Abstract
Despite the increasing adoption of insulin pumps and continuous glucose monitoring devices, most people with type 1 diabetes do not achieve their glycemic goals1. This could be related to a lack of expertise or inadequate time for clinicians to analyze complex sensor-augmented pump data. We tested whether frequent insulin dose adjustments guided by an automated artificial intelligence-based decision support system (AI-DSS) is as effective and safe as those guided by physicians in controlling glucose levels. ADVICE4U was a six-month, multicenter, multinational, parallel, randomized controlled, non-inferiority trial in 108 participants with type 1 diabetes, aged 10–21 years and using insulin pump therapy (ClinicalTrials.gov no. NCT03003806). Participants were randomized 1:1 to receive remote insulin dose adjustment every three weeks guided by either an AI-DSS, (AI-DSS arm, n = 54) or by physicians (physician arm, n = 54). The results for the primary efficacy measure—the percentage of time spent within the target glucose range (70–180 mg dl−1 (3.9–10.0 mmol l−1))—in the AI-DSS arm were statistically non-inferior to those in the physician arm (50.2 ± 11.1% versus 51.6 ± 11.3%, respectively, P < 1 × 10−7). The percentage of readings below 54 mg dl−1 (<3.0 mmol l−1) within the AI-DSS arm was statistically non-inferior to that in the physician arm (1.3 ± 1.4% versus 1.0 ± 0.9%, respectively, P < 0.0001). Three severe adverse events related to diabetes (two severe hypoglycemia, one diabetic ketoacidosis) were reported in the physician arm and none in the AI-DSS arm. In conclusion, use of an automated decision support tool for optimizing insulin pump settings was non-inferior to intensive insulin titration provided by physicians from specialized academic diabetes centers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Any requests for raw data (that is, glucose levels, insulin delivery, de-identified patient characteristics) will be reviewed by the NextDREAM consortium steering committee, which comprises the principal investigators of the different sites participating in this study. Applications for non-commercial use only will be considered and should be sent to the corresponding author (M.P.). Applications should outline how the specific use of the data would catalyze considerable advancement in the treatment and management of type 1 diabetes or improve care for those living with type 1 diabetes, including the specific purpose of developing therapies and technology that can be used by patients to help manage their disease and improve their health outcomes. Any data that can be shared will need approval from the NextDREAM consortium steering committee and a Material Transfer Agreement in place. All data shared will be de-identified.
Code availability
The code cannot be made available due to proprietary reasons.
References
Miller, K. M. et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D exchange clinic registry. Diabetes Care 38, 971–978 (2015).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Nathan, D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care 42, S1–S193 (2019).
Foster N. C. et al. Marked increases in CGM use has not prevented increases in HbA1c levels in participants in the T1D exchange (T1DX) clinic network. Diabetes 67 (2018); https://doi.org/10.2337/db18-1689-P
Edelman, S. V., Argento, N. B., Pettus, J. & Hirsch, I. B. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 41, 2265–2274 (2018).
Zisser, H. et al. Clinical performance of three bolus calculators in subjects with type 1 diabetes mellitus: a head-to-head-to-head comparison. Diabetes Technol. Ther. 12, 955–961 (2010).
Petitti, D. B. et al. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J. Pediatr. 155, 668–672 (2009).
The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 10, 1–19 (1987).
Davidson, M. B. How our current medical care system fails people with diabetes: lack of timely, appropriate clinical decisions. Diabetes Care 32, 370–372 (2009).
Bergenstal, R. M. et al. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet 393, 1138–1148 (2019).
Shalitin, S. et al. Using the Internet-based upload blood glucose monitoring and therapy management system in patients with type 1 diabetes. Acta Diabetol. 51, 247–256 (2014).
Pihoker, C. et al. ISPAD clinical practice consensus guidelines 2018: the delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr. Diabetes 19, 84–104 (2018).
Chiang, J. L. et al. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care 41, 2026–2044 (2018).
Markowitz, J. T., Volkening, L. K. & Laffel, L. M. B. Care utilization in a pediatric diabetes clinic: cancellations, parental attendance and mental health appointments. J. Pediatr. 164, 1384–1389 (2014).
Comellas, M. J. et al. Evaluation of a new digital automated glycemic pattern detection tool. Diabetes Technol. Ther. 19, 633–640 (2017).
Lu, H. et al. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Serv. Res. 15, 541 (2015).
Nimri, R. et al. Adjusting insulin doses in patients with type 1 diabetes who use insulin pump and continuous glucose monitoring: variations among countries and physicians. Diabetes Obes. Metab. 20, 2458–2466 (2018).
Beck, R. W. Downloading diabetes device data: empowering patients to download at home to achieve better outcomes. Diabetes Technol. Ther. 17, 536–537 (2015).
IDF Diabetes Atlas, 8th edn (International Diabetes Federation, accessed 3 December 2018); https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html
Patterson, C. C. et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 62, 408–417 (2019).
Vigersky, R. A. et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J. Clin. Endocrinol. Metab. 99, 3112–3121 (2014).
Hahn, S. Understanding noninferiority trials. Korean J. Pediatr. 55, 403–407 (2012).
Tamborlane, W. V. et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N. Engl. J. Med. 359, 1464–1476 (2008).
Aleppo, G. et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 40, 538–545 (2017).
Abramson, J. H. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 8, 1 (2011).
Acknowledgements
We thank the following colleagues for assistance in the study: David Steinberg and Sigal Fleishman of Tel Aviv University for the statistical analysis; the Data and Safety Monitoring Board (DSMB) members: David Maahs, Larry Fox, Jan Bolinder, and the study statistician David Steinberg; Clinlogix, Contract Research Organization: Ksenia Keller, Oliver Hautz, Hiroko Griffin, Jean-Luc Baeriswyl, Christian Dierker, Daniel Rider, Andrew Milczarek, Jennifer M. Kratz, Vanessa Okonkwo; DreaMed Diabetes Ltd: Eran Atlas, Ido Muller, Eran Agmon, Daniel Koch, Yaron Matiash, Tomer Segall, Anat Cohen, Moshe Lev, Igal Shtudiner; Glooko, Inc: Dave Conn, Jeff Chang, Holly McGarraugh, Makenzie Wells, Shannon Gillum-Collins, A.-J. Boyer; Prof. Katharine Barnard, PhD CPsychol AFBPsS, Bournemouth University, Bournemouth, UK BHR Ltd, Portsmouth, UK for developing the ‘Healthcare Professionals Post-Intervention Survey’. We also thank Chris Parkin for his assistance in editing this manuscript. We thank the patients and their families for their participation. Funding for the study was provided by The Leona M. and Harry B. Helmsley Charitable Trust through DreaMed Diabetes Ltd grant no. 2016PG-T1D050. The trial was conducted in collaboration with DreaMed Diabetes Ltd, which provided the DreaMed Advisor Pro, offered technical support to the sites and generated the glucose and insulin data from Glooko system uploads for statistical analysis by an external statistician. Glooko provided the Glooko Platform for the sites, conducted training and offered technical support. Dexcom provided the CGM devices (Dexcom G5) free of charge. DexCom had no role in the trial conduct. Insulet supplied six discounted Omnipod pumps. The DreaMed Advisor Pro development was funded by DreaMed Diabetes Ltd in addition to support from a grant by The Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Consortia
Contributions
M.P., the corresponding author, had full access to all the data in the trial and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.N., T.B., L.M.L., R.H.S., D.S., S.A.W., K.D., T.D. and M.P. contributed to the study concept and design, contributed data and advised on analysis or interpretation of the data. R.N. and M.P. wrote the first draft of the manuscript with the aid of an independent medical writer. R.N., T.B., L.M.L., R.H.S., D.S., S.A.W., K.D., T.D. and M.P. commented on and revised the manuscript and approved the submission.
Corresponding author
Ethics declarations
Competing interests
R.N. reports receiving grants from Helmsley Charitable Trust, Dexcom and Insulet, personal fees and others from DreaMed Diabetes Ltd, personal fees from Novo Nordisk and Eli Lilly and grants from Medtronic. In addition, R.N. owns DreaMed Diabetes Ltd stock and has a patent licensed by DreaMed Diabetes Ltd. T.B. reports receiving grants and personal fees from Novo Nordisk, Medtronic and Abbott, personal fees from Eli Lilly, Sanofi, Indigo, Roche, AstraZeneca and Dexcom and grants from Glusense and Zealandpharma. In addition, T.B. owns DreaMed Diabetes Ltd stocks. L.M.L. reports receiving grants from Helmsley Charitable Trust and personal fees from Eli Lilly, Novo Nordisk, Sanofi, Convatec, Roche, Insulogic, Dexcom, Insulet, AstraZeneca, Boehringer Ingelheim and Janssen. S.A.W. reports receiving grants from Helmsley Charitable Trust, grants and personal fees from Medtronic and personal fees from Insulet, Tandem, Eli Lilly, Sanofi and Zealand. T.D. reports receiving grants and personal fees from AstraZeneca, DexCom, Boehringer, Novo Nordisk, Medtronic, Sanofi, Eli Lilly and Insulet. In addition, T.D. owns DreaMed Diabetes Ltd stocks. M.P. reports receiving grants from Helmsley Charitable Trust, Dexcom and Insulet, personal fees and others from DreaMed Diabetes Ltd, grants and personal fees from Medtronic and Novo Nordisk, grants from Roche, Eli Lilly and Sanof, grants from Lexicon and OPKO and personal fees from RSP Systems and Qulab Medical. In addition, M.P. owns DreaMed Diabetes Ltd stock and has a patent licensed by DreaMed Diabetes Ltd. R.H.S., D.S. and K.D. declare no competing interests.
Additional information
Peer review information Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Exploratory outcomes: intent to treat cohort (N=108).
* Plus-minus values are means ±SD calculated over the entire 24-weeks study period. To convert the values for glucose to millimoles per liter, multiply by 0.05551. This category of SD is an average of the variability of sensor glucose measurements for each patient, rather than the variation in the mean glucose values among patients in the trial. ¥ Insulin information was recorded only for 52 patients per arm. § Two-sided ANCOVA adjusting for baseline level and number of valid days. Ϯ Randomization test.
Extended Data Fig. 2 Glycated hemoglobin levels at baseline, randomization, 12-weeks, and 24-weeks for per-protocol (n=30 per arm) cohort.
Values are means (markers) ± standard error (error bars). Black circles mark the AI-DSS arm and empty diamonds mark the physician arm. The results of the two-sided, paired t-test analysis are marked by asterisk symbol to show statistically significant difference between baseline and specific time point within AI-DSS arm and by pound symbol to show statistically significant difference between baseline and specific time point within physician arm, where one symbol denotes P<0.05 and two denotes P<0.01.
Extended Data Fig. 3 Study outcomes measurements: per protocol cohort (N=60).
* Plus-minus values are means ±SD. To convert the values for glucose to millimoles per liter, multiply by 0.05551. ¥ One-sided test using ANCOVA model adjusted for baseline % of time in range and number of valid days used in the analysis. § Non-inferiority randomization test. ϮTwo-sided paired t-test. ΨTwo-sided ANCOVA adjusting for baseline level and number of valid days.
Extended Data Fig. 4 Healthcare professionals post-intervention survey responses: physicians who used the AI-DSS during the study (n=13).
The scale of the reply was: 5—Strongly Agree, 4—Somewhat Agree, 3—Do not Agree or Disagree, 2—Somewhat Disagree, 1—Strongly Disagree. The Healthcare Professionals Post-Intervention Survey is a 50 items questionnaire. The questionnaire comprises: (a) 28 items for pertaining to the physician’s experience with the Advisor Pro use and recommendations. Each item is score on a 5-point scale range from 5 (strongly agree) to 1 (strongly disagree) (Extended Data Fig. 4) (b) 22 items are questions that asses the physician’s view regarding integration of the Advisor Pro into daily routine practice (14 items are yes/no questions and 8 items are open questions). The questionnaire was developed by Prof Katharine Barnard PhD CP sychol AFBPsS and reviewed by the investigators (first and third authors).
Extended Data Fig. 5 Description of the number and technical issues related to insulin dose recommendations in both study arms - intent to treat cohort.
1 In both groups, there were cases in which insulin dosing recommendation was not shared with the participant via the DMS system but in another method. 2 The reasons for missing scheduled recommendations were: physician did not give a recommendation for the physician arm (supplement to the number of given recommendation) and lack of minimal requirements needed for creating advisor recommendation such as lack of valid days for analysis for the AI-DSS arm (supplement to the number of recommendation by the AI-DSS). 3 In 7 cases, recommendation was given by the physician and not the Advisor. In 5 of these cases the minimal data requirement of the AI-DSS was not meet and in the other 2 cases there was a suspected pump clock shifting that prevented the system from providing a recommendation. 4 Technical issues that caused missing recommendation include: any technical failure related to pump, continuous glucose monitoring, diabetes management system or temporary algorithm faults that were resolved during the study. 5 Insufficient insulin and glucose data according to the system requirements prevent formation of recommendation in both trial arms. 6 Pump clock shifting is a safety layer of the advisor that prevent the formation of a recommendation.
Extended Data Fig. 6 AI-DSS data flow in the study.
1. participants upload devices data to DMS 2. AI-DSS pulled data from DMS after DMS requested advice and validated data 3. AI-DSS detected the source of the data: in case the data came from participants in the physician arm no advice was created and in case the data came from participants in the AI-DSS arm advice was created 4. AI-DSS report was sent via DMS to the physician 5. Physician could review new pump settings recommendations within AI-DSS report page using the DMS platform 6. Physician shared new pump settings and management tips with participant through smartphone.
Extended Data Fig. 7 Data management system population tracker with AI-DSS report.
a, Once the participant uploaded data to the DMS and algorithm has enough data, a new recommendation will be sent and physician could enter into the patient page and review it. Current basal rates are displayed on the left, for the physician arm—a duplicate of the current settings was displayed on the right, clinicians could click EDIT and make changes if necessary and add their own comments for behavioral tips. For the AI-DSS arm—the right side displayed the AI-DSS recommended changes (if necessary) in bold. Comments may be added from the AI-DSS. b, Current carbohydrate ratio values are displayed on the left, for the physician arm - a duplicate of the current settings was displayed on the right, clinicians could click EDIT and make changes if necessary and add their own comments for behavioral tips. For the AI-DSS arm—the right side displayed the AI-DSS recommended changes (if necessary), in bold. Comments may be added from the AI-DSS. c, Current correction factor (Insulin Sensitivity Factor) are displayed on the right, for the physician arm - a duplicate of the current settings was displayed on the right, clinicians could click EDIT and make changes if necessary and add their own comments for behavioral tips. For the AI-DSS arm—the right side displayed the AI-DSS recommended changes (if necessary), in bold. Comments may be added from the AI-DSS. d, The Ambulatory Glucose Profile, Logbook and Daily reports are displayed under each of the previous settings screens for guidance. e, Once all settings screens are completed, a summary of the recommendation will appear. This is what the patients sees on their mobile app once the physician shares the recommendations with them.
Extended Data Fig. 8 AI-DSS insulin pump recommendations presented on patient’s smartphone: new basal plan, new carbohydrates ratio, new correction factors and diabetes management tips.
The recommendation report for the patient is presented in the app, three screen shots from the application. The data in the figure related to virtual patient, Mia Foster.
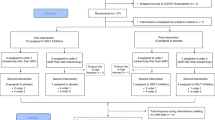
Extended Data Fig. 9 Study design scheme.
After a 3-week run-in period, participants were randomized to participate either at the IA-DSS or physician arm. Three weeks after randomization and every 3 weeks thereafter, all participants uploaded their insulin pump and continuous glucose monitoring data using the DMS. Participants then received insulin pump settings recommendations either from physician or AI-DSS according to their randomization for a 24-week period.
Extended Data Fig. 10 Study visit schedule.
Procedures conducted at each visit.
Supplementary information
Supplementary Information
Supplementary material
Rights and permissions
About this article
Cite this article
Nimri, R., Battelino, T., Laffel, L.M. et al. Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat Med 26, 1380–1384 (2020). https://doi.org/10.1038/s41591-020-1045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-1045-7
This article is cited by
-
AI-based diabetes care: risk prediction models and implementation concerns
npj Digital Medicine (2024)
-
Assisting the implementation of screening for type 1 diabetes by using artificial intelligence on publicly available data
Diabetologia (2024)
-
Prioritizing educational initiatives on emerging technologies for Italian pediatricians: bibliometric review and a survey
Italian Journal of Pediatrics (2023)
-
The importance of interpreting machine learning models for blood glucose prediction in diabetes: an analysis using SHAP
Scientific Reports (2023)
-
Artificial intelligence in diabetes mellitus and endocrine diseases — what can we expect?
Nature Reviews Endocrinology (2023)