Abstract
Although many studies support the association of obesity with neurodegenerative diseases, such as Parkinson’s disease (PD), there are limited data regarding the association between abdominal obesity and PD, with mixed findings. The aim of this study was to examine the association of waist circumference (WC) with the risk of PD incidence. We retrospectively analyzed a large-scale nationwide cohort of 6,925,646 individuals aged ≥40 years who underwent the Korean National Health Screening during 2009. We performed multivariable Cox proportional hazards regression to evaluate the association of WC and abdominal obesity with PD risk and calculated hazard ratios (HRs) with 95% confidence intervals (CIs) of PD incidence. During a median follow-up period of 8.35 years, 33,300 cases of PD developed. PD incidence was positively associated with increases in WC (P for trend < 0.001). The risk of PD incidence tended to elevate as WC increased (P for trend < 0.001), indicating that the adjusted HRs of PD incidence in the highest WC group versus the reference group was 1.16 (95% CI, 1.10–1.23), whereas it was 0.91 (95% CI 0.84–0.98) in the lowest WC group. Individuals with abdominal obesity were significantly associated with an increased PD risk (HR 1.10, 95% CI: 1.07–1.13). These associations persisted even after adjustment for body mass index and stratification by sex. Even among non-obese individuals, abdominal obesity was associated with a higher PD risk (adjusted HR 1.13, 95% CI: 1.09–1.18). Taken together, higher WC and abdominal obesity were associated with increased PD risk. Even in non-obese individuals, abdominal obesity was associated with an increased PD risk.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease following Alzheimer’s disease and affects 6.1 million individuals worldwide, as estimated in 20161. With the increasing aging population, environmental changes, and improved survival of patients with PD, the prevalence of PD is growing fast among all neurologic disorders globally2,3. In South Korea, there has been an upward trend in the age-standardized PD incidence (increasing from 13.6 in 2004 to 26.9 per 100,000 person-years in 2013), with approximately 111,311 individuals affected by PD in 20203.
The precise mechanism of neurodegeneration in PD is not yet fully understood, while aging is known to be the primary risk factor of PD4,5. Several environmental toxins, behavioral factors, and cardiometabolic parameters have also been reported as key factors affecting dopaminergic neuronal cell death at the substantia nigra through oxidative stress, mitochondrial dysfunction, protein misfolding, and some inflammatory mechanisms5,6,7. Of these, adiposity might be an underlying etiologic factor for age-related neurodegenerative pathologies of PD, since it plays a role in the depletion of striatal dopamine receptor availability8. The deleterious effect of general obesity (measured by body mass index [BMI]) on PD risk has been demonstrated in several epidemiologic studies;9,10,11 however, other studies reported opposite results or even null associations12,13,14,15,16,17,18. Moreover, studies using anthropometric parameters other than BMI are relatively limited16,19,20.
In light of abdominal obesity, Chen et al. prospectively examined two large cohorts of men and women in the United States (US), and concluded that a greater waist circumference (WC) was associated with future development of PD among never smokers20. Although another US-based cohort study showed null association between WC and PD risk, a European multi-center cohort study in 2019 revealed that female smokers had a 64% increase in the risk of PD development per 10 cm increase in WC16,19. Investigations on the association between central adiposity and PD risk have provided a closer look on the mechanism of PD through the perspective of the effect of adiposity-related inflammation on neurodegeneration. WC is known to be a more reliable parameter for abdominal and visceral accumulation of adipose tissue than BMI. However, most of the previous epidemiologic studies on this issue used European or North American data16,19,20 and some studies relied on self-measured WC data16,19. Although several studies now suggest a potential link between obesity and pathology of neurodegeneration, these few and contradictory findings on WC and PD development prompted us to assess the impact of central adiposity on PD risk. Therefore, we aimed to examine the association between WC, abdominal obesity, and PD risk using the large-scale cohort data of the South Korean population.
Results
Baseline characteristics
The proportions of participants according to the five WC categories were as follows: 2.9% (in group of <70 cm in males, <65 cm in females), 27.3% (70–80 cm in males, 65–75 cm in females), 47.2% (80–90 cm in males, 75–85 cm in females), 19.5% (90–100 cm in males, 85–95 cm in females), and 3.1% (≥100 cm in males, ≥95 cm in females), respectively. Table 1 shows the baseline clinical characteristics of the study population according to WC categories. Individuals in the larger WC levels were older (P < 0.001), and the distributions of income level and lifestyle habits, such as smoking status, alcohol consumption, and physical activity, were significantly different among WC categories (P < 0.001). The mean values of cardiometabolic parameters, such as BMI, systolic and diastolic blood pressures, total cholesterol, low-density lipoprotein cholesterol, and triglycerides were higher in individuals with greater WC categories (P < 0.001). Individuals in the larger WC categories were more likely to have comorbidities, such as obesity, hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease (P < 0.001).
Associations of WC and abdominal obesity with PD incidence
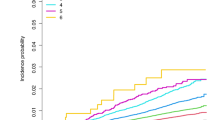
A total of 33,300 (0.48%) individuals were diagnosed with PD during the follow-up. Figure 1 shows the Kaplan–Meier curves for incidence probabilities of PD with respect to WC categories and abdominal obesity. The incidence probabilities significantly increased in the higher WC categories in total, male, and female participants (all log-rank P-value < 0.001). The plot showed that the cumulative PD incidence increased in total, male, and female participants with abdominal obesity compared with each group without abdominal obesity (all log-rank P-value < 0.001). Table 2 presents the adjusted hazard ratios (HRs) (95% confidence intervals [CIs]) of PD in five WC categories. Incrementally higher HRs of PD were observed with higher WC categories in the total, male, and female population irrespective of adjustment for confounding variables (all P for trend < 0.001). In total participants, compared with the second highest level of WC (70–80 cm in males and 65–75 cm in females) as the reference group, the smallest WC group was associated with a 9% lower PD risk (HR: 0.91, 95% CI: 0.84–0.98), and the largest WC category was associated with a 16% higher PD risk (1.16, 95% CI: 1.10–1.23). In male and female participants, the adjusted HRs of PD in model 2 were 25% (HR: 1.25, 95% CI: 1.14–1.36) and 15% (1.15, 95% CI: 1.07–1.23) higher in the largest WC group than in the reference groups. These associations remained significant after adjusting for baseline BMI.
Table 3 shows the adjusted HRs (95% CIs) of PD in the individuals with abdominal obesity compared with that of those without abdominal obesity. After adjusting for potential confounding variables (model 2), the HRs for PD increased significantly among individuals with abdominal obesity compared with those without in total (HR: 1.09, 95% CI: 1.07–1.13), male (1.11, 95% CI: 1.07–1.15), and female participants (1.10, 95% CI: 1.07–1.14). These associations persisted even after further adjusting for BMI (model 3).
Subgroup analysis
Figure 2 shows the associations between abdominal obesity and PD risk in subgroups stratified by age, smoking status, BMI, hypertension, and diabetes mellitus. The associations interacted with age differently between males and females. Positive associations between abdominal obesity and PD risk were stronger among males aged ≥65 years than those aged <65 years, while the associations were stronger among females aged <65 years than those aged ≥65 years. There was a significant interaction with smoking status in the association of abdominal obesity with PD risk in female individuals (P for interaction = 0.023), and the association was stronger in those currently smoking than those who were not. Although increased PD risks in abdominal obesity were observed regardless of the presence of general obesity based on BMI ≥ 25 kg/m2, there was no significant interaction with respect to obesity status. The association between abdominal obesity and PD risk was stronger in the total and female populations without hypertension than in those with hypertension (P for interaction < 0.001), and the association was stronger in females without diabetes mellitus than in those with diabetes mellitus (P for interaction = 0.048).
Associations between abdominal obesity and the risk of Parkinson's disease in subgroups stratified by age, smoking status, BMI, hypertension, and diabetes mellitus among total participants (A), males (B), and females (C) were analyzed. P-values for interaction were calculated using multivariable Cox proportional hazard regression models after adjusting for age, sex, smoking status, alcohol consumption, physical activity, income, hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease.
Discussion
This large-scale Asian cohort study investigated the association between professionally measured WC and PD risk. In this nationwide cohort, which included 6,925,646 participants aged ≥40 years with 8.35 years of follow-up, we found that higher WC and the presence of abdominal obesity was significantly associated with an elevated PD risk both in males and females. Notably, abdominal obesity was associated with an increased PD risk even in individuals without general obesity. Each subgroup based on age, smoking status, and comorbidities showed consistent results that abdominal obesity was associated with an increased PD risk, although the level of interaction was different according to sex.
Only a few studies have reported the association between WC and PD risk, with inconsistent findings. One recent large-scale European cohort study and another US health professional cohort study revealed a statistically significant positive association between WC and PD risk among women19,20. Furthermore, WC was positively associated with PD risk after multivariable adjustment in a nationwide cohort study examining metabolic syndrome and PD risk21. In contrast, a few population-based studies showed a null association between WC and PD risk16,17. Consistently with our findings, the Honolulu-Asia aging study has also reported that high subscapular skinfold thickness of participants was positively associated with PD risk, whereas the leanest participants were found to have the lowest PD incidence over 30 years of follow-up9.
Although pathophysiology under the association between central obesity and subsequent PD development as shown in our study and several prior studies has not been fully revealed, insulin resistance appears to act as a key modulator in the link between them. A strong link between obesity induced insulin resistance and neurodegeneration through brain insulin dysregulation has been demonstrated previously22,23. Central insulin effects are considered as neurotrophic, and insulin signaling impairment plays an important role in increasing intracellular protein aggregates, mainly alpha-synuclein protein in the substantia nigra22,23,24. In accordance with dysregulated insulin signaling, mitochondrial dysfunction in the brain is involved in metabolic disturbance and neurodegeneration25,26. A previous epidemiologic finding showed that metabolic syndrome, a cluster of abdominal obesity and insulin resistance, is associated with the PD risk21. In addition, obesity is associated with chronic inflammation states, which is characterized by elevated production of proinflammatory cytokines, thereby leading to oxidative stress and neuronal death26,27. Also, recent evidence on the effect of gut microbiota on α-synucleinopathy or neuroinflammation suggests a dysbiosis-mediated link between adiposity and PD pathogenesis, based on an established association between adiposity and altered gut microbiota28.
Besides insulin resistance, accumulated visceral adipose tissue acts as an endocrine organ secreting adipokines, such as leptin and adiponectin, which play an important role in neurodegeneration29,30,31. Although leptin primarily controls appetite via acting on hypothalamic regions, leptin receptors are expressed in extra-hypothalamic regions, including the substantia nigra. Leptin has a protective effect on dopaminergic neuron degeneration by mediating intracellular signaling via the JAK-STAT and ERK/CREB pathways, thus preserving the function of the dopamine system29,31,32. The level of adipokine is inversely associated with central fat distribution33, and individuals with abdominal obesity are suspected to have vulnerability in neuroprotection against 6-hydroxydopamine toxicity, which can accelerate dopaminergic neuron degeneration29,31.
Above all, even normal-weight individuals with abdominal obesity showed an increased PD risk in our results. While normal-weight central obesity and its associated negative health outcomes have been widely investigated33,34. Our results imply that neurodegenerative diseases, including PD, can be one of those poor health outcomes of abdominal obesity even in non-obese individuals through the deleterious effects of visceral adipose tissue.
Subgroup analysis in our study showed some interesting results. There was a prominently positive association between abdominal obesity and PD risk in female current smokers and the risk was 1.32-fold higher in female current smokers with abdominal obesity than in those without abdominal obesity. A few studies have investigated the relationship between central adiposity and PD risk according to smoking status, although the findings differed16,19,20. Chen et al. reported that the higher WC was associated with an increased PD risk among never smokers of both sexes and female ever smokers20. Another evidence has highlighted the smoking status as an effect modifier of PD risk in female smokers19; however, Palacios et al. did not observe any significant associations16. Current evidence in the genetics of PD may partly explain these contrasting findings, suggesting that ethnicity-specific mutations or variants may have different contributions to the etiologies of PD development3,35. The combination of smoking status and visceral fat distribution might have a different influence on PD risk depending on ethnicity as well as genetic factors36,37. Furthermore, epidemiologic characteristics of the Asian-Pacific region indicate a distinct female predominance of PD prevalence and low smoking rates among females3, which could also modify our findings that the PD risk among female smokers with abdominal obesity was more prominent than that among not currently smoking females. This interpretation could be applicable to our findings that males and females showed different interactions with age in the association between abdominal obesity and PD risk. Meanwhile, it is noteworthy that abdominal obesity increased the PD risk irrespective of the presence of diabetes mellitus in our findings, given that diabetes is known as one of risk factors of PD through shared genetic predisposition and pathophysiologic mechanisms38. Furthermore, abdominally obese females without diabetes mellitus or hypertension had an increased PD risk compared with abdominally nonobese females without the disease, indicating the possible role of abdominal obesity in PD development in these subgroups.
We acknowledge that some potential limitations should be considered when interpreting the current data. First, a reverse causality might exist between WC and PD risk because of the retrospective cohort design, although we considered a 1-year lag period in identifying PD to overcome this issue. Second, the operational diagnosis of PD based on claim database may cause a possibility of misdiagnosis. However, our diagnostic criteria using the rare and incurable disease registry in the NHIS database could identify PD cases more reliably. Third, the ethnic homogeneity of our data limits the generalizability of our study findings; thus, further studies will be needed in Asian populations with different ethnic backgrounds as well as other races. Fourth, due to the lack of information on the NHIS database, we could not adjust for some confounders affecting WC and PD incidence, such as nutritional intake, including coffee and caffeine intake, education, occupation, and history of exposure to toxic molecules including pesticides. Despite these limitations, our study has several strengths. This study determined the relationship between abdominal obesity and PD risk using the largest cohort database in Asia. The ethnic homogeneity of our study could be viewed as a strength since important differences in genetic factors, etiologies, and management of PD according to the regions are emerging. We confirmed the associations using very large-scale cohort data of South Koreans, which enabled us to consider various confounding variables and detailed subgroup analyses. Another strength of this study was the relatively long-term follow-up period of 8.3 years, which allowed us to more truly examine the causality of abdominal obesity and PD risk. Additionally, we had the WC measured directly by trained health professionals instead of using self-reported data.
In conclusion, in this large, nationwide cohort study comprising South Korean individuals aged ≥40 years, we found a positive association of WC and abdominal obesity with PD risk. WC represents central fat distribution and the level of visceral fat level more accurately than BMI, which makes WC a good indicator of insulin resistance. Our findings suggest that a higher WC and abdominal obesity reflect a PD risk in both obese and non-obese individuals. Of note, our findings can add clarity to the current evidence on the role of abdominal obesity in PD risk and provide valuable insights to risk reduction interventions of PD. Future research is needed to investigate the potential roles of visceral fat-driven adipokines and insulin resistance on PD and neurodegeneration.
Methods
Data sources
South Korea has launched a single, mandatory universal health insurance system called the National Health Insurance Service (NHIS) in 2000, which almost covers the entire South Korean population. The NHIS in South Korea contains electronic records for sociodemographic variables; health care utilization-related data for all insured people with disease diagnosis data based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM); and mortality data accumulated since 2002. Furthermore, the information on health screening results obtained through the free mandatory National Health Screening Program at least biennially for adults aged ≥19 years are included in the NHIS database. An individual researcher can use the NHIS database under permission granted from the NHIS with the appropriate research proposal for the use of data. The protocol for this study was approved by the NHIS review committee and Institutional Review Board of Korea University Anam Hospital in accordance with the Declaration of Helsinki of the World Medical Association (IRB No. 2019AN0201). The requirement for informed consent was waived as all data used in the analysis was anonymized and de-identified according to strict confidentiality guidelines.
Study participants
Of the 10,585,844 individuals who underwent the national health screening offered by the NHIS between January 1 and December 31 in 2009, we excluded 3,345,043 individuals aged <40 years, 274,452 with missing values for the analysis, and 12,786 diagnosed with PD between 2002 and study enrollment. We further excluded 27,917 individuals diagnosed with PD within 1 year after enrollment. Finally, a total of 6,925,646 individuals were included in the analysis.
Study outcome and follow-up
Newly diagnosed PD was identified on the basis of the ICD-10-CM code for PD (G20), with the PD registration code (V124) in the rare and incurable diseases registry in the NHIS database between January 2010 and December 2018. The rare and incurable disease registry, which also includes PD, has been implemented by the South Korean government since 2006 for copayment reduction by providing financial support to reduce patients’ medical expenses burden. Study participants were monitored until the date of PD diagnosis, date of death, or December 31, 2018, whichever came first. The median follow-up duration for the study endpoint was 8.35 years (interquartile range 8.11–8.60).
Definition of abdominal obesity and WC categories
WC was measured at the narrowest point between the inferior border of the rib cage and iliac crest during minimal respiration. The cutoff point for WC for abdominal obesity in South Koreans was defined as 90 cm for males and 85 cm for females39. WC was categorized into five levels as follows: <70, 70–80, 80–90, 90–100, and ≥100 cm in males; <65, 65–75, 75–85, 85–95, ≥95 cm in females.
Other variables
The data for each participant’s smoking status, alcohol consumption, and physical activity were obtained by self-report questionnaire. Smoking status was classified into current smokers or nonsmokers based on the participants’ smoking history. Current smokers were defined as individuals who answered they are currently smoking, and nonsmokers as those who had never smoked or had quit smoking. Participants who had consumed any amount of alcohol were defined as alcohol drinkers. Regular exercise was defined as working out, including either ≥30 min of moderate physical activity at least five times per week or ≥20 min of vigorous physical activity at least three times per week. Income level was dichotomized at the lower 25%, based on employee health insurance premiums reflecting a worker’s salary. BMI was calculated as a participant’s weight in kilograms divided by the square of their height in meters. Obesity was defined as BMI ≥ 25 kg/m2 based on the World Health Organization recommendations for Asian populations40. Systolic and diastolic blood pressure was measured using a standard mercury sphygmomanometer. Serum glucose, lipid, and creatinine levels were measured after an overnight fast. These health examinations were performed in hospitals certified by the NHIS under regular quality control.
The presence of comorbidities, such as hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease was defined based on the combination of health examination results and ICD-10-CM codes. Hypertension was defined as (a) systolic/diastolic blood pressure ≥140/90 mmHg or (b) having one or more claims for prescription of antihypertensive medications per year under ICD-10-CM codes I10–13 and I15. Diabetes mellitus was defined as (a) serum fasting glucose level ≥126 mg/dL or (b) having one or more claims for prescription of antidiabetic medication per year under ICD-10-CM code E10–14. Dyslipidemia was defined as (a) serum total cholesterol level ≥240 mg/dL or (b) having one or more claims for prescription of lipid-lowering agents per year under ICD-10-CM code E78. Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease equation41.
Statistical analyses
Baseline characteristics are presented as means ± standard deviations for continuous variables and numbers (percentages) for categorical variables according to baseline WC categories. One-way analysis of variance for continuous variables and Pearson’s chi-squared test for categorical variables were used to compare baseline characteristics among the five WC groups. The PD incidence rate was calculated as event number per 1,000 person-years. The cumulative PD incidence according to the five WC categories and the presence or absence of abdominal obesity was plotted using the Kaplan–Meier curves, and the log-rank test was performed to analyze differences among the groups. To investigate the association between WC, abdominal obesity, and PD risk, HRs and 95% CIs values were calculated using the multivariable Cox proportional hazards regression models. Model 1 was not adjusted. Model 2 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, income, hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease. We further adjusted for baseline BMI in model 3 in addition to the variables adjusted in model 2. These analyses were also performed after stratifying by sex. We performed subgroup analyses by age, smoking status, baseline BMI, hypertension, and diabetes mellitus to investigate the associations between abdominal obesity and PD development in these subgroups. P-value for interaction was calculated using Cox regression analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P < 0.05.
Data availability
This study was performed using the National Health Insurance System database (https://nhiss.nhis.or.kr/), and the results do not necessarily represent the opinion of the National Health Insurance Corporation. Restrictions apply to the availability of these data, which were used under license for this study.
References
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Lim, S. Y. et al. Parkinson’s disease in the Western Pacific Region. Lancet Neurol. 18, 865–879 (2019).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Ascherio, A. & Schwarzschild, M. A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272 (2016).
Abbott, R. D. et al. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J. Neurol. Suppl. 250, 30–39 (2003).
Pezzoli, G. & Cereda, E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80, 2035–2041 (2013).
Wang, G.-J. et al. Brain dopamine and obesity. Lancet 357, 354–357 (2001).
Abbott, R. D. et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology 59, 1051–1057 (2002).
Chen, J. et al. Meta-analysis: overweight, obesity, and Parkinson’ s disease. Int. J. Endocrinol. (2014).
Wang, Y. L. et al. Body mass index and risk of Parkinson’s disease: a dose-response meta-analysis of prospective studies. PLoS ONE 10, (2015).
Jeong, S. M. et al. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 35, 236–244 (2020).
Rahmani, J. et al. Body mass index and risk of Parkinson, Alzheimer, Dementia, and Dementia mortality: a systematic review and dose–response meta-analysis of cohort studies among 5 million participants. Nutr. Neurosci. 0, 1–9 (2020).
Logroscino, G., Sesso, H. D., Paffenbarger, R. S. & Lee, I. M. Body mass index and risk of Parkinson’s disease: a prospective cohort study. Am. J. Epidemiol. 166, 1186–1190 (2007).
Noyce, A. J. et al. Estimating the causal influence of body mass index on risk of Parkinson disease: a Mendelian randomisation study. PLoS Med. 14, e1002314 (2017).
Palacios, N. et al. Obesity, diabetes, and risk of Parkinson’s disease. Mov. Disord. 26, 2253–2259 (2011).
Park, J. H. et al. Association between body weight variability and incidence of Parkinson disease: a nationwide, population-based cohort study. Eur. J. Neurol. 28, 3626–3633 (2021).
Roos, E. et al. Body mass index, sitting time, and risk of Parkinson disease. Neurology 90, E1413–E1417 (2018).
Riso, L. et al. General and abdominal adiposity and the risk of Parkinson’s disease: a prospective cohort study. Park. Relat. Disord. 62, 98–104 (2019).
Chen, H. et al. Obesity and the Risk of Parkinson’s Disease. Am. J. Epidemiol. 159, 547–555 (2004).
Nam, G. E. et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med 15, 1–15 (2018).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Sci. (80-.) 289, 2122–2125 (2000).
Ghasemi, R. et al. Brain insulin dysregulation: Implication for neurological and neuropsychiatric disorders. Mol. Neurobiol. 47, 1046–1065 (2013).
Lee, S. H., Zabolotny, J. M., Huang, H., Lee, H. & Kim, Y. B. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol. Metab. 5, 589–601 (2016).
De Mello, N. P. et al. Insulin and autophagy in neurodegeneration. Front. Neurosci. 13, 1–17 (2019).
Schell, M., Wardelmann, K. & Kleinridders, A. Untangling the effect of insulin action on brain mitochondria and metabolism. J. Neuroendocrinol. 1–14 (2021). https://doi.org/10.1111/jne.12932.
Spielman, L. J., Little, J. P. & Klegeris, A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 273, 8–21 (2014).
Romano, S. et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park. Dis. 7, (2021).
Fulton, S. et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811–822 (2006).
Zou, X. et al. Role of leptin in mood disorder and neurodegenerative disease. Front. Neurosci. 13, 1–8 (2019).
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Weng, Z. et al. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J. Biol. Chem. 282, 34479–34491 (2007).
Gavrila, A. et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J. Clin. Endocrinol. Metab. 88, 4823–4831 (2003).
Sun, Y. et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw. Open 2, 1–13 (2019).
Sahakyan, K. R. et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann. Intern. Med. 163, 827–835 (2015).
Schleinitz, D., Böttcher, Y., Blüher, M. & Kovacs, P. The genetics of fat distribution. Diabetologia 57, 1276–1286 (2014).
Fehlert, E. et al. Genetic determination of body fat distribution and the attributive influence on metabolism. Obesity 25, 1277–1283 (2017).
De Pablo-Fernandez, E., Goldacre, R., Pakpoor, J., Noyce, A. J. & Warner, T. T. Association between diabetes and subsequent Parkinson disease. Neurology 91, e139 LP–e139142 (2018).
Kim, B.-Y. et al. 2020 Korean society for the study of obesity guidelines for the management of obesity in Korea. J. Obes. Metab. Syndr. 30, 81–92 (2021).
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004).
Levey, A. S. Chronic kidney disease epidemiology collaboration. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53, 766–772 (2007).
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1I1A1A01061757). Also, this study was supported by the Seokchunnanum Foundation (No. SCY2112P). These funding sources had no role in the design and execution of our study, analysis and interpretation of the data, and decision to submit the results.
Author information
Authors and Affiliations
Contributions
K.Y.P.: Writing of the first draft; G.E.N.: Conception, Organization, and Execution of research project, Design of statistical analysis, Review and Critique of the draft; K.H.: Design, Conception and Execution of statistical analysis, Review and Critique of the draft; H.K.P.: Review and Critique of the draft; H.S.H.: Conception of research design, Review and Critique of the draft;
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, KY., Nam, G.E., Han, K. et al. Waist circumference and risk of Parkinson’s disease. npj Parkinsons Dis. 8, 89 (2022). https://doi.org/10.1038/s41531-022-00353-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00353-4
This article is cited by
-
Lebensstil und Parkinson-Risiko
Journal für Klinische Endokrinologie und Stoffwechsel (2022)