (2012).
Sepehri, Z. et al. Human Toll like receptor 4 gene expression of PBMCs in diabetes mellitus type 2 patients. Cell Mol Biol (Noisy-le-grand) 61, 92–95 (2015).
Ahmad, R. et al. Elevated expression of the toll like receptors 2 and 4 in obese individuals: its significance for obesity-induced inflammation. Journal of inflammation (London, England) 9, 48 (2012).
Andrews, M., Soto, N. & Arredondo, M. [Effect of metformin on the expression of tumor necrosis factor-alpha, Toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients]. Revista medica de Chile 140, 1377–1382 (2012).
Dasu, M. R., Park, S., Devaraj, S. & Jialal, I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 150, 3457–3464 (2009).
Dasu, M. R., Riosvelasco, A. C. & Jialal, I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 202, 76–83 (2009).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England journal of medicine 348, 383–393 (2003).
Gaede, P. H. et al. The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2 diabetes. Ugeskrift for laeger 165, 2658–2661 (2003).
Gaede, P., Vedel, P., Parving, H. H. & Pedersen, O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353, 617–622 (1999).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine 358, 2560–2572 (2008).
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine 359, 1577–1589 (2008).
Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clinical diabetes: a publication of the American Diabetes Association 34, 3-21 (2016).
Siednienko, J. & Miggin, S. M. Expression analysis of the Toll-like receptors in human peripheral blood mononuclear cells. Methods in molecular biology Clifton, N.J 517, 3–14 (2009).
Acknowledgements
This work was funded, in part, by Endocrine Society of Ireland. The study describe in our manuscript was selected for an oral communication at The Endocrine Society’s 96th Annual Meeting & Expo, June 21–24, 2014 – Chicago. LINK: http://press.endocrine.org/doi/book/10.1210/endo-meetings.2014.OABA.16
Author information
Authors and Affiliations
Contributions
A.M., J.S., A.N. and T.G. researched data. S.G. researched data, wrote the manuscript and reviewed/edited the manuscript. S.H. and S.M. co-wrote the manuscript and reviewed/edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, S., Maratha, A., Siednienko, J. et al. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Sci Rep 7, 7633 (2017). https://doi.org/10.1038/s41598-017-07230-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07230-8
This article is cited by
-
Advancements of fish-derived peptides for mucormycosis: a novel strategy to treat diabetic compilation
Molecular Biology Reports (2023)
-
Need for improving immunization status and preventive care in diabetes mellitus patients
Wiener klinische Wochenschrift (2023)
-
Association of IL-10 (− 1082 A/G) and IL-6 (− 174 G/C) gene polymorphism with type 2 diabetes mellitus in Ethiopia population
BMC Endocrine Disorders (2021)
-
Macrophage accumulation within coronary arterial wall in diabetic patients with acute coronary syndrome: a study with in-vivo intravascular imaging modalities
Cardiovascular Diabetology (2020)
-
Serum cytokine dependent hematopoietic cell linker (CLNK) as a predictor for the duration of illness in type 2 diabetes mellitus
Journal of Diabetes & Metabolic Disorders (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.