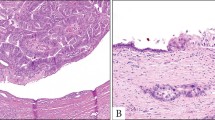
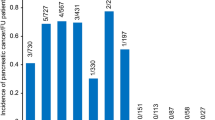
Abstract
Pancreatic cystic neoplasms (PCN) are a heterogeneous group of pancreatic cysts that include intraductal papillary mucinous neoplasms, mucinous cystic neoplasms, serous cystic neoplasms and other rare cystic lesions, all with different biological behaviours and variable risk of progression to malignancy. As more pancreatic cysts are incidentally discovered on routine cross-sectional imaging, optimal surveillance for patients with PCN is becoming an increasingly common clinical problem, highlighting the need to balance cancer prevention with the risk of (surgical) overtreatment. This Review summarizes the latest developments in the diagnosis and management of PCN, including the quality of available evidence. Also discussed are the most important differences between the PCN guidelines from the American Gastroenterological Association, the International Association of Pancreatology and the European Study Group on Cystic Tumours of the Pancreas, including diagnostic and follow-up strategies and indications for surgery. Finally, new developments in the management of patients with PCN are addressed.
Key points
-
Pancreatic cysts are increasingly diagnosed and, although most are benign, some can develop into pancreatic cancer; uniform guidelines for diagnosis, treatment and follow-up of pancreatic cysts are therefore urgently required.
-
In revised guidelines, obstructive jaundice, a contrast-enhanced mural nodule or solid component ≥5 mm, a dilated pancreatic duct or positive cytology for advanced neoplasia are absolute indications for resection in patients with intraductal papillary mucinous neoplasms (IPMN).
-
In European guidelines, both a mucinous cystic neoplasm (MCN) and IPMN <40 mm are treated conservatively when other risk factors are absent.
-
In international and American guidelines, an MCN of any size is an absolute indication for resection; in the international guidelines, an IPMN >30 mm is a relative indication for resection.
-
Lifelong surveillance is indicated for patients with IPMN and MCN without risk factors, as long as they are fit and willing to undergo surgery.
-
Follow-up for IPMN after pancreatectomy is warranted because of the risk of developing recurrent disease, although evidence on the best surveillance interval is lacking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tanaka, M. et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12, 183–197 (2012). An update to the 2006 guidelines, in which extensive research leads to new insights and the dichotomization of risk stratification (high-risk stigmata and worrisome features), recommending immediate resection in the case of high-risk features and a conservative approach in the case of worrisome features.
Del Chiaro, M. et al. European Experts Consensus Statement on cystic tumours of the pancreas. Dig. Liver. Dis. 45, 703–711 (2013). The European response to the Tanaka et al. (2012) guidelines, distinguishing absolute and relative indications for surgery and simplifying the surveillance intervals to 6 months in the first year and yearly afterwards.
Tanaka, M. et al. Revisions of International Consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17, 738–753 (2017). This article gives minor revisions and updates to the International Association of Pancreatology guideline according to the recent literature.
European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67, 789–804 (2018). This is the first evidence-based guideline on management of PCN, in which growth rate >5 mm per year, new-onset diabetes mellitus and acute pancreatitis caused IPMN were added to the list of relative indications for resection.
Ikeda, M. et al. Morphologic changes in the pancreas detected by screening ultrasonography in a mass survey, with special reference to main duct dilatation, cyst formation, and calcification. Pancreas 9, 508–512 (1994).
Laffan, T. A. et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am. J. Roentgenol. 191, 802–807 (2008).
de Jong, K. et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 8, 806–811 (2010).
Zhang, X. M., Mitchell, D. G., Dohke, M., Holland, G. A. & Parker, L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 223, 547–553 (2002).
Lee, K. S., Sekhar, A., Rofsky, N. M. & Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 105, 2079–2084 (2010).
Kromrey, M. L. et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 67, 138–145 (2018). This is a prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts showing that the prevalence of pancreatic cysts in the general population is 49.1% and that prevalence, number and maximum size of pancreatic cysts increases significantly with the age of the patients.
Kimura, W., Nagai, H., Kuroda, A., Muto, T. & Esaki, Y. Analysis of small cystic lesions of the pancreas. Int. J. Pancreatol. 18, 197–206 (1995).
Zaheer, A., Pokharel, S. S., Wolfgang, C., Fishman, E. K. & Horton, K. M. Incidentally detected cystic lesions of the pancreas on CT: review of literature and management suggestions. Abdom. Imaging 38, 331–341 (2013).
Capurso, G. et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am. J. Gastroenterol. 108, 1003–1009 (2013).
Lee, S. Y. et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J. Gastroenterol. Hepatol. 20, 1379–1384 (2005).
Crippa, S. et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin. Gastroenterol. Hepatol. 8, 213–219 (2010).
Hwang, D. W. et al. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch. Surg. 397, 93–102 (2012).
Salvia, R. et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann. Surg. 239, 678–685; discussion 685–677 (2004).
Ohno, E. et al. Natural history of pancreatic cystic lesions: a multicenter prospective observational study for evaluating the risk of pancreatic cancer. J. Gastroenterol. Hepatol. 33, 320–328 (2018).
Nagai, K. et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J. Surg. 32, 271–278; discussion 279–280 (2008).
Ridtitid, W. et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest. Endosc. 84, 436–445 (2016).
Moris, M. et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology, carcinoembryonic antigen, and amylase in intraductal papillary mucinous neoplasm. Pancreas 45, 870–875 (2016).
Jang, J. Y. et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann. Surg. Oncol. 15, 199–205 (2008).
Kanno, A. et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J. Gastroenterol. 45, 952–959 (2010).
Rodriguez, J. R. et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 133, 72–79 (2007).
Schmidt, C. M. et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann. Surg. 246, 644–651; discussion 651–644. (2007).
Waters, J. A. et al. CT vs MRCP: optimal classification of IPMN type and extent. J. Gastrointest. Surg. 12, 101–109 (2008).
Suzuki, Y. et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 28, 241–246 (2004).
Schnelldorfer, T. et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch. Surg. 143, 639–646; discussion 646 (2008).
Kim, S. C. et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J. Hepatobiliary Pancreat. Surg. 15, 183–188 (2008).
Ohno, E. et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann. Surg. 249, 628–634 (2009).
Nara, S. et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas 38, 8–16 (2009).
Marchegiani, G. et al. IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann. Surg. 261, 976–983 (2015).
Crippa, S. et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut 66, 495–506 (2017).
Tanno, S. et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 10, 173–178 (2010).
Thornton, G. D. et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 13, 48–57 (2013).
Felsenstein, M. et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut 67, 1652–1662 (2018).
Jang, K. T. et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. Am. J. Surg. Pathol. 39, 179–187 (2015).
Zamboni, G. et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am. J. Surg. Pathol. 23, 410–422 (1999).
Sarr, M. G. et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann. Surg. 231, 205–212 (2000).
Reddy, R. P. et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin. Gastroenterol. Hepatol. 2, 1026–1031 (2004).
Goh, B. K. et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J. Surg. 30, 2236–2245 (2006).
Park, J. W. et al. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology 14, 131–136 (2014).
Lee, S. E., Jang, J. Y., Hwang, D. W., Park, K. W. & Kim, S. W. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch. Surg. 143, 1218–1221 (2008).
Koh, Y. X., Chok, A. Y., Zheng, H. L., Tan, C. S. & Goh, B. K. A systematic review and meta-analysis of the clinicopathologic characteristics of cystic versus solid pancreatic neuroendocrine neoplasms. Surgery 156, 83–96.e2 (2014).
Patra, K. C., Bardeesy, N. & Mizukami, Y. Diversity of precursor lesions for pancreatic cancer: the genetics and biology of intraductal papillary mucinous neoplasm. Clin. Transl. Gastroenterol. 8, e86 (2017).
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Howlader, N. SEER cancer statistics review, 1975–2011. NIH http://seer.cancer.gov/csr/1975_2011/ (2014).
Vege, S. S. et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148, 819–822 (2015).These American Gastroenterological Association guidelines suggest that patients with both a solid component and a dilated pancreatic duct and/or cytology positive for malignancy should undergo surgery to reduce the risk of mortality from invasive cancer; these guidelines have led to discussion owing to their recommendation to discontinue surveillance in the case of no significant change in the cyst during 5 years follow-up.
Hruban R. H. et al. in WHO Classification Tumours Digestive System 4th edn (eds Bosman F. T., Carneiro F. & Hruban R. H.) 280–330 (International Agency for Research on Cancer, 2010).
Bosman F. T., Carneiro F. & Hruban R. H. (eds) WHO Classification Tumours Digestive System (International Agency for Research on Cancer, 2010).
Basturk, O. et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am. J. Surg. Pathol. 39, 1730–1741 (2015).
Furukawa, T. et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 447, 794–799 (2005).
Koh, Y. X. et al. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery 157, 496–509 (2015).
Schaberg, K. B., DiMaio, M. A. & Longacre, T. A. Intraductal papillary mucinous neoplasms often contain epithelium from multiple subtypes and/or are unclassifiable. Am. J. Surg. Pathol. 40, 44–50 (2016).
Adsay, V. et al. Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of Verona consensus meeting. Ann. Surg. 263, 162–177 (2016).
Tsutsumi, K. et al. A history of acute pancreatitis in intraductal papillary mucinous neoplasms of the pancreas is a potential predictive factor for malignant papillary subtype. Pancreatology 10, 707–712 (2010).
Ringold, D. A. et al. Pancreatitis is frequent among patients with side-branch intraductal papillary mucinous neoplasia diagnosed by EUS. Gastrointest. Endosc. 70, 488–494 (2009).
Pelletier, A. L. et al. Acute pancreatitis in patients operated on for intraductal papillary mucinous neoplasms of the pancreas: frequency, severity, and clinicopathologic correlations. Pancreas 39, 658–661 (2010).
Sodickson, A. et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 251, 175–184 (2009).
Berland, L. L. et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 7, 754–773 (2010).
Pilleul, F. et al. Preoperative evaluation of intraductal papillary mucinous tumors performed by pancreatic magnetic resonance imaging and correlated with surgical and histopathologic findings. J. Magn. Reson. Imaging 21, 237–244 (2005).
Sugiyama, M. & Atomi, Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann. Surg. 228, 685–691 (1998).
Postlewait, L. M. et al. Association of preoperative risk factors with malignancy in pancreatic mucinous cystic neoplasms: a multicenter study. JAMA Surg. 152, 19–25 (2017). This multicentre retrospective study identifies HGD or invasive cancer to be present in 14.9% of resected MCN, for which risks include gender, pancreatic head and neck location, larger size, solid component or nodules, and duct dilatation.
Keane, M. G. et al. Risk of malignancy in resected pancreatic mucinous cystic neoplasms. Br. J. Surg. 105, 439–446 (2018).
Kimura, W. et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 41, 380–387 (2012).
Dietrich, C. F. et al. Serous pancreatic neoplasia, data and review. World J. Gastroenterol. 23, 5567–5578 (2017).
Leite, I. et al. Unilocular macrocystic serous cystadenoma of the pancreas-atypical features: a case report. Clin. Imaging 38, 336–339 (2014).
Papavramidis, T. & Papavramidis, S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J. Am. Coll. Surg. 200, 965–972 (2005).
Ligneau, B. et al. Cystic endocrine tumors of the pancreas: clinical, radiologic, and histopathologic features in 13 cases. Am. J. Surg. Pathol. 25, 752–760 (2001).
Lewis, R. B., Lattin, G. E. Jr. & Paal, E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics 30, 1445–1464 (2010).
Rastegar, N. et al. Incremental value of secretin-enhanced magnetic resonance cholangiopancreatography in detecting ductal communication in a population with high prevalence of small pancreatic cysts. Eur. J. Radiol. 84, 575–580 (2015).
Chey, W. Y. & Chang, T. M. Secretin, 100 years later. J. Gastroenterol. 38, 1025–1035 (2003).
Carbognin, G. et al. Collateral branches IPMTs: secretin-enhanced MRCP. Abdom. Imaging 32, 374–380 (2007).
Akisik, M. F. et al. Dynamic secretin-enhanced MR cholangiopancreatography. Radiographics 26, 665–677 (2006).
Yamashita, Y. et al. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J. Ultrasound Med. 32, 61–68 (2013).
Fujita, M. et al. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc. Ultrasound 5, 377–383 (2016).
Yamamoto, N. et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy 48, 26–34 (2016).
Kamata, K. et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy 48, 35–41 (2016).
Fusaroli, P. et al. Contrast harmonic-endoscopic ultrasound is useful to identify neoplastic features of pancreatic cysts (with videos). Pancreas 45, 265–268 (2016).
Marchegiani, G. et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 International Guidelines on IPMN of the pancreas. Surgery 163, 1272–1279 (2018). This meta-analysis includes 70 studies with 2,297 resected IPMN and reports a positive predictive value of an enhancing mural nodule on contrast-enhanced EUS of 62% for the presence of advanced neoplasia at final pathology.
Ahmad, N. A. et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest. Endosc. 58, 59–64 (2003).
Fusaroli, P. et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J. Gastroenterol. Hepatol. 27, 1063–1069 (2012).
Krishna, S. G. et al. Validation of diagnostic characteristics of needle based confocal laser endomicroscopy in differentiation of pancreatic cystic lesions. Endosc. Int. Open 4, E1124–E1135 (2016).
Nakai, Y. et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest. Endosc. 81, 1204–1214 (2015).
Napoleon, B. et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy 47, 26–32 (2015).
Modi, R. M., Swanson, B., Muscarella, P. 2nd, Conwell, D. L. & Krishna, S. G. Novel techniques for diagnosis of serous cystadenoma: fern pattern of vascularity confirmed by in vivo and ex vivo confocal laser endomicroscopy. Gastrointest. Endosc. 85, 258–259 (2017).
Konda, V. J. et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 45, 1006–1013 (2013).
Modi, R. M., Kamboj, A. K., Swanson, B., Conwell, D. L. & Krishna, S. G. Novel technique for diagnosis of mucinous cystic neoplasms: in vivo and ex vivo confocal laser endomicroscopy. VideoGIE 2, 55–56 (2017).
Napoleon, B. et al. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg. Endosc. 30, 2603–2612 (2016).
Dumonceau, J. M. et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline — updated January 2017. Endoscopy 49, 695–714 (2017).
Polkowski, M. et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline – March 2017. Endoscopy 49, 989–1006 (2017).
Yoon, W. J. et al. Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE study. Endoscopy 46, 382–387 (2014).
Leung, K. K. et al. Pancreatic cystic neoplasm: the role of cyst morphology, cyst fluid analysis, and expectant management. Ann. Surg. Oncol. 16, 2818–2824 (2009).
Bick, B. L. et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy 47, 626–631 (2015).
Oh, S. H. et al. The combination of cyst fluid carcinoembryonic antigen, cytology and viscosity increases the diagnostic accuracy of mucinous pancreatic cysts. Gut Liver 11, 283–289 (2017).
Khamaysi, I. et al. Differentiation of pancreatic cyst types by analysis of rheological behavior of pancreatic cyst fluid. Sci. Rep. 7, 45589 (2017).
Pitman, M. B. et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 118, 434–440 (2010).
Genevay, M. et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann. Surg. 254, 977–983 (2011).
Hoda, R. S., Lu, R., Arpin, R. N. 3rd, Rosenbaum, M. W. & Pitman, M. B. Risk of malignancy in pancreatic cysts with cytology of high-grade epithelial atypia. Cancer Cytopathol. 126, 773–781 (2018).
Mittal, C. et al. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest. Endosc. 87, 1263–1269 (2018).
Attili, F. et al. Endoscopic ultrasound-guided histological diagnosis of a mucinous non-neoplastic pancreatic cyst using a specially designed through-the-needle microforceps. Endoscopy 48 Suppl 1, E188–E189 (2016).
Pham, K. D., Engjom, T., Gjelberg Kollesete, H. & Helgeland, L. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle micro forceps. Endoscopy 48 Suppl 1, E125–E126 (2016).
Basar, O. et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest. Endosc. 88, 79–86 (2018).
Carethers, J. B. C. in Textbook of Gastroenterology Vol. 1 (eds Yamada T. et al) (Lippincott, Williams and Wilkins, 1999).
Brugge, W. R. et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 126, 1330–1336 (2004).
van Huijgevoort, N. C. M. et al. Su1347 — the diagnostic accuracy of carcinoembryonic antigen in differentiating mucinous and non-mucinous pancreatic cystic neoplasms — a systematic review and individual patient data meta-analysis. Gastroenterology 154 (Suppl. 1), S-528 (2018).
Al-Rashdan, A. et al. Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience. J. Gastrointest. Oncol. 2, 208–214 (2011).
van der Waaij, L. A., van Dullemen, H. M. & Porte, R. J. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest. Endosc. 62, 383–389 (2005).
Park, W. G. et al. Metabolomics derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest. Endosc. 78, 295–302.e2 (2013).
Zikos, T. et al. Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts. Am. J. Gastroenterol. 110, 909–914 (2015).
Carr, R. A. et al. Pancreatic cyst fluid glucose: rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery 163, 600–605 (2018).
Wu, J. et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl Acad. Sci. U.S.A. 108, 21188–21193 (2011).
Singhi, A. D. et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 67, 2131–2141 (2018). This prospective study evaluates preoperative pancreatic cyst fluid DNA testing and shows that preoperative next-generation sequencing of pancreatic cyst fluid for KRAS or GNAS mutations is highly sensitive for IPMN and specific for MCN.
Nikiforova, M. N. et al. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod. Pathol. 26, 1478–1487 (2013).
Singhi, A. D. et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin. Cancer Res. 20, 4381–4389 (2014).
Wu, J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl Med. 3, 92ra66 (2011).
Yu, J. et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 66, 1677–1687 (2017).
Jones, M. et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest. Endosc. 83, 140–148 (2016).
Pea, A. et al. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann. Surg. 266, 133–141 (2017).
Kanda, M. et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin. Gastroenterol. Hepatol. 11, 719–730.e5 (2013).
Schonleben, F. et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin. Cancer Res. 12, 3851–3855 (2006).
Garcia-Carracedo, D. et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin. Cancer Res. 19, 6830–6841 (2013).
Garcia-Carracedo, D. et al. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas 43, 245–249 (2014).
Del Chiaro, M. et al. Main duct dilatation is the best predictor of high-grade dysplasia or invasion in intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003174 (2019).
Hackert, T. et al. Main-duct intraductal papillary mucinous neoplasm: high cancer risk in duct diameter of 5 to 9 mm. Ann. Surg. 262, 875–880; discussion 880–881 (2015).
Del Chiaro, M. & Schulick, R. D. Main-duct intraductal papillary mucinous neoplasm. High cancer risk in duct diameter of 5 to 9 mm. Ann. Surg. 266, e86 (2017).
Canto, M. I. & Hruban, R. H. Managing pancreatic cysts: less is more? Gastroenterology 148, 688–691 (2015).
Fernandez-Del Castillo, C. & Tanaka, M. Management of pancreatic cysts: the evidence is not here yet. Gastroenterology 148, 685–687 (2015).
Crippa, S. et al. Active surveillance beyond 5 years is required for presumed branch-duct intraductal papillary mucinous neoplasms undergoing non-operative management. Am. J. Gastroenterol. 112, 1153–1161 (2017).
Lawrence, S. A. et al. Should patients with cystic lesions of the pancreas undergo long-term radiographic surveillance? Results of 3024 patients evaluated at a single institution. Ann. Surg. 266, 536–544 (2017).
Brook, O. R. et al. Delayed growth in incidental pancreatic cysts: are the current American College of Radiology recommendations for follow-up appropriate? Radiology 278, 752–761 (2016).
Netherlands Trial Register. TrialRegister.nl https://www.trialregister.nl/trial/4365 (2019).
Han, Y. et al. Progression of pancreatic branch duct intraductal papillary mucinous neoplasm associates with cyst size. Gastroenterology 154, 576–584 (2018).
Jais, B. et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumours of the Pancreas). Gut 65, 305–312 (2016).
Falconi, M. et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103, 153–171 (2016).
Partelli, S. et al. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br. J. Surg. 104, 34–41 (2017).
Netherlands Trial Register. TrialRegister.nl https://www.trialregister.nl/trial/6510 (2019).
Palanivelu, C. et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 104, 1443–1450 (2017).
Poves, I. et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann. Surg. 268, 731–739 (2018).
van Hilst, J. et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 4, 199–207 (2019).
de Rooij, T. et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann. Surg. 269, 2–9 (2019).
Allen, P. J. et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann. Surg. 244, 572–582 (2006).
Clancy, T. E. Surgery for pancreatic cancer. Hematol. Oncol. Clin. North Am. 29, 701–716 (2015).
McPhee, J. T. et al. Perioperative mortality for pancreatectomy: a national perspective. Ann. Surg. 246, 246–253 (2007).
Amini, N., Spolverato, G., Kim, Y. & Pawlik, T. M. Trends in hospital volume and failure to rescue for pancreatic surgery. J. Gastrointest. Surg. 19, 1581–1592 (2015).
Goudard, Y. et al. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg. 149, 356–363 (2014).
Iacono, C. et al. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br. J. Surg. 100, 873–885 (2013).
Xu, S. B., Zhu, Y. P., Zhou, W., Xie, K. & Mou, Y. P. Patients get more long-term benefit from central pancreatectomy than distal resection: a meta-analysis. Eur. J. Surg. Oncol. 39, 567–574 (2013).
Xiao, W. et al. The role of central pancreatectomy in pancreatic surgery: a systematic review and meta-analysis. HPB 20, 896–904 (2018).
Nilsson, L. N. et al. Nature and management of pancreatic mucinous cystic neoplasm (MCN): a systematic review of the literature. Pancreatology 16, 1028–1036 (2016).
Lekkerkerker, S. J. et al. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest. Endosc. 85, 1025–1031 (2017).
Singhi, A. D. et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest. Endosc. 83, 1107–1117.e2 (2016).
Scholten, L. et al. Surgical management of intraductal papillary mucinous neoplasm with main duct involvement: an international expert survey and case-vignette study. Surgery. 164, 17–23 (2018).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37 (Suppl. 1), S81–S90 (2014).
Hart, P. A. et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 1, 226–237 (2016).
Scholten, L. et al. New-onset diabetes after pancreatoduodenectomy: a systematic review and meta-analysis. Surgery. 164, 6–16 (2018).
El-Khatib, F. H. et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 389, 369–380 (2017).
Blauw, H., van Bon, A. C., Koops, R. & DeVries, J. H., PCDIAB consortium. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes. Metab. 18, 671–677 (2016).
Castle, J. R. et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 33, 1282–1287 (2010).
El-Khatib, F. H., Jiang, J. & Damiano, E. R. Adaptive closed-loop control provides blood-glucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic Swine. J. Diabetes Sci. Technol. 1, 181–192 (2007).
Haidar, A. et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 185, 297–305 (2013).
Castle, J. R., Engle, J. M., El Youssef, J., Massoud, R. G. & Ward, W. K. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J. Diabetes Sci. Technol. 4, 1305–1310 (2010).
Weinzimer, S. A. et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 35, 1994–1999 (2012).
Russell, S. J. et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N. Engl. J. Med. 371, 313–325 (2014).
Fazlalizadeh, R. et al. Total pancreatectomy and islet autotransplantation: a decade nationwide analysis. World J. Transpl. 6, 233–238 (2016).
Lohr, J. M., Oliver, M. R. & Frulloni, L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European Gastroenterol. J. 1, 79–83 (2013).
Gianotti, L. et al. Nutritional support and therapy in pancreatic surgery: a position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 164, 1035–1048 (2018).
Imrie, C. W., Connett, G., Hall, R. I. & Charnley, R. M. Review article: enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment. Pharmacol. Ther. 32 Suppl 1, 1–25 (2010).
Tamura, K. et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: a retrospective review. Ann. Surg. 259, 360–368 (2014).
Yamaguchi, J. et al. Positive surgical margins in surgically treated unifocal and multifocal IPMN. Int. J. Surg. 28, 51–55 (2016).
Fujii, T. et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery 148, 285–290 (2010).
Park, J. et al. Risk factors associated with the postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 40, 46–51 (2011).
Nara, S. et al. Clinical significance of frozen section analysis during resection of intraductal papillary mucinous neoplasm: should a positive pancreatic margin for adenoma or borderline lesion be resected additionally? J. Am. Coll. Surg. 209, 614–621 (2009).
Landa, J., Allen, P., D’Angelica, M. & Schwartz, L. H. Recurrence patterns of intraductal papillary mucinous neoplasms of the pancreas on enhanced computed tomography. J. Comput. Assist. Tomogr. 33, 838–843 (2009).
Leng, K. M., Wang, Z. D., Zhao, J. B., Cui, Y. F. & Zhong, X. Y. Impact of pancreatic margin status and lymph node metastases on recurrence after resection for invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Dig. Surg. 29, 213–225 (2012).
Moriya, T. & Traverso, W. Fate of the pancreatic remnant after resection for an intraductal papillary mucinous neoplasm: a longitudinal level II cohort study. Arch. Surg. 147, 528–534 (2012).
Kang, M. J. et al. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann. Surg. 260, 356–363 (2014).
Yokoyama, Y. et al. Clinicopathologic features of re-resected cases of intraductal papillary mucinous neoplasms (IPMNs). Surgery 142, 136–142 (2007).
Cuillerier, E. et al. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am. J. Gastroenterol. 95, 441–445 (2000).
He, J. et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J. Am. Coll. Surg. 216, 657–667 (2013).
Rezaee, N. et al. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB 18, 236–246 (2016).
Uehara, H. et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 57, 1561–1565 (2008).
Yamaguchi, K. et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40, 571–580 (2011).
Al Efishat, M. et al. Progression patterns in the remnant pancreas after resection of non-invasive or micro-invasive intraductal papillary mucinous neoplasms (IPMN). Ann. Surg. Oncol. 25, 1752–1759 (2018).
Blackham, A. U. et al. Patterns of recurrence and long-term outcomes in patients who underwent pancreatectomy for intraductal papillary mucinous neoplasms with high grade dysplasia: implications for surveillance and future management guidelines. HPB 19, 603–610 (2017).
Yan, L. et al. A large multicenter study of recurrence after surgical resection of branch-duct intraductal papillary mucinous neoplasm of the pancreas. Minerva Gastroenterol. Dietol. 63, 50–54 (2017).
Winter, J. M. et al. Recurrence and survival after resection of small intraductal papillary mucinous neoplasm-associated carcinomas (</=20-mm invasive component): a multi-institutional analysis. Ann. Surg. 263, 793–801 (2016).
Marchegiani, G. et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann. Surg. 262, 1108–1114 (2015).
Yogi, T. et al. Risk factors for postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas based on a long-term follow-up study: proposals for follow-up strategies. J. Hepatobiliary Pancreat. Sci. 22, 757–765 (2015).
Xourafas, D., Tavakkoli, A., Clancy, T. E. & Ashley, S. W. Noninvasive intraductal papillary mucinous neoplasms and mucinous cystic neoplasms: recurrence rates and postoperative imaging follow-up. Surgery 157, 473–483 (2015).
Yuan, C. et al. Data analysis of 36 cases with intraductal papillary mucinous neoplasm of the pancreas for their clinicopathological features, diagnosis, and treatment. Chin. Med. J. 127, 4087–4091 (2014).
Frankel, T. L. et al. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB 15, 814–821 (2013).
Winner, M. et al. Predictors of recurrence in intraductal papillary mucinous neoplasm: experience with 183 pancreatic resections. J. Gastrointest. Surg. 17, 1618–1626 (2013).
Passot, G. et al. Recurrences after surgical resection of intraductal papillary mucinous neoplasm of the pancreas: a single-center study of recurrence predictive factors. Pancreas 41, 137–141 (2012).
Miller, J. R. et al. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB 13, 759–766 (2011).
White, R. et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J. Am. Coll. Surg. 204, 987–993; discussion 993–995 (2007).
Raut, C. P. et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann. Surg. Oncol. 13, 582–594 (2006).
Ducreux, M. et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26 Suppl 5, v56–v68 (2015).
Kaiser, J. et al. Enucleation: a treatment alternative for branch duct intraductal papillary mucinous neoplasms. Surgery 161, 602–610 (2017).
Oh, H. C. et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 140, 172–179 (2011).
Gan, S. I., Thompson, C. C., Lauwers, G. Y., Bounds, B. C. & Brugge, W. R. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest. Endosc. 61, 746–752 (2005).
DeWitt, J., McGreevy, K., Schmidt, C. M. & Brugge, W. R. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest. Endosc. 70, 710–723 (2009).
Moyer, M. T. et al. The safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology 153, 1295–1303 (2017).
Choi, J. H. et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy 49, 866–873 (2017).
Oh, H. C. & Seo, D. W. Endoscopic ultrasonography-guided pancreatic cyst ablation (with video). J. Hepatobiliary Pancreat. Sci. 22, 16–19 (2015).
Ho, K. Y. & Brugge, W. R., EUS 2008 Working Group. EUS 2008 Working Group document: evaluation of EUS-guided pancreatic-cyst ablation. Gastrointest. Endosc. 69 (Suppl. 2), S22–S27 (2009).
Gomez, V. et al. EUS-guided ethanol lavage does not reliably ablate pancreatic cystic neoplasms (with video). Gastrointest. Endosc. 83, 914–920 (2016).
DeWitt, J., DiMaio, C. J. & Brugge, W. R. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest. Endosc. 72, 862–866 (2010).
Castellano-Megias, V. M., Andres, C. I., Lopez-Alonso, G. & Colina-Ruizdelgado, F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J. Gastrointest. Oncol. 6, 311–324 (2014).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and provided a substantial contribution to discussion of the content. N.C.M.v.H. wrote the article. M.d.C., C.L.W., J.E.v.H. and M.G.B. contributed to reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed using the terms “pancreatic cystic neoplasm” combined with “classification”, “diagnosis”, “endoscopic ultrasound”, “cyst fluid analysis”, “guidelines”, “treatment”, “surgery”, “recurrence” and “surveillance”. We selected full-text articles in English published in the previous 10 years, but exceptions were made for older, highly cited papers. We aimed to describe the results of prospective studies, since randomized controlled trials are lacking in this field, but other studies are also referenced. In addition, references list of the finally included articles were checked manually for studies that had not been identified by the primary search. Some recommendations are based on the recently revised guidelines of the International Association of Pancreatology3 and the European Study Group on Cystic Tumours of the Pancreas4.
Supplementary information
Rights and permissions
About this article
Cite this article
van Huijgevoort, N.C.M., del Chiaro, M., Wolfgang, C.L. et al. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol 16, 676–689 (2019). https://doi.org/10.1038/s41575-019-0195-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0195-x
This article is cited by
-
Clinical characteristics and survival prediction of surgical patients with invasive pancreatic cystic neoplasm: a large retrospective study over two decades
World Journal of Surgical Oncology (2023)
-
Limitations and prospects in the management of IPMN: a retrospective, single-center observational study
BMC Surgery (2023)
-
Short-term clinical outcomes of laparoscopic duodenum-preserving pancreatic head resection for the management of pancreatic-head cystic neoplasms
BMC Surgery (2023)
-
Clinical outcomes of minimally invasive duodenum-preserving pancreatic head resection
BMC Surgery (2023)
-
Cyst fluid glycoproteins accurately distinguishing malignancies of pancreatic cystic neoplasm
Signal Transduction and Targeted Therapy (2023)