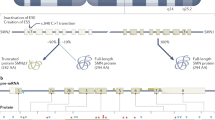
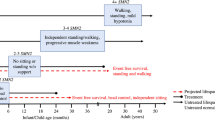
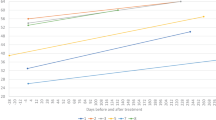
Abstract
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease caused by deletion or mutation of SMN1. Four subtypes exist, characterized by different clinical severities. New therapeutic approaches have become available in the past few years, dramatically changing the natural history of all SMA subtypes, including substantial clinical improvement with the severe and advanced SMA type 1 variant. Trials have now demonstrated that phenotypic rescue is even more dramatic when pre-symptomatic patients are treated, and emerging real-world data are demonstrating the benefits of intervention even in the chronic phase of the condition. Here, we critically review how the field is rapidly evolving in response to the new therapies and questions that the new treatments have posed, including the effects of treatment at different ages and stages of disease, new phenotypes and long-term outcomes in patients who would not have survived without treatment, and decisions of who to treat and when. We also discuss how the outcomes associated with different timing of therapeutic intervention are contributing to our understanding of the biology and pathogenesis of SMA.
Key points
-
Drugs that increase levels of survival motor neuron (SMN) protein are revolutionizing the disease course and treatment of spinal muscular atrophy (SMA) across the spectrum of the disease.
-
Clinical responses in patients with symptomatic SMA can reach levels that were unanticipated, but the longer the disease duration and the greater the severity, the more modest the response.
-
Clinical trials and biomarker studies are providing new information about disease processes, highlighting prenatal onset in children with severe SMA and in pre-symptomatic patients with two copies of SMN2.
-
Some of the drugs that have been developed address SMN deficiency only in the CNS, whereas others address deficiency in the CNS and periphery, which could increase the benefit.
-
The phenotypes of treated patients with SMA are evolving before our eyes; from their observation, we could learn more about disease processes and treatment effects.
-
Which of the available drugs works best in the long term for particular subgroups of patients remains unclear, as does whether combination treatments will be superior to monotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kolb, S. J. & Kissel, J. T. Spinal muscular atrophy: a timely review. Arch. Neurol. 68, 979–984 (2011).
Sangare, M. et al. Genetics of low spinal muscular atrophy carrier frequency in sub-Saharan Africa. Ann. Neurol. 75, 525–532 (2014).
Cusin, V., Clermont, O., Gerard, B., Chantereau, D. & Elion, J. Prevalence of SMN1 deletion and duplication in carrier and normal populations: implication for genetic counselling. J. Med. Genet. 40, e39 (2003).
Vorster, E., Essop, F. B., Rodda, J. L. & Krause, A. Spinal muscular atrophy in the Black South African population: a matter of rearrangement? Front. Genet. 11, 54 (2020).
Dubowitz, V. Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. Eur. J. Paediatr. Neurol. 3, 49–51 (1999).
Finkel, R., Bertini, E., Muntoni, F., Mercuri, E. & ENMC SMA Workshop Study Group. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7–9 November 2014, Heemskerk, The Netherlands. Neuromuscul. Disord. 25, 593–602 (2015).
Burghes, A. H. & Beattie, C. E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10, 597–609 (2009).
Feldkotter, M., Schwarzer, V., Wirth, R., Wienker, T. F. & Wirth, B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 70, 358–368 (2002).
Lefebvre, S. et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 16, 265–269 (1997).
Calucho, M. et al. Correlation between SMA type and SMN2 copy number revisited: san analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 28, 208–215 (2018). This large study demonstrates the correlation between SMN2 copy number and clinical outcomes.
Wirth, B., Karakaya, M., Kye, M. J. & Mendoza-Ferreira, N. Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu. Rev. Genomics Hum. Genet. 21, 231–261 (2020).
Bernal, S. et al. The c.859G>C variant in the SMN2 gene is associated with types II and III SMA and originates from a common ancestor. J. Med. Genet. 47, 640–642 (2010).
Riessland, M. et al. Neurocalcin δ suppression protects against spinal muscular atrophy in humans and across species by restoring impaired endocytosis. Am. J. Hum. Genet. 100, 297–315 (2017).
Hosseinibarkooie, S., Schneider, S. & Wirth, B. Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev. Proteom. 14, 581–592 (2017).
Hosseinibarkooie, S. et al. The power of human protective modifiers: PLS3 and CORO1C unravel impaired endocytosis in spinal muscular atrophy and rescue SMA phenotype. Am. J. Hum. Genet. 99, 647–665 (2016). This study identifies genes that can modify SMA disease severity in preclinical models.
Finkel, R. S. et al. Diagnosis and management of spinal muscular atrophy: Part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 28, 197–207 (2018).
Mercuri, E. et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 28, 103–115 (2018).
Farrar, M. A. et al. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 81, 355–368 (2017).
Sumner, C. J. & Crawford, T. O. Two breakthrough gene-targeted treatments for spinal muscular atrophy: challenges remain. J. Clin. Invest. 128, 3219–3227 (2018).
Passini, M. A. et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl Med. 3, 72ra18 (2011).
Hua, Y. et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 24, 1634–1644 (2010).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017). This paper presents a seminal phase III trial of the antisense oligonucleotide nusinersen in infants with SMA-I.
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378, 625–635 (2018). This paper presents a seminal phase III trial of nusinersen in children with chronic forms of SMA-II and SMA-III.
Darras, B. T. et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology 92, e2492–e2506 (2019).
De Vivo, D. C. et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul. Disord. 29, 842–856 (2019). This paper presents the first study of nusinersen in pre-symptomatic infants with SMA-I and SMA-II who were identified through family studies or newborn screening.
Sivaramakrishnan, M. et al. Binding to SMN2 pre-mRNA–protein complex elicits specificity for small molecule splicing modifiers. Nat. Commun. 8, 1476 (2017). This paper presents the identification of a small molecule that can selectively induce SMN2 exon 7 retention in the mRNA.
Poirier, A. et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 6, e00447 (2018). This paper presents the first-in-human study of an AAV replacement therapy for infants with SMA-I.
Ratni, H. et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA). J. Med. Chem. 61, 6501–6517 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02913482 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02908685 (2020).
Baranello, G. et al. FIREFISH Part 1: early clinical results following a significant increase of SMN protein in SMA type 1 babies treated with RG7916. INeuromuscular Disord. 28, S109 (2018).
Mercuri, E. M. et al. SUNFISH Part 1: RG7916 treatment results in a sustained increase of SMN protein levels and the first clinical efficacy results in patients with type 2 or 3 SMA. Neuromuscul. Disord. 28, S108 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03779334 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03032172 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02268552 (2020).
Foust, K. D. et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 228, 271–274 (2010).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03306277 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03461289 (2020).
Day, J. W. et al. Onasemnogene abeparvovec-xioi gene-replacement therapy for spinal muscular atrophy type 1 (SMA1): phase 3 US study (STR1VE) update (1828). Neurology 94, 1828 (2020).
Strauss, K. A. et al. Onasemnogene abeparvovec-xioi gene-replacement therapy in presymptomatic spinal muscular atrophy: SPR1NT study update (2384). Neurology 94, 2384 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03505099 (2020).
European Medicines Agency. Zolgensma summary of product characteristics https://www.ema.europa.eu/en/documents/product-information/zolgensma-epar-product-information_en.pdf (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03381729 (2020).
Novartis. Novartis announces AVXS-101 intrathecal study update https://www.novartis.com/news/media-releases/novartis-announces-avxs-101-intrathecal-study-update (2019).
Mendell, J. R. et al. Gene-replacement therapy in spinal muscular atrophy type 1: long-term follow-up from the onasemnogene abeparvovec-xioi phase 1/2a clinical trial (1808). Neurology 94, 1808 (2020).
Ronzitti, G. et al. Human, immune responses to adeno-associated virus (AAV) vectors. Front. Immunol. 11, 670 (2020).
Mariot, V. et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 8, 1859 (2017).
Zhou, H. et al. Myostatin inhibition in combination with antisense oligonucleotide therapy improves outcomes in spinal muscular atrophy. J. Cachexia Sarcopenia Muscle 11, 768–782 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03819660 (2020).
Harding, B. N. et al. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J. Neuropathol. Exp. Neurol. 74, 15–24 (2015).
Finkel, R. S. Electrophysiological and motor function scale association in a pre-symptomatic infant with spinal muscular atrophy type I. Neuromuscul. Disord. 23, 112–115 (2013).
Swoboda, K. J. et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 57, 704–712 (2005).
Swoboda, K. J. et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of l-carnitine and valproic acid in spinal muscular atrophy. PLoS ONE 5, e12140 (2010).
Kang, P. B. et al. The motor neuron response to SMN1 deficiency in spinal muscular atrophy. Muscle Nerve 49, 636–644 (2014).
Kolb, S. J. et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann. Clin. Transl Neurol. 3, 132–145 (2016).
Kolb, S. J. et al. Natural history of infantile-onset spinal muscular atrophy. Ann. Neurol. 82, 883–891 (2017).
Pane, M. et al. Longitudinal assessments in discordant twins with SMA. Neuromuscul. Disord. 27, 890–893 (2017).
Ramos, D. M. et al. Age-dependent SMN expression in disease-relevant tissue and implications for SMA treatment. J. Clin. Invest. 129, 4817–4831 (2019). This study demonstrates developmental expression of SMN with implications for disease pathogenesis and response to SMN augmentation therapies.
Cifuentes-Diaz, C. et al. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet. 11, 1439–1447 (2002).
Kariya, S. et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 17, 2552–2569 (2008).
Murray, L. M. et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 17, 949–962 (2008).
Kong, L. et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. 29, 842–851 (2009).
Braun, S., Croizat, B., Lagrange, M. C., Warter, J. M. & Poindron, P. Constitutive muscular abnormalities in culture in spinal muscular atrophy. Lancet 345, 694–695 (1995).
Arnold, A. S. et al. Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Lab. Invest. 84, 1271–1278 (2004).
Wishart, T. M. et al. Dysregulation of ubiquitin homeostasis and β-catenin signaling promote spinal muscular atrophy. J. Clin. Invest. 124, 1821–1834 (2014).
Martinez-Hernandez, R. et al. Synaptic defects in type I spinal muscular atrophy in human development. J. Pathol. 229, 49–61 (2013).
Wadman, R. I., Vrancken, A. F., van den Berg, L. H. & van der Pol, W. L. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology 79, 2050–2055 (2012).
Pera, M. C. et al. 6MWT can identify type 3 SMA patients with neuromuscular junction dysfunction. Neuromuscul. Disord. 27, 879–882 (2017).
Montes, J. et al. A randomized, controlled clinical trial of exercise in patients with spinal muscular atrophy: methods and baseline characteristics. J. Neuromuscul. Dis. 1, 151–161 (2014).
Montes, J. et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology 74, 833–838 (2010).
Montes, J. et al. Leg muscle function and fatigue during walking in spinal muscular atrophy type 3. Muscle Nerve 50, 34–39 (2014).
Mazzone, E. et al. Six minute walk test in type III spinal muscular atrophy: a 12-month longitudinal study. Neuromuscul. Disord. 23, 624–628 (2013).
Montes, J. et al. Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy. Muscle Nerve 60, 409–414 (2019).
Ghazanfari, N., Morsch, M., Tse, N., Reddel, S. W. & Phillips, W. D. Effects of the β2-adrenoceptor agonist, albuterol, in a mouse model of anti-MuSK myasthenia gravis. PLoS ONE 9, e87840 (2014).
Kinali, M. et al. Pilot trial of albuterol in spinal muscular atrophy. Neurology 59, 609–610 (2002).
Pane, M. et al. Daily salbutamol in young patients with SMA type II. Neuromuscul. Disord. 18, 536–540 (2008).
Khirani, S. et al. Effect of salbutamol on respiratory muscle strength in spinal muscular atrophy. Pediatr. Neurol. 73, 78–87 (2017).
Pera, M. C. et al. Does albuterol have an effect on neuromuscular junction dysfunction in spinal muscular atrophy? Neuromuscul. Disord. 28, 863–864 (2018).
Stam, M. et al. Protocol for a phase II, monocentre, double-blind, placebo-controlled, cross-over trial to assess efficacy of pyridostigmine in patients with spinal muscular atrophy types 2–4 (SPACE trial). BMJ Open 8, e019932 (2018).
Messina, S. et al. Expanded access program with nusinersen in SMA type I in Italy: strengths and pitfalls of a successful experience. Neuromuscul. Disord. 27, 1084–1086 (2017).
Walter, M. C. et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—a prospective observational study. J. Neuromuscul. Dis. 6, 453–465 (2019).
Pane, M. et al. Nusinersen in type 1 spinal muscular atrophy: twelve-month real-world data. Ann. Neurol. 86, 443–451 (2019).
Aragon-Gawinska, K. et al. Sitting in patients with spinal muscular atrophy type 1 treated with nusinersen. Dev. Med. Child. Neurol. 62, 310–314 (2020).
Pechmann, A. et al. Evaluation of children with SMA type 1 under treatment with nusinersen within the expanded access program in Germany. J. Neuromuscul. Dis. 5, 135–143 (2018).
Pechmann, A., Langer, T., Wider, S. & Kirschner, J. Single-center experience with intrathecal administration of nusinersen in children with spinal muscular atrophy type 1. Eur. J. Paediatr. Neurol. 22, 122–127 (2018).
Sansone, V. A. et al. Respiratory needs in patients with type 1 spinal muscular atrophy treated with nusinersen. J. Pediatr. 219, 223–228 (2020).
LoMauro, A. et al. Effect of nusinersen on respiratory muscle function in different subtypes of type 1 spinal muscular atrophy. Am. J. Respir. Crit. Care Med. 200, 1547–1550 (2019).
Hagenacker, T. et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 19, 317–325 (2020).
Mercuri, E. & Sansone, V. Nusinersen in adults with spinal muscular atrophy: new challenges. Lancet Neurol. 19, 283–284 (2020).
Finkel, R. S. et al. RESTORE: a prospective multinational registry of patients with genetically confirmed spinal muscular atrophy—rationale and study design. Neuromuscul. Dis. 7, 145–152 (2020).
Messina, S. et al. A critical review of patient and parent caregiver oriented tools to assess health-related quality of life, activity of daily living and caregiver burden in spinal muscular atrophy. Neuromuscul. Disord. 29, 940–950 (2019).
Landfeldt, E. et al. Quality of life of patients with spinal muscular atrophy: a systematic review. Eur. J. Paediatr. Neurol. 23, 347–356 (2019).
Mercuri, E. E. A. Patient and parent oriented tools to assess health-related quality of life, activity of daily living and caregiver burden in SMA. Neuromuscul. Disord. 29, 940–950 (2020).
Pasternak, A. et al. Rasch analysis of the pediatric evaluation of disability inventory-computer adaptive test (PEDI-CAT) item bank for children and young adults with spinal muscular atrophy. Muscle Nerve 54, 1097–1107 (2016).
Mongiovi, P. et al. Patient Reported Impact of Symptoms in Spinal Muscular Atrophy (PRISM-SMA). Neurology 91, e1206–e1214 (2018).
Chen, T. H. New and developing therapies in spinal muscular atrophy: from genotype to phenotype to treatment and where do we stand? Int. J. Mol. Sci. 21, 3297 (2020).
Schorling, D. C., Pechmann, A. & Kirschner, J. Advances in treatment of spinal muscular atrophy — new phenotypes, new challenges, new implications for care. J. Neuromuscul. Dis. 7, 1–13 (2020).
Tizzano, E. F. & Finkel, R. S. Spinal muscular atrophy: a changing phenotype beyond the clinical trials. Neuromuscul. Disord. 27, 883–889 (2017).
National Institute for Health and Care Excellence. Nusinersin for treating spinal muscular atrophy. Technology appraisal guidance [TA588]. NICE https://www.nice.org.uk/guidance/ta588 (2019).
Yeo, C. J. J. & Darras, B. T. Overturning the paradigm of spinal muscular atrophy as just a motor neuron disease. Pediatr. Neurol. 109, 12–19 (2020). This paper presents a comprehensive review of the implications of peripheral SMN deficiency for patients with SMA.
Pechmann, A. et al. Treatment with nusinersen — challenges regarding the indication for children with SMA type 1. Neuromuscul. Dis. 7, 41–46 (2020).
Ziegler, A. et al. Recommendations for gene therapy of spinal muscular atrophy with onasemnogene abeparvovec-AVXS-101: consensus paper of the German representatives of the Society for Pediatric Neurology (GNP) and the German treatment centers with collaboration of the medical scientific advisory board of the German Society for Muscular Diseases (DGM)]. Nervenarzt. 91, 518–529 (2020).
Salazar, R. et al. Quantitative evaluation of lower extremity joint contractures in spinal muscular atrophy: implications for motor function. Pediatr. Phys. Ther. 30, 209–215 (2018).
Crawford, T. O. et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE 7, e33572 (2012).
Finkel, R. S. et al. Candidate proteins, metabolites and transcripts in the Biomarkers for Spinal Muscular Atrophy (BforSMA) clinical study. PLoS ONE 7, e35462 (2012).
Lee, Y., Lee, B. H., Yip, W., Chou, P. & Yip, B. S. Neurofilament proteins as prognostic biomarkers in neurological disorders. Curr. Pharm. Des. 25, 4560–4569 (2020).
Catapano, F. et al. Altered levels of microRNA-9, -206, and -132 in spinal muscular atrophy and their response to antisense oligonucleotide therapy. Mol. Ther. Nucleic Acids 5, e331 (2016).
Also-Rallo, E. et al. Treatment of spinal muscular atrophy cells with drugs that upregulate SMN expression reveals inter- and intra-patient variability. Eur. J. Hum. Genet. 19, 1059–1065 (2011).
Wilson, J. M. & Jungner, Y. G. Principles and practice of mass screening for disease. Bol. Oficina Sanit. Panam. 65, 281–393 (1968).
Health Resources and Services Administration. Newborn screening for spinal muscular atrophy. A summary of the evidence and advisory committee decision https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/rusp/previous-nominations/sma-consumer-summary.pdf (2018).
Glascock, J. et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J. Neuromuscul. Dis. 5, 145–158 (2018).
Glascock, J. et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J. Neuromuscul. Dis. 7, 97–100 (2020).
Schorling, D. C. et al. Discrepancy in redetermination of SMN2 copy numbers in children with SMA. Neurology 93, 267–269 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02644668 (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
E.M. is involved as an investigator in clinical trials for AveXis, Biogen, Roche and Scholar Rock. He has received honoraria for participating in symposia and advisory boards for AveXis, Biogen, Roche and Scholar Rock. His institution receives funding from Biogen for the coordination of an Italian registry for SMA, iSMAC. M.C.P. is involved as an investigator in clinical trials for AveXis, Biogen, Roche and Scholar Rock. She has received honoraria for participating in symposia and advisory boards for Biogen and Roche. Her institution receives funding from Biogen for the coordination of an Italian registry for SMA, iSMAC. M.S. is involved as an investigator in clinical trials for AveXis, Biogen and Roche. She has received honoraria for participating in symposia and advisory boards for AveXis, Biogen and Roche. Her institution receives funding from Biogen for the coordination of a UK-wide registry for SMA, SMA REACH UK. R.F. is involved as an investigator in clinical trials for AveXis, Biogen, Cytokinetics, Ionis, Roche and Scholar Rock. He has received honoraria for participating in symposia and advisory boards for AveXis, Biogen, Cytokinetics, Ionis, Roche and Scholar Rock. His institution receives funding from Biogen for the coordination of a US registry for SMA, iSMAC. F.M. is involved as an investigator in clinical trials for AveXis, Biogen and Roche. He has received honoraria for participating in symposia and advisory boards for AveXis, Biogen and Roche. His institution receives funding from Biogen for the coordination of a UK-wide registry for SMA, SMA REACH UK.
Additional information
Peer review information
Nature Reviews Neurology thanks J. Melki, H. Nishio, B. Wirth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mercuri, E., Pera, M.C., Scoto, M. et al. Spinal muscular atrophy — insights and challenges in the treatment era. Nat Rev Neurol 16, 706–715 (2020). https://doi.org/10.1038/s41582-020-00413-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00413-4
This article is cited by
-
Administration of adipose-derived stem cells extracellular vesicles in a murine model of spinal muscular atrophy: effects of a new potential therapeutic strategy
Stem Cell Research & Therapy (2024)
-
Optimized MLPA workflow for spinal muscular atrophy diagnosis: identification of a novel variant, NC_000005.10:g.(70919941_70927324)del in isolated exon 1 of SMN1 gene through long-range PCR
BMC Neurology (2024)
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases
Signal Transduction and Targeted Therapy (2024)
-
Large-scale whole-exome sequencing of neuropsychiatric diseases and traits in 350,770 adults
Nature Human Behaviour (2024)
-
Screening of Spinal Muscular Atrophy Carriers and Prenatal Diagnosis in Pregnant Women in Yancheng, China
Biochemical Genetics (2024)