Abstract
Background:
Promising therapeutic results of the prostate-specific membrane antigen (PSMA) ligand have been shown when labelling with lutetium-177 (177Lu). We performed a systematic review and meta-analysis to assess the therapeutic response of 177Lu-PSMA in the treatment of metastatic castration-resistant prostate cancer (mCRPC).
Methods:
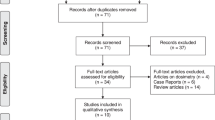
A systematic review was conducted using electronic databases up to December 2016. Two reviewers independently extracted data and assessed methodological quality. The main outcome of interest was antitumour biochemical response of 177Lu-PSMA, analysing two measures: ‘any PSA decline’ and ‘>50% decline’ from baseline. A random-effects meta-analysis was used to calculate the pooled proportion across studies. The I2 statistic was calculated in each case to investigate the extent of heterogeneity across the studies. A sensitivity analysis was conducted removing two studies, which were presented as abstracts and proportions were summarised by chemical type (177Lu-J591/DKZ/I&T). All analyses were conducted using Stata v14.
Results:
A total of 10 studies were included in the analysis giving a total sample size of 369, 220 (of 334 analysable) experienced any PSA decline. The pooled proportion of patients with any PSA decline was 68% (95% confidence interval (CI): 61–74). The I2 statistic was 39.1% (P=0.11) suggesting minor heterogeneity between results. The pooled proportion of patients with >50% PSA decline was 37% (95% CI: 22–52). The I2 statistic was 91.0% (P<0.001) suggesting substantial heterogeneity between results. On subgroup analysis, a higher proportion of patients in the 177Lu-DKZ/I&T subgroup had a PSA decline >50%, however, it can be seen that the 177Lu-DKZ/I&T subgroup had a substantial amount of heterogeneity across studies.
Conclusions:
This review suggests promising early results for the treatment of mCRPC, especially from patients treated with the more recently developed radioligands. Overall, our meta-analysis showed that approximately two-thirds of patients had a biochemical response. Randomised-controlled trials would be necessary to verify its effectiveness against current systemic therapies and create an ideal treatment protocol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Conford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic and castration-resistant prostate cancer. Eur Urol 2017; 71: 630–642.
Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the bad berka experience 2013. J Nucl Med 2016; 57: 97S–104S.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med 2016; 57: 1006–1013.
Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 1998; 58: 4055–4060.
Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumour vascular endothelium. Cancer Res 1997; 57: 3629–3634.
Vallabhajosula S, Nikolopoulou A, Jhanwar YS, Kaur G, Tagawa ST, Nanus DM et al. Radioimmunotherapy of metastatic prostate cancer with 177Lu-DOTA-huJ591 anti-prostate specific membrane antigen specific monoclonal antibody. Curr Radiopharm 2016; 9: 44–53.
Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003; 170: 1717–1721.
Kularatne SA, Zhou Z, Yang J, Post CB, Low PS . Design, synthesis, and pre-clinical evaluation of prostate specific membrane antigen targeted 99mTc-radioimmaging agents. Mol Pharm 2009; 6: 790–800.
Zhang AX, Murelli RP, Barinka C, Michel J, Cocleaza A, Jorgensen WL et al. A remote arene-bidning site on prostate specific membrane antigen revealed by antibody-recruiting small molecules. J Am Chem Soc 2010; 132: 12711–12716.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA targeted theranostic concept and first proof of concept human studies. J Nucl Med 2015; 56: 1169–1176.
Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N et al. Pre-clinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res 2009; 69: 6932–6940.
Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W et al. Preclinical evaluation of tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med 2015; 56: 914–920.
Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ . Phase I trial of 177lutetium labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostatecancer. J Clin Oncol 2005; 23: 4591–4601.
Tagawa ST, Milowsky M, Morris M, Vallabhajosula S, Christos P, Akhtar NH et al. Phase II study of Lutetium-177-labelled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 2013; 46: 220–231.
Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 2015; 5: 1–8.
Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane for imaging and therapy in patients with metastatic castration-resistant prostatecancer. J Urol 2016; 196: 382–391.
Fendler WP, Reinhardt S, Ilhan H, Delker A, Boning G, Gildehaus FJ et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2016; 8: 3581–3590.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58: 85–90.
Tagawa ST, Batra J, Vallabhajosula S, Jhanwar Y, Christos PJ, Emmerich L et al Final results of 2-dose fractionation of 177Lu-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC). Conference: 2016 Annual Meeting of the American Society of Clinical Oncology, ASCO. J Clin Oncol 2016; 34 (abstract 5022).
Rathore H, Shah H, Aland P, Chaudhuri P, Bharadwaj T, Kale C et al. Assessment of response, clinical evaluation and toxicity of radioligand therapy (RLT) with 177-Lutetium-DKFZ-617-labeled prostate specific membrane antigen (177-Lu-DKFZ-617-PSMA) for metastatic castrate resistant prostate cancer (mCRPC): an initial experience in Jaslok. Conference: 29th Annual Congress of the European Association of Nuclear Medicine, EANM 2016, Spain. Eur J Nucl Med Mol Imaging 2016; 43: S414.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging 2017; 44: 81–91.
Kirby M, Hirst C, Crawford ED . Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65: 1180–1192.
Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care, Ontario clinical practice guideline. J Clin Oncol 2014; 32: 3436–3448.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197.
Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012; 61: 1079–1092.
Kiess A, Minn I, Chen Y, Hobbs R, Sgouros G, Mease RC et al. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med 2015; 56: 1401–1407.
Tagawa ST, Akhtar NH, Nikolopoulou A, Vallabhajosula S, Osborne J, Beltran H et al. Phase I trial of fractionated-dose 177Lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer (CRPC). Conference: European Cancer Congress, ECC 2013, Amsterdam, Netherlands. Eur J Cancer 2013; 49: S701–S702.
Tagawa ST, Whang YE, Kaur G, Vallabhajosula S, Christos PJ, Nikolopoulou A et al. Phase I trial of docetaxel/prednisone plus fractionated dose radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody 177Lu-J591 in patients with metastatic, castration-resistant prostate cancer (mCRPC). Conference: Annual Meeting of the American Society of Clinical Oncology, ASCO, Chicago, United States, 2014. J Clin Oncol 2014; 32 (Suppl 1): 15.
Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics 2015; 5: 1388–1401.
De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154.
Scher HI, Morris MJ, Basch E, Heller G . End points and outcomes in castration resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol 2011; 29: 3695–3704.
Evans-Axelsson S, Timmermand O, Bjartell A, Strand SE, Elgqvist J . Radioimmunotherapy for prostate cancer: current status and future possibilities. Semin Nucl Med 2016; 46: 165–179.
Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996; 48: 326–334.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010; 11: 1066–1073.
Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 2015; 75: 4688–4696.
Tosoian JJ, Gorin MA, Rowe SP, Andreas D, Szabo Z, Pienta KJ et al. Correlation of PSMA-targeted 18F-DCFPyL PET/CT findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin Genitourin Cancer 2017; 15: e65–e68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Calopedos, R., Chalasani, V., Asher, R. et al. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 20, 352–360 (2017). https://doi.org/10.1038/pcan.2017.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2017.23
This article is cited by
-
Adoption of Lutetium-177 PSMA radioligand therapy for metastatic castration resistant prostate cancer: a total population analysis in Germany from 2016 to 2020
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Editor’ summary: A paradigm shift in castration-resistant prostate cancer management
Prostate Cancer and Prostatic Diseases (2022)
-
Real-World Data Analysis of Efficacy and Survival After Lutetium-177 Labelled PSMA Ligand Therapy in Metastatic Castration-Resistant Prostate Cancer
Targeted Oncology (2021)
-
177Lu-PSMA-RLT of metastatic castration-resistant prostate cancer: limitations and improvements
Annals of Nuclear Medicine (2021)
-
PSMA: a game changer in the diagnosis and treatment of advanced prostate cancer
Medical Oncology (2021)