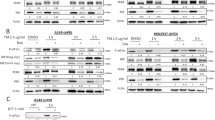
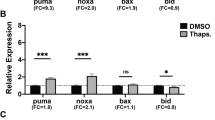
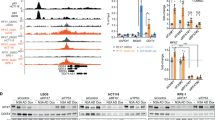
Abstract
Reactive oxygen species (ROS) are produced in growth factor-signaling pathways leading to cell proliferation, but the mechanisms leading to ROS generation and the targets of ROS signals are not well understood. Using a focused siRNA screen to identify redox-related proteins required for growth factor-induced cell cycle entry, we show that two ROS-generating proteins, the NADPH oxidases NOX4 and DUOX2, are required for platelet-derived growth factor (PDGF) induced retinoblastoma protein (Rb) phosphorylation in normal human fibroblasts. Unexpectedly, NOX4 and DUOX2 knockdown did not inhibit the early signaling pathways leading to cyclin D1 upregulation. However, hours after growth factor stimulation, NOX4 and DUOX2 knockdown reduced ERK1 phosphorylation and increased levels of the tumor suppressor protein p53 and a cell cycle inhibitor protein p21 (Waf1/Cip1) that is transcriptionally regulated by p53. Co-knockdown of NOX4 or DUOX2 with either p53 or with p21 overcame the inhibition of Rb phosphorylation that occurred with NOX4 or DUOX2 knockdown alone. Our results argue that rather than primarily affecting growth factor receptor signaling, NOX4 and DUOX2 regulate cell cycle entry as part of a p53-dependent checkpoint for proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB et al. (1997). Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272: 217–221.
Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A et al. (2000). Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem 275: 10527–10531.
Burch PM, Heintz NH . (2005). Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal 7: 741–751.
Burhans WC, Heintz NH . (2009). The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radic Biol Med 47: 1282–1293.
Chambard JC, Lefloch R, Pouyssegur J, Lenormand P . (2007). ERK implication in cell cycle regulation. Biochimica Et Biophysica Acta 1773: 1299–1310.
Chen K, Kirber MT, Xiao H, Yang Y, Keaney Jr JF . (2008). Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139.
Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S et al. (2005). NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907.
Hainaut P, Mann K . (2001). Zinc binding and redox control of p53 structure and function. Antioxid Redox Signal 3: 611–623.
Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T et al. (2008). Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17.
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y et al. (2005). AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18: 283–293.
Keyse SM . (2008). Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27: 253–261.
Lambeth JD . (2004). NOX enzymes and the biology of reactive oxygen. Nat Rev 4: 181–189.
Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y et al. (2001). Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894.
Laurent E, McCoy III JW, Macina RA, Liu W, Cheng G, Robine S et al. (2008). Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer 123: 100–107.
Lee SR, Kwon KS, Kim SR, Rhee SG . (1998). Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273: 15366–15372.
Li M, Zhou JY, Ge Y, Matherly LH, Wu GS . (2003). The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. J Biol Chem 278: 41059–41068.
Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM . (1996). A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev 10: 934–947.
Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell Jr JE et al. (2005). STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241.
Liu YX, Wang J, Guo J, Wu J, Lieberman HB, Yin Y . (2008). DUSP1 is controlled by p53 during the cellular response to oxidative stress. Mol Cancer Res 6: 624–633.
Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G et al. (2004). The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24: 1844–1854.
Meng TC, Fukada T, Tonks NK . (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399.
Myers JW, Jones JT, Meyer T, Ferrell Jr JE . (2003). Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nat Biotechnol 21: 324–328.
Park HS, Jin DK, Shin SM, Jang MK, Longo N, Park JW et al. (2005). Impaired generation of reactive oxygen species in leprechaunism through downregulation of Nox4. Diabetes 54: 3175–3181.
Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ et al. (2004). Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol 24: 4384–4394.
Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A . (2006). NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484.
Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH . (2006). Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal 8: 1447–1459.
Rhee SG, Bae YS, Lee SR, Kwon J . (2000). Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000: PE1.
Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K et al. (2006). Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673.
Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P et al. (2007). Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1543–L1555.
Sun XZ, Vinci C, Makmura L, Han S, Tran D, Nguyen J et al. (2003). Formation of disulfide bond in p53 correlates with inhibition of DNA binding and tetramerization. Antioxid Redox Signal 5: 655–665.
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T . (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science (New York, NY) 270: 296–299.
Szatrowski TP, Nathan CF . (1991). Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798.
Trachootham D, Alexandre J, Huang P . (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8: 579–591.
Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS . (2007). Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry 46: 7765–7780.
Ward JP . (2008). Oxygen sensors in context. Biochimica Et Biophysica Acta 1777: 1–14.
Wilkinson DS, Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA et al. (2005). A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene. Mol Cell Biol 25: 1200–1212.
Wilkinson DS, Tsai WW, Schumacher MA, Barton MC . (2008). Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol Cell Biol 28: 1988–1998.
Winterbourn CC . (2008). Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286.
Winterbourn CC, Hampton MB . (2008). Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561.
Wu GS . (2004). The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther 3: 156–161.
Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y et al. (2009). NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res 69: 2647–2654.
Yao G, Lee TJ, Mori S, Nevins JR, You L . (2008). A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol 10: 476–482.
Zarkowska T, Mittnacht S . (1997). Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem 272: 12738–12746.
Acknowledgements
We thank Dr Won Do Heo for his assistance with the cell cycle entry assay, Dr Dan Kaplan, Dr Sean Collins and Dr Karlene Cimprich for their critical reading of the article and members of the Meyer lab and Renee Paulsen for helpful discussions. This work was supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (AS), by a Helen Hay Whitney Foundation/Paul Sigler Agouron Institute post-doctoral fellowship (AS) and by an NIH grant R33 CA 120732 (TM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Salmeen, A., Park, B. & Meyer, T. The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway. Oncogene 29, 4473–4484 (2010). https://doi.org/10.1038/onc.2010.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.200
Keywords
This article is cited by
-
G protein-coupled receptor kinase 2 modifies the cellular reaction to cisplatin through interactions with NADPH oxidase 4
Molecular and Cellular Biochemistry (2021)
-
Poldip2 knockdown inhibits vascular smooth muscle proliferation and neointima formation by regulating the expression of PCNA and p21
Laboratory Investigation (2019)
-
Selection scan reveals three new loci related to high altitude adaptation in Native Andeans
Scientific Reports (2018)
-
The defense and signaling role of NADPH oxidases in eukaryotic cells
Wiener Medizinische Wochenschrift (2018)
-
Cytotoxin-induced NADPH oxides activation: roles in regulation of cell death
Archives of Toxicology (2015)