Abstract
Background
To report the 3-year results of a prospective, single arm, multicenter, international clinical study with the second generation of the temporary implantable nitinol device (iTIND; Medi-Tate Ltd®, Israel) on men suffering lower urinary tract symptoms (LUTS) secondary to benign prostatic obstruction (BPO).
Methods
Eighty-one men with symptomatic BPO (IPSS ≥ 10, peak urinary flow <12 ml/s, and prostate volume <75 ml) were enrolled in this study between December 2014 and December 2016. Subjects were washed-out 1 month for alpha-blockers and 6 months for 5-ARIs. The implantation was performed under light sedation and the removal 5–7 days later with topical anesthesia. Perioperative results including OR-time, pain (VAS) postoperative complications (Clavien–Dindo-Grading System), functional results (Qmax, IPSS, PVR) and quality of life (QoL) were assessed at 1, 3, 6 months, 1, 2, and 3 years. Sexual and ejaculatory function were evaluated using two yes/no questions.
Results
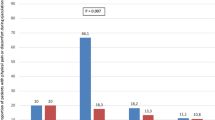
Thirty-six month functional results were available for 50 patients and demonstrated that iTIND efficacy remained stable through 3 years, with averages IPSS, QOL, Qmax and PVR of 8.55 + 6.38, 1.76 + 1.32, 15.2 + 6.59 ml/s and 9.38 + 17.4 ml, improved from baseline by −58.2, −55.6, +114.7, and −85.4% (all significantly different from their corresponding baseline values, p < 0.0001). Even considering the Intention to Treat analysis (ITT), the 36-month results confirmed significant improvements of the functional outcomes if compared with baselines values (all p < 0.0001). No late post-operative complications were observed between 12 and 36 months. Sexual function was stable through 3 years, with no reports of sexual or ejaculatory dysfunctions. No patients underwent alternative treatments between 24 and 36 months.
Conclusion
Treatment of BPO-related LUTS with iTIND demonstrated a significant and durable reduction in symptoms and improvement of functional parameters and quality of life at 3 years of follow-up. No late post-operative complications, ejaculatory dysfunction or additional treatment failures were observed between 24 and 36 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mazur DJ, Helfand BT, McVary KT. Influences of neuroregulatory factors on the development of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction in aging men. Urol Clin North Am. 2012;39:77–88.
Song Q, Abrams P, Sun Y. Beyond prostate, beyond surgery and beyond urology: the “3Bs” of managing non-neurogenic male lower urinary tract symptoms. Asian J Urol. 2019;6:169–73.
Cindolo L, Pirozzi L, Fanizza C, Romero M, Tubaro A, Autorino R, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68:418–25.
Hordijk IMJ, Steffens MG, Hak E, Blanker MH. Continuation rates of alpha-blockers mono-therapy in adult men, prescribed by urologists or general practitioners: a pharmacy-based study. World J Urol. 2019;37:1659–64.
Alexander CE, Scullion MM, Omar MI, Yuan Y, Mamoulakis C, N’Dow JM, et al. Bipolar versus monopolar transurethral resection of the prostate for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database Syst Rev. 2019;12:CD009629.
Cacciamani GE, Cuhna F, Tafuri A, Shakir A, Cocci A, Gill K, et al. Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: systematic review and pooled-analysis of randomized clinical trials. Minerva Urol Nefrol. 2019;71:427.
Gratzke C, Bachmann A, Descazeaud A, Drake JM, Madersbacher S, Mamoulakis C, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–109.
Thangasamy IA, Chalasani V, Bachmann A, Woo HH. Photoselective vaporisation of the prostate using 80-W and 120-W laser versus transurethral resection of the prostate for benign prostatic hyperplasia: a systematic review with meta-analysis from 2002 to 2012. Eur Urol. 2012;62:315–23.
Rapisarda S, Russo GI, Osman NI, Chapple CR, Morgia G, Tubaro A, et al. The use of laser as a therapeutic modality as compared to TURP for the small prostate ≤40 mL: a collaborative review. Minerva Urol Nefrol. 2019;71:569–75.
Porpiglia F, Fiori C, Bertolo R, Garrou D, Cattaneo G, Amparore D. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up. BJU Int. 2015;116:278–87.
Porpiglia F, Fiori C, Bertolo R, Giordano A, Checcucci E, Garrou D, et al. 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int. 2018;122:106–12.
Porpiglia F, Fiori C, Amparore D, Kadner G, Manit A, Valerio M, et al. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int. 2019;123:1061–9.
Kadner G, Valerio M, Giannakis I, Manit A, Lumen N, Ho BSH, et al. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World J Urol. 2020. https://doi.org/10.1007/s00345-020-03140-z. [Epub online ahead of print].
Sun Y, Peng B, Lei GL, Wei Q, Yang L. Study of phosphodiesterase 5 inhibitors and α-adrenoceptor antagonists used alone or in combination for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Minerva Urol Nefrol. 2020;72:13–21.
Zhang Y, Yuan P, Ma D, Gao X, Wei C, Liu Z, et al. Efficacy and safety of enucleation vs. resection of prostate for treatment of benign prostatic hyperplasia: a meta-analysis of randomized controlled trials. Prostate Cancer Prostatic Dis. 2019;22:493–508.
Kallidonis P, Adamou C, Kotsiris D, Ntasiotis P, Verze P, Athanasppoulos A, et al. Combination therapy with alpha-blocker and phosphodiesterase-5 inhibitor for improving lower urinary tract symptoms and erectile dysfunction in comparison with monotherapy: a systematic review and meta-analysis. Eur Urol Focus. 2020;6:537–58.
Magistro G, Chapple CR, Elhilali M, Gilling P, McVary KT, Roehrborn CG, et al. Emerging minimally invasive treatment options for male lower urinary tract symptoms. Eur Urol. 2017;72:986–99.
Sønksen J, Barber NJ, Speakman MJ, Berges R, Wetteraurer U, Greene D, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68:643–52.
Chin PT, Bolton DM, Jack G, Rashid P, Thavaeelan J, Yu RJ, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2012;79:5–11.
Dixon C, Cedano ER, Pacik D, Vit V, Varga G, Wagrell L, et al. Efficacy and safety of Rezūm system water vapor treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2015;86:1042–7.
Roehrborn CG, Rukstalis DB, Barkin J, Gange SN, Shore ND, Giddens JL, et al. Three year results of the prostatic urethral L.I.F.T. study. Can J Urol. 2015;22:7772–82.
Rukstalis D, Grier D, Stroup SP, Tutrone R, deSouza E, Freedman S, et al. Prostatic Urethral Lift (PUL) for obstructive median lobes: 12 month results of the MedLift Study. Prostate Cancer Prostatic Dis. 2019;22:411–9.
Roos NP, Wennberg JE, Malenka DJ, Fisher ES, McPherson K, Andersen TF, et al. Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N. Engl J Med. 1989;320:1120–4.
Madersbacher S, Lackner J, Brössner C, Rohlich M, Stancik I, Williger M, et al. Reoperation, myocardial infarction and mortality after transurethral and open prostatectomy: a nation-wide, long-term analysis of 23,123 cases. Eur Urol. 2005;47:499–504.
Gilfrich C, Leicht H, Fahlenbrach C, Jeschke E, Popken G, Stoltzengurg JU, et al. Morbidity and mortality after surgery for lower urinary tract symptoms: a study of 95,577 cases from a nationwide German health insurance database. Prostate Cancer Prostatic Dis. 2016;19:406–11.
Welk B, Reid J, Ordon M, Razvi H, Campbell J. Population-based assessment of re-treatment and healthcare utilisation after photoselective vaporisation of the prostate or electrosurgical transurethral resection of the prostate. BJU Int. 2019;124:1047–54.
Rieken M, Ebinger Mundorff N, Bonkat G, Wyler S, Bachmann A. Complications of laser prostatectomy: a review of recent data. World J Urol. 2010;28:53–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amparore, D., Fiori, C., Valerio, M. et al. 3-Year results following treatment with the second generation of the temporary implantable nitinol device in men with LUTS secondary to benign prostatic obstruction. Prostate Cancer Prostatic Dis 24, 349–357 (2021). https://doi.org/10.1038/s41391-020-00281-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-00281-5
This article is cited by
-
Impact of minimally invasive surgical procedures for Male Lower Urinary Tract Symptoms due to benign prostatic hyperplasia on ejaculatory function: a systematic review
Prostate Cancer and Prostatic Diseases (2024)
-
Ejaculation sparing of classic and minimally invasive surgical treatments of LUTS/BPH
Prostate Cancer and Prostatic Diseases (2024)
-
Primary bladder neck obstruction in men—new perspectives in physiopathology
Prostate Cancer and Prostatic Diseases (2024)
-
Minimalinvasive Therapien des benignen Prostatasyndroms
Die Urologie (2024)
-
Treating LUTS in Men with Benign Prostatic Obstruction: A Review Article
Drugs & Aging (2023)