Abstract
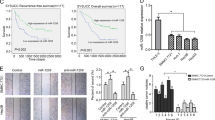
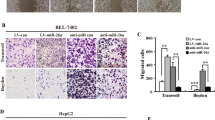
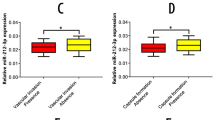
Metastasis is responsible for the rapid recurrence and poor survival of malignancies. Epithelial–mesenchymal transition (EMT) has a critical role in metastasis. Increasing evidence indicates that EMT can be regulated by microRNAs (miRNAs). miR-148a is a liver-abundant miRNA. However, the role of miR-148a in the development of liver cancer remains largely unknown. In this study, we found that, compared with normal livers, miR-148a was significantly decreased in hepatocellular carcinoma (HCC) tissues, especially in those with the portal vein tumor thrombus. An in vitro transwell assay and an in vivo orthotopic liver xenograft model showed that the restoration of miR-148a expression significantly repressed the migration and pulmonary metastasis of hepatoma cells. Linear regression analysis revealed a positive correlation between the expression of miR-148a and the mRNA level of E-cadherin gene in human HCC tissues. Both gain- and loss-of-function studies disclosed that miR-148a promoted the expression of epithelial marker (E-cadherin) and reduced the levels of mesenchymal markers (N-cadherin, fibronectin or vimentin) in hepatoma cells. These data suggest that miR-148a may suppress EMT and cancer metastasis. Further mechanistic investigations showed that miR-148a directly inhibited Met expression by binding to its 3′-UTR. Moreover, the reintroduction of miR-148a attenuated the downstream signaling of Met, like activated phosphorylation of AKT-Ser473 and inhibitory phosphorylation of GSK-3β-Ser9, and consequently reduced the nuclear accumulation of Snail, a transcription factor that promotes EMT. Taken together, miR-148a may negatively regulate Met/Snail signaling and therefore inhibit the EMT and metastasis of hepatoma cells. These findings highlight the significance of miR-148a downregulation in tumor progression and implicate miR-148a as an attractive candidate for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Polyak K, Weinberg RA . Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–273.
Iorio MV, Croce CM . MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2012; 4: 143–159.
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008; 10: 593–601.
Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology 2012; 56: 1792–1803.
Meng Z, Fu X, Chen X, Zeng S, Tian Y, Jove R et al. miR-194 is a marker of hepatic epithelial cells and suppresses metastasis of liver cancer cells in mice. Hepatology 2010; 52: 2148–2157.
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 2012; 61: 278–289.
Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol 2011; 195: 417–433.
Kumarswamy R, Mudduluru G, Ceppi P, Muppala S, Kozlowski M, Niklinski J et al. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer 2012; 130: 2044–2053.
Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J 2011; 440: 23–31.
Zhao X, Dou W, He L, Liang S, Tie J, Liu C et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene 2013; 32: 1363–1372.
Xu Q, Sun Q, Zhang J, Yu J, Chen W, Zhang Z . Downregulation of miR-153 contributes to epithelial-mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis 2013; 34: 539–549.
Khew-Goodall Y, Goodall GJ . Myc-modulated miR-9 makes more metastases. Nat Cell Biol 2010; 12: 209–211.
Papadimitriou E, Vasilaki E, Vorvis C, Iliopoulos D, Moustakas A, Kardassis D et al. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-beta and miR-24: role in epithelial-to-mesenchymal transition. Oncogene 2012; 31: 2862–2875.
Vetter G, Saumet A, Moes M, Vallar L, Le Bechec A, Laurini C et al. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene 2010; 29: 4436–4448.
Liffers ST, Munding JB, Vogt M, Kuhlmann JD, Verdoodt B, Nambiar S et al. MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest 2011; 91: 1472–1479.
Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A et al. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem 2010; 285: 19076–19084.
Zhang H, Li Y, Huang Q, Ren X, Hu H, Sheng H et al. MiR-148a promotes apoptosis by targeting Bcl-2 in colorectal cancer. Cell Death Differ 2011; 18: 1702–1710.
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J Mol Cell Biol 2013; 5: 3–13.
Yu J, Li Q, Xu Q, Liu L, Jiang B . MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res 2011; 25: 170–177.
Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res 2011; 17: 7574–7583.
Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res 2004; 14: 2486–2494.
Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 2008; 47: 897–907.
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G . Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12: 89–103.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2: 76–83.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6: 931–940.
Peinado H, Olmeda D, Cano A . Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–428.
Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA . Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology 2002; 36: 692–701.
Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 2009; 50: 1464–1474.
You H, Ding W, Dang H, Jiang Y, Rountree CB . c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology 2011; 54: 879–889.
Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology 2008; 135: 2128–2140.
Braconi C, Huang N, Patel T . MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 2010; 51: 881–890.
Duursma AM, Kedde M, Schrier M, le Sage C, Agami R . miR-148 targets human DNMT3b protein coding region. RNA 2008; 14: 872–877.
Gailhouste L, Gomez-Santos L, Hagiwara K, Hatada I, Kitagawa N, Kawaharada K et al. MiR-148a plays a pivotal role in the liver by promoting the hepatospecific phenotype and suppressing the invasiveness of transformed cells. Hepatology (e-pub ahead of print 26 March 2013; doi:10.1002/hep.26422).
Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y et al. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res 2009; 69: 1135–1142.
Acknowledgements
This study was supported by grants from Ministry of Science and Technology of China (2010CB912803, 2011CB811305), Ministry of Health of China (2012ZX10002011), National Natural Science Foundation of China (81101593, 30925036), Ministry of Education of China (20110171120043).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zhang, JP., Zeng, C., Xu, L. et al. MicroRNA-148a suppresses the epithelial–mesenchymal transition and metastasis of hepatoma cells by targeting Met/Snail signaling. Oncogene 33, 4069–4076 (2014). https://doi.org/10.1038/onc.2013.369
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.369
Keywords
This article is cited by
-
The lncRNA HOTAIR: a pleiotropic regulator of epithelial cell plasticity
Journal of Experimental & Clinical Cancer Research (2023)
-
Emerging role of MicroRNA-Based theranostics in Hepatocellular Carcinoma
Molecular Biology Reports (2023)
-
Portal vein tumor thrombosis in hepatocellular carcinoma: molecular mechanism and therapy
Clinical & Experimental Metastasis (2023)
-
Down-regulation of ZNF252P-AS1 alleviates ovarian cancer progression by binding miR-324-3p to downregulate LY6K
Journal of Ovarian Research (2022)
-
IKBKE phosphorylates and stabilizes Snail to promote breast cancer invasion and metastasis
Cell Death & Differentiation (2022)