Key Points
-
Prostate-specific membrane antigen (PSMA) is a promising and specific target for prostate cancer imaging
-
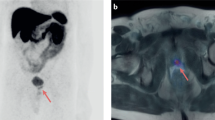
PSMA–PET imaging can add molecular information to multiparametric MRI and, therefore, delineate suspicious lesions for targeted biopsies, especially in patients whose biopsy samples are tumour-negative
-
PSMA–PET imaging shows increased specificity and sensitivity compared with current standard imaging (CT, MRI and bone scintigraphy) in patients with primary intermediate-risk or high-risk prostate cancer
-
PSMA–PET imaging improves detection of metastatic lesions even at low serum PSA values in biochemically recurrent prostate cancer
-
Enhanced detection of prostate cancer lesions might enable improved patient-tailored therapy planning and, therefore, lead to improved therapy outcomes
Abstract
Currently, the findings of imaging procedures used for detection or staging of prostate cancer depend on morphology of lymph nodes or bone metabolism and do not always meet diagnostic needs. Prostate-specific membrane antigen (PSMA), a transmembrane protein that has considerable overexpression on most prostate cancer cells, has gained increasing interest as a target molecule for imaging. To date, several small compounds for labelling PSMA have been developed and are currently being investigated as imaging probes for PET with the 68Ga-labelled PSMA inhibitor Glu-NH-CO-NH-Lys(Ahx)-HBED-CC being the most widely studied agent. 68Ga-PSMA–PET imaging in combination with multiparametric MRI (mpMRI) might provide additional molecular information on cancer localization within the prostate. In patients with primary prostate cancer of intermediate-risk to high-risk, PSMA-based imaging has been reported to improve detection of metastatic disease compared with CT or mpMRI, rendering additional cross-sectional imaging or bone scintigraphy unnecessary. Furthermore, in patients with biochemically recurrent prostate cancer, use of 68Ga-PSMA–PET imaging has been shown to increase detection of metastatic sites, even at low serum PSA values, compared with conventional imaging or PET examination with different tracers. Thus, although current knowledge is still limited and derived mostly from retrospective series, PSMA-based imaging holds great promise to improve prostate cancer management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
American Urological Association. Guideline for the management of clinically localized prostate cancer (2007). [online], (2007).
European Association of Urology. Guidelines on Prostate Cancer. http://uroweb.org/guideline/prostate-cancer/ (2015).
Barentsz, J. O. et al. ESUR prostate MR guidelines 2012. Eur. Radiol. 22, 746–757 (2012).
Baco, E. et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur. Urol. 67, 787–794 (2015).
Valerio, M. et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur. Urol. 68, 8–19 (2015).
Dianat, S. S., Carter, H. B. & Macura, K. J. Performance of multiparametric magnetic resonance imaging in the evaluation and management of clinically low-risk prostate cancer. Urol. Oncol. 32, 39.e1–39.e10 (2014).
Reisaeter, L. A. et al. 1.5-T multiparametric MRI using PI-RADS: a region by region analysis to localize the index-tumor of prostate cancer in patients undergoing prostatectomy. Acta Radiol. 56, 500–511 (2015).
Hoeks, C. M. et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 266, 207–217 (2013).
Schimmoller, L. et al. MR-sequences for prostate cancer diagnostics: validation based on the PI-RADS scoring system and targeted MR-guided in-bore biopsy. Eur. Radiol. 24, 2582–2589 (2014).
National Comprehensive Cancer Network. Prostate cancer. ">[online], (2015).
Umbehr, M. H., Muntener, M., Hany, T., Sulser, T. & Bachmann, L. M. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur. Urol. 64, 106–117 (2013).
Yu, C. Y., Desai, B., Ji, L., Groshen, S. & Jadvar, H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am. J. Nucl. Med. Mol. Imaging 4, 580–601 (2014).
Souvatzoglou, M. et al. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin. Cancer Res. 17, 3751–3759 (2011).
Brogsitter, C., Zophel, K. & Kotzerke, J. 18F-choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 40, S18–S27 (2013).
Evangelista, L., Guttilla, A., Zattoni, F., Muzzio, P. C. & Zattoni, F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol. 63, 1040–1048 (2013).
Beresford, M. J., Gillatt, D., Benson, R. J. & Ajithkumar, T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. (R. Coll. Radiol.) 22, 46–55 (2010).
Krause, B. J. et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 35, 18–23 (2008).
Schoder, H. et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. 11, 4761–4769 (2005).
Afshar-Oromieh, A., Haberkorn, U., Eder, M., Eisenhut, M. & Zechmann, C. M. [68Ga]gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nuclear Med. Mol. Imaging 39, 1085–1086 (2012).
Leek, J. et al. Prostate-specific membrane antigen: evidence for the existence of a second related human gene. Br. J. Cancer 72, 583–588 (1995).
O'Keefe, D. S. et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim. Biophys. Acta 1443, 113–127 (1998).
DeMarzo, A. M., Nelson, W. G., Isaacs, W. B. & Epstein, J. I. Pathological and molecular aspects of prostate cancer. Lancet 361, 955–964 (2003).
Grauer, L. S. et al. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSM′ protein in the LNCaP prostatic carcinoma cell line. Cancer Res. 58, 4787–4789 (1998).
Heston, W. D. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urology 49, 104–112 (1997).
Huang, E., Teh, B. S., Mody, D. R., Carpenter, L. S. & Butler, E. B. Prostate adenocarcinoma presenting with inguinal lymphadenopathy. Urology 61, 463 (2003).
Wu, L. M., Xu, J. R., Ye, Y. Q., Lu, Q. & Hu, J. N. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am. J. Roentgenol. 199, 103–110 (2012).
Birtle, A. J. et al. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of <10 ng/mL. BJU Int. 96, 303–307 (2005).
Evans, M. J. et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl Acad. Sci. USA 108, 9578–9582 (2011).
Chang, S. S. et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 5, 2674–2681 (1999).
Chang, S. S. et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59, 3192–3198 (1999).
Chang, S. S., Reuter, V. E., Heston, W. D. & Gaudin, P. B. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology 57, 801–805 (2001).
Haffner, M. C. et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 40, 1754–1761 (2009).
Samplaski, M. K., Heston, W., Elson, P., Magi-Galluzzi, C. & Hansel, D. E. Folate hydrolase (prostate-specific membrane [corrected] antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod. Pathol. 24, 1521–1529 (2011).
Silver, D. A., Pellicer, I., Fair, W. R., Heston, W. D. & Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85 (1997).
Carter, R. E., Feldman, A. R. & Coyle, J. T. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Natl Acad. Sci. USA 93, 749–753 (1996).
Robinson, M. B., Blakely, R. D., Couto, R. & Coyle, J. T. Hydrolysis of the brain dipeptide N-acetyl-l-aspartyl-l-glutamate. J. Biol. Chem. 262, 14498–14506 (1987).
Rowe, S. P. et al. Detection of 18F-FDG PET/CT occult lesions with 18F-DCFPyL PET/CT in a patient with metastatic renal cell carcinoma. Clin. Nucl. Med. 41, 83–85 (2015).
Rowe, S. P. et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted F-DCFPyL PET/CT. Ann. Nucl. Med. 29, 877–882 (2015).
Verburg, F. A., Krohn, T., Heinzel, A., Mottaghy, F. M. & Behrendt, F. F. First evidence of PSMA expression in differentiated thyroid cancer using [68Ga]PSMA-HBED-CC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 42, 1622–1623 (2015).
Krohn, T. et al. [68Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 42, 210–214 (2015).
Schwenck, J. et al. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 42, 170–171 (2015).
Rischpler, C., Maurer, T., Schwaiger, M. & Eiber, M. Intense PSMA-expression using 68Ga-PSMA PET/CT in a paravertebral schwannoma mimicking prostate cancer metastasis. Eur. J. Nucl. Med. Mol. Imaging 43, 193–194 (2016).
Ghosh, A. & Heston, W. D. W. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 91, 528–539 (2004).
Schülke, N. et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc. Natl Acad. Sci. USA 100, 12590–12595 (2003).
Commandeur, L. C. & Parsons, J. R. Degradation of halogenated aromatic compounds. Biodegradation 1, 207–220 (1990).
Bostwick, D. G., Pacelli, A., Blute, M., Roche, P. & Murphy, G. P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82, 2256–2261 (1998).
Mannweiler, S. et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 15, 167–172 (2009).
Troyer, J. K., Beckett, M. L. & Wright, G. L. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer. 62, 552–558 (1995).
Maurer, T. et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 68, 530–534 (2015).
Chang, S. S. Overview of prostate-specific membrane antigen. Rev. Urol. 6, S13–S18 (2004).
Rajasekaran, S. A. et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell 14, 4835–4845 (2003).
Liu, H. et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 58, 4055–4060 (1998).
Eder, M. et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 23, 688–697 (2012).
Ghosh, A. & Heston, W. D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 91, 528–539 (2004).
Troyer, J. K., Beckett, M. L. & Wright, G. L. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 30, 232–242 (1997).
Tagawa, S. T. et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer 116, 1075–1083 (2010).
Elsässer-Beile, U. et al. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J. Nuclear Med. 50, 606–611 (2009).
Holland, J. P. et al. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 51, 1293–1300 (2010).
Wiehr, S. et al. Pharmacokinetics and PET imaging properties of two recombinant anti-PSMA antibody fragments in comparison to their parental antibody. Prostate 74, 743–755 (2014).
Luthi-Carter, R., Barczak, A. K., Speno, H. & Coyle, J. T. Molecular characterization of human brain N-acetylated α-linked acidic dipeptidase (NAALADase). J. Pharmacol. Exp. Ther. 286, 1020–1025 (1998).
Tiffany, C. W., Lapidus, R. G., Merion, A., Calvin, D. C. & Slusher, B. S. Characterization of the enzymatic activity of PSM: comparison with brain NAALADase. Prostate 39, 28–35 (1999).
Wang, H. et al. Bioisosterism of urea-based GCPII inhibitors: synthesis and structure–activity relationship studies. Bioorg. Med. Chem. Lett. 20, 392–397 (2010).
Mease, R. C., Foss, C. A. & Pomper, M. G. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr. Top. Med. Chem. 13, 951–962 (2013).
Foss, C. A. et al. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 11, 4022–4028 (2005).
Hillier, S. M. et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 69, 6932–6940 (2009).
Maresca, K. P. et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J. Med. Chem. 52, 347–357 (2009).
Kularatne, S. A., Zhou, Z., Yang, J., Post, C. B. & Low, P. S. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted 99mTc-radioimaging agents. Mol. Pharm. 6, 790–800 (2009).
Lu, G. et al. Synthesis and SAR of 99mTc/Re-labeled small molecule prostate specific membrane antigen inhibitors with novel polar chelates. Bioorg. Med. Chem. Lett. 23, 1557–1563 (2013).
Chen, Y. et al. 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin. Cancer Res. 17, 7645–7653 (2011).
Rowe, S. P. et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J. Nucl. Med. 56, 1003–1010 (2015).
Banerjee, S. R. et al. A modular strategy to prepare multivalent inhibitors of prostate-specific membrane antigen (PSMA). Oncotarget 2, 1244–1253 (2011).
Afshar-Oromieh, A. et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 197–209 (2015).
Afshar-Oromieh, A. et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 41, 11–20 (2014).
Afshar-Oromieh, A. et al. PET/MRI with a 68Ga-PSMA ligand for the detection of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 1629–1630 (2013).
Weineisen, M., Simecek, J., Schottelius, M., Schwaiger, M. & Wester, H.-J. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 4, 63 (2014).
Afshar-Oromieh, A. et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 40, 486–495 (2013).
Roesch, F. & Riss, P. J. The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr. Top. Med. Chem. 10, 1633–1668 (2010).
Schottelius, M., Wirtz, M., Eiber, M., Maurer, T. & Wester, H. J. [111In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 5, 68 (2015).
Weineisen, M. et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 56, 1169–1176 (2015).
Weineisen, M., Simecek, J., Schottelius, M., Schwaiger, M. & Wester, H. J. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 4, 63 (2014).
Benešová, M. et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 56, 914–920 (2015).
Afshar-Oromieh, A. et al. The novel theranostic PSMA-ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry and first evaluation of tumor lesions. J. Nucl. Med. 56, 1697–1705 (2015).
Delker, A. et al. Dosimetry for Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 43, 42–51 (2015).
Ahmadzadehfar, H. et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 5, 114 (2015).
Dietlein, M. et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol. Imaging Biol. 17, 575–584 (2015).
Malik, N. et al. Radiofluorination of PSMA-HBED via Al18F2+ chelation and biological evaluations in vitro. Mol. Imaging Biol. 17, 777–785 (2015).
Mease, R. C. et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-l-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin. Cancer Res. 14, 3036–3043 (2008).
Cho, S. Y. et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 53, 1883–1891 (2012).
Szabo, Z. et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol. Imaging Biol. 17, 565–574 (2015).
Futterer, J. J. et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 241, 449–458 (2006).
Lim, H. K., Kim, J. K., Kim, K. A. & Cho, K. S. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection — a multireader study. Radiology 250, 145–151 (2009).
Sciarra, A. et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin. Cancer Res. 16, 1875–1883 (2010).
Tan, C. H., Wei, W., Johnson, V. & Kundra, V. Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. AJR Am. J. Roentgenol. 199, 822–829 (2012).
Issa, B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J. Magn. Reson. Imaging 16, 196–200 (2002).
Jacobs, M. A., Ouwerkerk, R., Petrowski, K. & Macura, K. J. Diffusion-weighted imaging with apparent diffusion coefficient mapping and spectroscopy in prostate cancer. Top. Magn. Reson. Imaging 19, 261–272 (2008).
Manenti, G. et al. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol. Med. 111, 1124–1133 (2006).
Puech, P. et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology 74, 1094–1099 (2009).
Chen, Y. J. et al. Washout gradient in dynamic contrast-enhanced MRI is associated with tumor aggressiveness of prostate cancer. J. Magn. Reson. Imaging 36, 912–919 (2012).
Oto, A. et al. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am. J. Roentgenol. 197, 1382–1390 (2011).
Yerram, N. K. et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 110, E783–E788 (2012).
Souvatzoglou, M. et al. PET/MR in prostate cancer: technical aspects and potential diagnostic value. Eur. J. Nucl. Med. Mol. Imaging 40, S79–S88 (2013).
Souvatzoglou, M. et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 1486–1499 (2013).
Eiber, M. et al. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom. Imaging 40, 1769–1771 (2014).
Eiber, M. et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol.(in the press).
Storz, E. et al. PSMA-PET/MRI-guided transrectal fusion biopsy for the detection of prostate cancer. Eur. Urol. Suppl. 14, E217 (2015).
Zettinig, O. et al. Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int. J. Comput. Assist. Radiol. Surg. 10, 1997–2007 (2015).
Maurer, T. et al. Diagnostic efficacy of 68Gallium-PSMA-PET compared to conventional imaging in lymph node staging of of 130 consecutive patients with intermediate to high-risk prostate cancer. J. Urol. http://dx.doi.org/10.1016/j.juro.2015.12.025 (2015).
Lecouvet, F. E. et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 62, 68–75 (2012).
Heesakkers, R. A. et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 9, 850–856 (2008).
Hovels, A. M. et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol. 63, 387–395 (2008).
Akduman, E. I. et al. Comparison between malignant and benign abdominal lymph nodes on diffusion-weighted imaging. Acad. Radiol. 15, 641–646 (2008).
Eiber, M. et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest. Radiol. 45, 15–23 (2010).
Kim, J. K., Kim, K. A., Park, B. W., Kim, N. & Cho, K. S. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J. Magn. Reson. Imaging 28, 714–719 (2008).
Jadvar, H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur. J. Nucl. Med. Mol. Imaging 40, S5–S10 (2013).
Mohsen, B. et al. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 112, 1062–1072 (2013).
Pinaquy, J. B. et al. Comparative effectiveness of [18F]-fluorocholine PET-CT and pelvic MRI with diffusion-weighted imaging for staging in patients with high-risk prostate cancer. Prostate 75, 323–331 (2015).
Schumacher, M. C., Radecka, E., Hellstrom, M., Jacobsson, H. & Sundin, A. [11C]acetate positron emission tomography-computed tomography imaging of prostate cancer lymph-node metastases correlated with histopathological findings after extended lymphadenectomy. Scand. J. Urol. 49, 35–42 (2015).
de Jong, I. J., Pruim, J., Elsinga, P. H., Vaalburg, W. & Mensink, H. J. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J. Nucl. Med. 44, 331–335 (2003).
Kotzerke, J. et al. Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur. J. Nucl. Med. 27, 1415–1419 (2000).
Beheshti, M. et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254, 925–933 (2010).
Kjolhede, H. et al. 18F-fluorocholine PET/CT compared with extended pelvic lymph node dissection in high-risk prostate cancer. World J. Urol. 32, 965–970 (2014).
Chakraborty, P. S., Kumar, R., Tripathi, M., Das, C. J. & Bal, C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: a novel radiotracer for imaging of prostate carcinoma. Clin. Nucl. Med. 40, 328–329 (2015).
Kabasakal, L. et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 36, 582–587 (2015).
Maurer, T. et al. Positron emission tomography/magnetic resonance imaging with 68Gallium-labeled ligand of prostate-specific membrane antigen: promising novel option in prostate cancer imaging? Int. J. Urol. 21, 1286–1288 (2014).
Pfister, D. et al. Early salvage radiotherapy following radical prostatectomy. Eur. Urol. 65, 1034–1043 (2014).
King, C. R. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int. J. Radiat. Oncol. Biol. Phys. 84, 104–111 (2012).
Rouviere, O., Vitry, T. & Lyonnet, D. Imaging of prostate cancer local recurrences: why and how? Eur. Radiol 20, 1254–1266 (2010).
Eiber, M. et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 56, 668–674 (2015).
Castellucci, P. et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur. J. Nucl. Med. Mol. Imaging 38, 55–63 (2011).
Castellucci, P. & Picchio, M. 11C-choline PET/CT and PSA kinetics. Eur. J. Nucl. Med. Mol. Imaging 40, S36–S40 (2013).
Graute, V. et al. Relationship between PSA kinetics and [18F]fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 39, 271–282 (2012).
Maurer, T. et al. PET imaging with 68Gallium-labelled ligand of prostate-specific membrane antigen (68Ga-HBED-PSMA) for staging of biochemical recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 33 (Suppl.),5023 (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
EU Clinical Trials Register. clinicaltrialsregister.eu [online], (2015).
Author information
Authors and Affiliations
Contributions
T.M. and M.E. contributed equally to the manuscript, researched data for and wrote the article. All authors made a substantial contribution to discussions of content and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.M. declares a familial association with Celgene® (Celgene Corporation, USA); grants or funding from Acelity® (KCI Licensing, Inc.), Bayer® (Bayer Aktiengesellschaft, Germany) and Takeda Oncology® (Takeda Pharmaceutical Company Limited, Japan); consultation for DLR® (Deutsches Luft-und Raumfahrtzentrum, Germany) and honoraria from Astellas® (Astellas US LLC, USA), Janssen Cilag (Johnson & Johnson, USA) and Sanofi® (Sanofi Corporation, France). M.E. declares grants or funding from Bayer® and honoraria Astellas® and Janssen Cilag. M.S. and J.E.G. declare no competing interests.
Rights and permissions
About this article
Cite this article
Maurer, T., Eiber, M., Schwaiger, M. et al. Current use of PSMA–PET in prostate cancer management. Nat Rev Urol 13, 226–235 (2016). https://doi.org/10.1038/nrurol.2016.26
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.26
This article is cited by
-
Exploring the impact of PEGylation on pharmacokinetics: a size-dependent effect of polyethylene glycol on prostate-specific membrane antigen inhibitors
EJNMMI Research (2024)
-
Deep learning–based whole-body characterization of prostate cancer lesions on [68Ga]Ga-PSMA-11 PET/CT in patients with post-prostatectomy recurrence
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Total-body PET/CT with half-dose [68 Ga]Ga-PSMA-11 for biochemical recurrent prostate cancer: comparable diagnostic value to short axial field-of-view PET/CT with full-dose [68 Ga]Ga-PSMA-11
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Heterogeneity of prostate-specific membrane antigen (PSMA) and PSMA-ligand uptake detection combining autoradiography and postoperative pathology in primary prostate cancer
EJNMMI Research (2023)
-
The relationship between biochemical recurrence and number of lymph nodes removed during surgery for localized prostate cancer
BMC Urology (2023)