Key Points
-
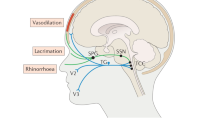
Calcitonin gene-related peptide (CGRP) is the best-validated therapeutic target for migraine
-
Monoclonal antibodies (mAbs) against CGRP or its receptor hold promise for migraine prevention, and small-molecule CGRP antagonists hold promise for acute and/or preventive treatment
-
Four mAbs targeting CGRP or its receptor have been effective in phase II studies and are being studied in phase III trials for migraine prevention
-
Non-oral systems to deliver triptans and dihydroergotamine mesylate for acute migraine treatment bypass hurdles posed by nausea, gastric stasis, and first-pass metabolism
-
Noninvasive electrical and electromagnetic neurostimulators for primary headache disorders are available for clinical use, but regulatory approval varies by country, and patients might be required to cover costs
-
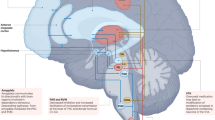
For patients with intractable, medically refractory primary headache disorders, implantable neurostimulators targeting peripheral nerves, the sphenopalatine ganglion, and high cervical spinal cord are being studied
Abstract
The primary headache disorders, which include migraine, cluster headache and tension-type headache, are among the most common diseases and leading causes of disability worldwide. The available treatment options for primary headache disorders have unsatisfactory rates of efficacy, tolerability and patient adherence. In this Review, we discuss promising new approaches for the prevention of primary headache disorders, such as monoclonal antibodies targeting calcitonin gene-related peptide (CGRP) or its receptor, and small-molecule CGRP receptor antagonists. Neuromodulation approaches employing noninvasive or implantable devices also show promise for treating primary headache disorders. Noninvasive treatments, such as transcranial magnetic stimulation and transcutaneous peripheral nerve stimulation, are delivered by devices that patients can self-administer. Implantable devices targeting the occipital nerves, sphenopalatine ganglion or high cervical spinal cord are placed using percutaneous and/or surgical procedures, and are powered either wirelessly or by surgically implanted batteries. These new and emerging treatments have the potential to address unmet patient needs and reduce headache-associated disability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Steiner, T. J., Stovner, L. J. & Birbeck, G. L. Migraine: the seventh disabler. Cephalalgia 33, 289–290 (2013).
Steiner, T. J., Stovner, L. J. & Birbeck, G. L. Migraine: the seventh disabler. Headache 53, 227–229 (2013).
Steiner, T. J., Stovner, L. J. & Birbeck, G. L. Migraine: the seventh disabler. J. Headache Pain 14, 1 (2013).
Steiner, T. J. et al. Headache disorders are third cause of disability worldwide. J. Headache Pain 16, 58 (2015).
Forouzanfar, M. H. et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 2287–2323 (2015).
Ekbom, K., Svensson, D. A., Pedersen, N. L. & Waldenlind, E. Lifetime prevalence and concordance risk of cluster headache in the Swedish twin population. Neurology 67, 798–803 (2006).
Torelli, P., Beghi, E. & Manzoni, G. C. Cluster headache prevalence in the Italian general population. Neurology 64, 469–474 (2005).
Sjaastad, O. & Bakketeig, L. S. Cluster headache prevalence. Vaga study of headache epidemiology. Cephalalgia 23, 528–533 (2003).
Tonon, C. et al. Prevalence and incidence of cluster headache in the Republic of San Marino. Neurology 58, 1407–1409 (2002).
Evers, S., Fischera, M., May, A. & Berger, K. Prevalence of cluster headache in Germany: results of the epidemiological DMKG study. J. Neurol. Neurosurg. Psychiatry 78, 1289–1290 (2007).
Rozen, T. D. & Fishman, R. S. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache 52, 99–113 (2012).
Hepp, Z. et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 35, 478–488 (2015).
Mason, R. T. et al. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 308, 653–655 (1984).
Brain, S. D., Williams, T. J., Tippins, J. R., Morris, H. R. & MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 313, 54–56 (1985).
Walker, C. S. et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann. Clin. Transl Neurol. 2, 595–608 (2015).
Lassen, L. H. et al. CGRP may play a causative role in migraine. Cephalalgia 22, 54–61 (2002).
Hansen, J. M., Hauge, A. W., Olesen, J. & Ashina, M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30, 1179–1186 (2010).
Underwood, E. Feature: Will antibodies finally put an end to migraines? Science http://www.sciencemag.org/news/2016/01/feature-will-antibodies-finally-put-end-migraines (2016).
Bigal, M. E. et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 14, 1081–1090 (2015).
Goadsby, P. J. & Edvinsson, L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 33, 48–56 (1993).
Cady, R. K., Vause, C. V., Ho, T. W., Bigal, M. E. & Durham, P. L. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 49, 1258–1266 (2009).
Cernuda-Morollón, E. et al. CGRP and VIP levels as predictors of efficacy of onabotulinumtoxin type A in chronic migraine. Headache 54, 987–995 (2014).
Cady, R. et al. An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache 54, 269–277 (2014).
Connor, K. M. et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73, 970–977 (2009).
Ho, T. W. et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 83, 958–966 (2014).
Hewitt, D. J. et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 31, 712–722 (2011).
Marcus, R. et al. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 34, 114–125 (2014).
Olesen, J. et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 350, 1104–1110 (2004).
Shi, L. et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J. Pharmacol. Exp. Ther. 356, 223–231 (2016).
Ramos, M. L. & Pascual, J. AMG 334 CGRP antibody for migraine: time to celebrate? Lancet Neurol. 15, 347–349 (2016).
Dodick, D. et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 13, 885–892 (2014).
Dodick, D. et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 13, 1100–1107 (2014).
Bigal, M. E. et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 14, 1091–1100 (2015).
Sun, H. et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. http://dx.doi.org/10.1016/s1474-4422(16)00019-3 (2016).
Bigal, M. E. et al. TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology http://dx.doi.org/10.1212/wnl.0000000000002801 (2016).
US National Library of Medicine. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT02614183 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02614196 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02614261 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02397473 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02438826 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02576951 (2016).
Smith, J. et al. OR01 — proof of concept clinical trial of ALD403, ananti-calcitonin gene-related peptide (CGRP) antibody in the prevention of migraine — 6 month data. International Headache Society abstracts. Cephalalgia 35 (6 Suppl.), 4–5 (2015).
[No authors listed.] Alder reports phase 2b trial of ALD403 meets primary and secondary endpoints demonstrating migraine prevention in patients with chronic migraine. Alder http://investor.alderbio.com/releasedetail.cfm?releaseid=962238 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02559895 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02629861 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02621931 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02638103 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01952574 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02456740 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02483585 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02066415(2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02174861 (2016).
Lenz, R. et al. Prevention of episodic migraine with AMG 334, a human anti-calcitonin gene-related peptide receptor monoclonal antibody: phase 2 study results and 52-week analysis of open-label extension (S26.002). Neurology 86 (16 Suppl.), S26.002 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02630459 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02575833 (2016).
Voss,T. et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 38, 887–898 (2001).
Goldstein, D. J. et al. Selective serotonin 1F (5-HT(1F)) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet 358, 1230–1234 (2001).
Ferrari, M. D. et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan — a randomised proof-of-concept trial. Cephalalgia 30, 1170–1178 (2010).
Farkkila, M. et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 11, 405–413 (2012).
Reuter, U., Israel, H. & Neeb, L. The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migraine. Ther. Adv. Neurol. Disord. 8, 46–54 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00883051 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02439320 (2016).
Obaidi, M. et al. Improved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powder. Headache 53, 1323–1333 (2013).
Cady, R. et al. A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (the TARGET study). Headache 55, 88–100 (2015).
Tepper, S. J. et al. AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder versus 100 mg oral sumatriptan in the acute treatment of migraines (the COMPASS study): a comparative randomized clinical trial across multiple attacks. Headache 55, 621–635 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02571049 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02583425 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02569853 (2016).
Siegel, S. J. et al. A unique iontophoretic patch for optimal transdermal delivery of sumatriptan. Pharm. Res. 24, 1919–1926 (2007).
Vikelis, M., Mitsikostas, D. D. & Rapoport, A. M. Sumatriptan transdermal iontophoretic patch (NP101-Zelrix): review of pharmacology, clinical efficacy, and safety in the acute treatment of migraine. Neuropsychiatr. Dis. Treat. 8, 429–434 (2012).
Hurtukova, D. Urgent – Zecuity (sumatriptan iontophoretic transdermal system) suspensionof marketing. Teva Pharmaceuticals http://www.fda.gov/downloads/Drugs/DrugSafety/UCM506332.pdf (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00623636 (2016).
Aurora, S. et al. MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache 51, 507–517 (2011).
Tepper, S. J. et al. MAP0004, orally inhaled dihydroergotamine for acute treatment of migraine: efficacy of early and late treatments. Mayo Clin. Proc. 86, 948–955 (2011).
Tepper, S. J. et al. Efficacy and safety of MAP0004, orally inhaled DHE in treating migraines with and without allodynia. Headache 52, 37–47 (2012).
Armer, T. A., Lynch, M., Moutvic, R. & Singer, A. Toxicological assessment of dihydroergotamine after chronic inhalation in dogs. Toxicol. Pathol. 39, 544–552 (2011).
Cook, R. O., Shrewsbury, S. B. & Ramadan, N. M. Reduced adverse event profile of orally inhaled DHE (MAP0004) versus IV DHE: potential mechanism. Headache 49, 1423–1434 (2009).
Kellerman, D. et al. Lack of drug interaction between the migraine drug MAP0004 (orally inhaled dihydroergotamine) and a CYP3A4 inhibitor in humans. Cephalalgia 32, 150–158 (2012).
Kori, S., Kellerman, D. J., Voloshko, P. & Haugen, G. Effects of a supratherapeutic dose of investigational orally inhaled dihydroergotamine (MAP0004) on QT interval: a randomized, double-blind, active- and placebo-controlled crossover study in healthy volunteers. Clin. Ther. 34, 1920–1928 (2012).
Noveck, R. J. et al. Assessing acute systemic effects of an inhaled drug with serial echocardiography: a placebo-controlled comparison of inhaled and intravenous dihydroergotamine. Drug Des. Devel. Ther. 7, 619–625 (2013).
Shrewsbury, S. B., Cook, R. O., Taylor, G., Edwards, C. & Ramadan, N. M. Safety and pharmacokinetics of dihydroergotamine mesylate administered via a novel (Tempo) inhaler. Headache 48, 355–367 (2008).
Shrewsbury, S. B., Kori, S. H., Miller, S. D., Pedinoff, A. & Weinstein, S. Randomized, double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of MAP0004 (orally-inhaled DHE) in adult asthmatics. Curr. Med. Res. Opin. 24, 1977–1985 (2008).
Andreou, A. P., Sprenger, T. & Goadsby, P. J. Cortical modulation of thalamic function during cortical spreading depression — unraveling a new central mechanism involved in migraine aura. J. Headache Pain 14, I6 (2013).
Andreou, A. P. et al. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain http://dx.doi.org/10.1093/brain/aww118 (2016).
Teepker, M. et al. Low-frequency rTMS of the vertex in the prophylactic treatment of migraine. Cephalalgia 30, 137–144 (2010).
Misra, U. K., Kalita, J. & Bhoi, S. K. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J. Neurol. 260, 2793–2801 (2013).
Conforto, A. B. et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia 34, 464–472 (2014).
Lipton, R. B. et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 9, 373–380 (2010).
Bhola, R. et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J. Headache Pain 16, 535 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02357381 (2016).
Schoenen, J., Roberta, B., Magis, D. & Coppola, G. Noninvasive neurostimulation methods for migraine therapy: the available evidence. Cephalalgia http://dx.doi.org/10.1177/0333102416636022 (2016).
Auvichayapat, P. et al. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J. Med. Assoc. Thai. 95, 1003–1012 (2012).
Dasilva, A. F. et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache 52, 1283–1295 (2012).
Vigano, A. et al. Transcranial direct current stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J. Headache Pain 14, 23 (2013).
Antal, A., Kriener, N., Lang, N., Boros, K. & Paulus, W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 31, 820–828 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02562222 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02562196 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02122757 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02120326 (2016).
Schoenen, J. et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80, 697–704 (2013).
[No authors listed.] Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 85, 1997 (2015).
Magis, D., Sava, S., d'Elia, T. S., Baschi, R. & Schoenen, J. Safety and patients' satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population. J. Headache Pain 14, 95 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02590939 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02411513 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02438553 (2016).
National Institute of Health and Care Excellence. Interventional procedure overview of transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine. NICE https://www.nice.org.uk/guidance/ipg552 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01701245 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01958125 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01792817 (2016).
Gaul, C. et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia 36, 534–546 (2016).
Silberstein, S. D. et al. Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache 56, 1317–1332 (2016).
Barbanti, P. et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J. Headache Pain 16, 61 (2015).
Goadsby, P. J., Grosberg, B. M., Mauskop, A., Cady, R. & Simmons, K. A. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 34, 986–993 (2014).
Silberstein, S. D. et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: the EVENT study. Neurology 87, 1–10 (2016).
Magis, D., Gérard, P. & Schoenen, J. Transcutaneous vagus nerve stimulation (tVNS) for headache prophylaxis: initial experience. J. Headache Pain 14, P198 (2013).
Straube, A., Ellrich, J., Eren, O., Blum, B. & Ruscheweyh, R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J. Headache Pain 16, 543 (2015).
Fontaine, D., Vandersteen, C., Magis, D. & Lanteri-Minet, M. Neuromodulation in cluster headache. Adv. Techn. Stand. Neurosurg. 42, 3–21 (2015).
Wilbrink, L. A. et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: rationale and protocol of a randomised trial. Cephalalgia 33, 1238–1247 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01151631 (2016).
Burns, B., Watkins, L. & Goadsby, P. J. Treatment of hemicrania continua by occipital nerve stimulation with a bion device: long-term follow-up of a crossover study. Lancet Neurol. 7, 1001–1012 (2008).
Lambru, G., Shanahan, P., Watkins, L. & Matharu, M. S. Occipital nerve stimulation in the treatment of medically intractable SUNCT and SUNA. Pain Physician 17, 29–41 (2014).
Saper, J. R. et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 31, 271–285 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00200109 (2016).
Silberstein, S. D. et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 32, 1165–1179 (2012).
Dodick, D. W. et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 35, 344–358 (2015).
Janiski, H. Important product usage information. St. Jude Medical https://www.swissmedic.ch/recalllists_dl/10612/Vk_20141022_21-e1.pdf (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01775735 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02725554 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02729480 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01255813 (2016).
Schoenen, J. et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia 33, 816–830 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02168764 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01540799 (2016).
Wolter, T., Kiemen, A. & Kaube, H. High cervical spinal cord stimulation for chronic cluster headache. Cephalalgia 31, 1170–1180 (2011).
Gaul, C., Jurgens, T. & May, A. Concerning high cervical spinal cord stimulation for chronic cluster headache. Cephalalgia 31, 1588–1589 (2011).
De Agostino, R., Federspiel, B., Cesnulis, E. & Sandor, P. S. High-cervical spinal cord stimulation for medically intractable chronic migraine. Neuromodulation 18, 289–296 (2015).
Kapural, L. et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology 123, 851–860 (2015).
Arcioni, R. et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: a prospective, open-label, exploratory study. Eur. J. Pain 20, 70–78 (2016).
BioMed Central. HF10™ spinal cord stimulation in the treatment of refractory chronic migraine. ISRCTN registry http://www.isrctn.com/ISRCTN94247798 (2016).
Mitsikostas, D. D. et al. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J. Headache Pain 15, 79 (2014).
Bartsch, T., Levy, M. J., Knight, Y. E. & Goadsby, P. J. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain 109, 367–378 (2004).
Holland, P. R., Akerman, S. & Goadsby, P. J. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur. J. Neurosci. 24, 2825–2833 (2006).
Holland, P. R., Akerman, S. & Goadsby, P. J. Orexin 1 receptor activation attenuates neurogenic dural vasodilation in an animal model of trigeminovascular nociception. J. Pharmacol. Exp. Ther. 315, 1380–1385 (2005).
Hoffmann, J. et al. Evidence for orexinergic mechanisms in migraine. Neurobiol. Dis. 74, 137–143 (2015).
Herring, W. J. et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology 79, 2265–2274 (2012).
Chabi, A. et al. Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis. Cephalalgia 35, 379–388 (2015).
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33, 629–808 (2013).
Martelletti, P. et al. Refractory chronic migraine: a consensus statement on clinical definition from the European Headache Federation. J. Headache Pain 15, 47 (2014).
Cady, R. A novel intranasal breath-powered delivery system for sumatriptan: a review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine. Expert Opin. Drug Deliv. 12, 1565–1577 (2015).
Cady, R. The pharmacokinetics and clinical efficacy of AVP-825: a potential advancement for acute treatment of migraine. Expert Opin. Pharmacother. 16, 2039–2051 (2015).
Goldstein, J. et al. A sumatriptan iontophoretic transdermal system for the acute treatment of migraine. Headache 52, 1402–1410 (2012).
Bigal, M. E., Lipton, R. B., Newman, L. C., Pierce, M. W. & Silberstein, S. D. Sumatriptan iontophoretic transdermal system reduces treatment-emergent nausea and is effective in patients with and without nausea at baseline — results from a randomized controlled trial. Headache 55, 1124–1132 (2015).
Smith, T. R. et al. Twelve-month tolerability and efficacy study of NP101, the sumatriptan iontophoretic transdermal system. Headache 52, 612–624 (2012).
[No authors listed.] Zosano Pharma announces positive phase 1 results for Its ZP-Triptan Patch program for treatment of migraine. Zozano Pharma https://globenewswire.com/news-release/2015/11/02/782591/0/en/Zosano-Pharma-Announces-Positive-Phase-1-Results-for-Its-ZP-Triptan-Patch-Program-for-Treatment-of-Migraine.html (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02745392 (2016).
Acorda Therapeutics, Inc. Acorda presents phase 1 data on CVT-427 for acute treatment of migraine at 58th Annual Scientific Meeting of the American Headache Society. Business Wire http://www.businesswire.com/news/home/20160609005119/en/ (2016).
House, D. W. Acorda on go with CVT-427 phase 2. Acorda Therapeutics, Inc. http://seekingalpha.com/news/3165610-acorda-go-cvtminus-427-phase-2 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02609945 (2016).
Biocentury Online Intellegence. Atogepant (AGN-241689, MK-8031). Biocentury.com http://www.biocentury.com/products/mk-8031 (2016).
Grace, P. M. et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience 280, 299–317 (2014).
Grace, P. M., Maier, S. F. & Watkins, L. R. Opioid-induced central immune signaling: implications for opioid analgesia. Headache 55, 475–489 (2015).
Poulsen, J. N., Larsen, F., Duroux, M. & Gazerani, P. Primary culture of trigeminal satellite glial cells: a cell-based platform to study morphology and function of peripheral glia. Int. J. Physiol. Pathophysiol. Pharmacol. 6, 1–12 (2014).
Cooper, Z. D. et al. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict. Biol. http://dx.doi.org/10.1111/adb.12261 (2015).
Johnson, J. L. et al. Glial attenuation with ibudilast in the treatment of medication overuse headache: a double-blind, randomized, placebo-controlled pilot trial of efficacy and safety. Headache 55, 1192–1208 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01389193 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02686034 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02378844 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01630044 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01899040 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02307071 (2016).
Author information
Authors and Affiliations
Contributions
N.M.S. and A.M.R. contributed equally to researching data for the article, discussion of the article's content, and writing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.M.S. received an American Headache Society travel grant with funding from Avanir. A.M.R. serves on the speaker's bureaus of Avanir, Depomed and Teva, consults for Eli Lilly and serves on the advisory boards of Autonomic Technologies, Doctor Reddy's, Pernix, Teva and Zosano.
Glossary
- CGRP receptor
-
The calcitonin gene-related peptide (CGRP) receptor is a complex between the calcitonin receptor-like receptor and the receptor activity-modifying protein 1 (RAMP1). Expression of the CGRP receptor has been confirmed in the vasculature, the trigeminal ganglion, and the spinal trigeminal complex of the brainstem.
- LY2951742
-
A fully humanized anti-calcitonin gene-related peptide IgG4 monoclonal antibody with a 28-day half-life (also known as galcanezumab).
- Fast-track status
-
Fast-track status is granted by the FDA to investigational drugs anticipated to fill an unmet need in treating a serious condition.
- ALD403
-
A genetically engineered, desialylated, humanized anti-calcitonin gene-related peptide IgG1 antibody with a 31-day half-life.
- TEV-48125
-
A fully humanized anti-calcitonin gene-related peptide IgG2a monoclonal antibody with a half-life of 40–48 days (previously known as LBR-101).
- AMG 334
-
A human IgG2 monoclonal antibody that is targeted against the calcitonin gene-related peptide receptor. AMG 334 has a half-life of 21 days.
- Ubrogepant
-
An orally administered small-molecule calcitonin gene-related peptide antagonist for the acute treatment of migraine (also known as MK-1602).
- MK-8031
-
An orally administered small-molecule calcitonin gene-related peptide antagonist for the prevention of migraine.
- Acute treatments
-
Treatments that are used as needed while experiencing a headache, with the purpose of aborting the headache. These treatments include simple analgesics, triptans, and ergot derivatives such as dihydroergotamine mesylate.
- Paraesthesia
-
An abnormal sensation, often described as a 'pins and needles' feeling, that can be caused by medications or by damage, compression, or stimulation of peripheral nerves.
- Double-dummy
-
A double-dummy study is a form of double-blind study that is used when the two treatments being studied cannot be made to appear identical. For example, it can be used to compare an inhaled medication with an oral medication. All participants are administered two treatments — one inhaled and one oral. Participants are randomly assigned to receive either the active inhaled treatment and placebo oral treatment or the placebo inhaled treatment and active oral treatment.
- Atypical triptan sensations
-
Atypical triptan sensations are often reported following the use of triptans. Atypical triptan sensations include tightness and tingling in the chest, limbs and face. They are benign, but can be mistaken for much more serious conditions.
- Cutaneous allodynia
-
In cutaneous allodynia, central sensitization to pain causes normally non-noxious tactile stimuli to skin (such as showering, shaving, brushing the hair or wearing tight clothing) to be experienced as painful.
- Status migrainosus
-
A migraine attack lasting more than 72h.
- Hemicrania continua
-
A primary headache disorder that results in continuous pain in one side of the face and head, with associated ipsilateral autonomic symptoms.
- Lead migration
-
The displacement of an electrical neurostimulation lead following implantation. Lead migration is the most common complication following the surgical implantation of a peripheral nerve stimulator or spinal cord stimulator.
- Sphenopalatine ganglion
-
The sphenopalatine ganglion, also known as the pterygopalatine ganglion, sits in the pterygopalatine fossa and contains parasympathetic nerve bodies and postsynaptic sympathetic fibres.
Rights and permissions
About this article
Cite this article
Schuster, N., Rapoport, A. New strategies for the treatment and prevention of primary headache disorders. Nat Rev Neurol 12, 635–650 (2016). https://doi.org/10.1038/nrneurol.2016.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.143
This article is cited by
-
A comprehensive review on biomarkers associated with painful temporomandibular disorders
International Journal of Oral Science (2021)
-
Neurotransmitter and tryptophan metabolite concentration changes in the complete Freund’s adjuvant model of orofacial pain
The Journal of Headache and Pain (2020)
-
Fremanezumab blocks CGRP induced dilatation in human cerebral, middle meningeal and abdominal arteries
The Journal of Headache and Pain (2018)
-
Migraine and cluster headache – the common link
The Journal of Headache and Pain (2018)
-
Cluster headache
Nature Reviews Disease Primers (2018)