Key Points
-
Atherosclerotic cardiovascular disease is the leading cause of death worldwide; the disease process is accelerated in patients with chronic kidney disease
-
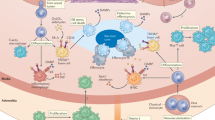
Hypercholesterolemia leads to accumulation of plasma LDL in the artery wall; LDL and its components elicit vascular inflammation that drives the build-up of lipid-laden atherosclerotic plaques
-
Several small populations of innate immune cells have important roles at different stages of atherosclerosis development, but macrophages are the main innate immune effector cell type in the plaque
-
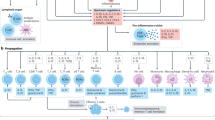
T cells that respond to autoantigenic components of LDL particles orchestrate plaque development; T helper type 1 (TH1) cells promote atherosclerosis, regulatory T cells are protective and TH17 cells promote plaque stability
-
Many connections between the immune system and metabolism exist; acute inflammation induces hypertriglyceridemia, whereas chronic inflammation has more complex effects
-
Novel approaches such as anti-inflammatory therapies, T-cell-based treatments or vaccination against LDL, could potentially reduce cardiovascular inflammation and protect against the development of atherosclerosis
Abstract
Cardiovascular disease is the leading cause of death worldwide, both in the general population and among patients with chronic kidney disease (CKD). In most cases, the underlying cause of the cardiovascular event is atherosclerosis — a chronic inflammatory disease. CKD accelerates atherosclerosis via augmentation of inflammation, perturbation of lipid metabolism, and other mechanisms. In the artery wall, subendothelial retention of plasma lipoproteins triggers monocyte-derived macrophages and T helper type 1 (TH1) cells to form atherosclerotic plaques. Inflammation is initiated by innate immune reactions to modified lipoproteins and is perpetuated by TH1 cells that react to autoantigens from the apolipoprotein B100 protein of LDL. Other T cells are also active in atherosclerotic lesions; regulatory T cells inhibit pathological inflammation, whereas TH17 cells can promote plaque fibrosis. The slow build-up of atherosclerotic plaques is asymptomatic, but plaque rupture or endothelial erosion can induce thrombus formation, leading to myocardial infarction or ischaemic stroke. Targeting risk factors for atherosclerosis has reduced mortality, but a need exists for novel therapies to stabilize plaques and to treat arterial inflammation. Patients with CKD would likely benefit from such preventive measures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Schiffrin, E. L., Lipman, M. L. & Mann, J. F. Chronic kidney disease: effects on the cardiovascular system. Circulation 116, 85–97 (2007).
Hakeem, A., Bhatti, S. & Chang, S. M. Screening and risk stratification of coronary artery disease in end-stage renal disease. JACC Cardiovasc. Imaging 7, 715–728 (2014).
Stenvinkel, P. et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 55, 1899–1911 (1999).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Skalen, K. et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754 (2002).
Williams, K. J. & Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15, 551–561 (1995).
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 92, 3893–3897 (1995). Discovery of the cellular immune response in the atherosclerotic plaque.
Frostegard, J. et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43 (1999). Study demonstrating that atherosclerosis is a T H 1-type disease.
Virchow, R. in Gesammelte Abhandlungen zur Wissenschaftlichen Medicin (Meidinger & Sohn Corp, 1856).
Anitschkoff, N. Über Veränderungen der Kaninchen-Aorta bei experimentelle Cholesterolinsteatose. Beitr. Path. Anat. 56, 379–391 (in German) (1913).
de Beer, F. C. et al. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br. Heart J. 47, 239–243 (1982).
Liuzzo, G. et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 331, 417–424 (1994). Study demonstrating that CRP is a biomarker for cardiovascular disease.
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 336, 973–979 (1997).
Hirschfield, G. M. et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 102, 8309–8314 (2005).
Tennent, G. A. et al. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis 196, 248–255 (2008).
Morrone, G. et al. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J. Biol. Chem. 263, 12554–12558 (1988).
Hoefer, I. E. et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 36, 2635–2642 (2015).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008). Report that patients with elevated CRP levels benefit from statin therapy.
Qureshi, A. R. et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J. Am. Soc. Nephrol. 13 (Suppl. 1), S28–S36 (2002).
Tong, M. et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin. J. Am. Soc. Nephrol. 2, 889–897 (2007).
Norata, G. D., Garlanda, C. & Catapano, A. L. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc. Med. 20, 35–40 (2010).
Deban, L. et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 11, 328–334 (2010).
Norata, G. D. et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation 120, 699–708 (2009).
Muhlestein, J. B. et al. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97, 633–636 (1998).
Caligiuri, G., Rottenberg, M., Nicoletti, A., Wigzell, H. & Hansson, G. K. Chlamydia pneumoniae infection does not induce or modify atherosclerosis in mice. Circulation 103, 2834–2838 (2001). Study demonstrating that Chlamydia pneumoniae does not cause atherosclerosis.
Wright, S. D. et al. Infectious agents are not necessary for murine atherogenesis. J. Exp. Med. 191, 1437–1442 (2000).
Andraws, R., Berger, J. S. & Brown, D. L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA 293, 2641–2647 (2005). Study demonstrating that antibiotics do not prevent myocardial infarction.
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011). Study linking gut metabolism to cardiovascular disease.
Schunkert, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Harismendy, O. et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature 470, 264–268 (2011).
Lusis, A. J. Genetics of atherosclerosis. Trends Genet. 28, 267–275 (2012).
Davies, R. W. et al. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ. Cardiovasc. Genet. 5, 217–225 (2012).
Swanberg, M. et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat. Genet. 37, 486–494 (2005).
Bjorkbacka, H. et al. Weak associations between human leucocyte antigen genotype and acute myocardial infarction. J. Intern. Med. 268, 50–58 (2010).
Pentikainen, M. O., Oorni, K., Ala-Korpela, M. & Kovanen, P. T. Modified LDL — trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 247, 359–370 (2000).
Cybulsky, M. I. & Gimbrone, M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788–791 (1991). Discovery that the leukocyte-recruiting adhesion molecule VCAM-1 is induced during atherogenesis.
Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L. & Ross, R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 18, 842–851 (1998).
Chevre, R. et al. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ. Res. 114, 770–779 (2014).
McArdle, S., Mikulski, Z. & Ley, K. Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis. J. Exp. Med. 213, 1117–1131 (2016).
Fowler, S., Shio, H. & Haley, N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab. Invest. 41, 372–378 (1979).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Goldstein, J. L., Ho, Y. K., Basu, S. K. & Brown, M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl Acad. Sci. USA 76, 333–337 (1979). Discovery of scavenger receptor expression on macrophages.
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013). State-of-the-art review of the role of macrophages in atherosclerosis.
Kunjathoor, V. V. et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988 (2002).
Buono, C., Anzinger, J. J., Amar, M. & Kruth, H. S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Invest. 119, 1373–1381 (2009).
Park, Y. M., Febbraio, M. & Silverstein, R. L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 (2009).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Chinetti-Gbaguidi, G. et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ. Res. 108, 985–995 (2011).
Kadl, A. et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 107, 737–746 (2010).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Rong, J. X., Shapiro, M., Trogan, E. & Fisher, E. A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl Acad. Sci. USA 100, 13531–13536 (2003).
Feil, S. et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 115, 662–667 (2014). Evidence for transdifferentiation accounting for foam cells of lesions.
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015). Further evidence that smooth muscle cells can develop into macrophage-like cells.
Allahverdian, S., Chehroudi, A. C., McManus, B. M., Abraham, T. & Francis, G. A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129, 1551–1559 (2014).
Jonasson, L., Holm, J., Skalli, O., Bondjers, G. & Hansson, G. K. Regional accumulations of T-cells, macrophages, and smooth-muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 (1986). Discovery of immune cells in atherosclerosis.
Richardson, P. D., Davies, M. J. & Born, G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 2, 941–944 (1989).
Naghavi, M. et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108, 1664–1672 (2003).
van der Wal, A. C., Becker, A. E., van der Loos, C. M. & Das, P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89, 36–44 (1994).
Kubo, T. et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50, 933–939 (2007).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Libby, P. & Pasterkamp, G. Requiem for the 'vulnerable plaque'. Eur. Heart J. 36, 2984–2987 (2015). Provocative review on the changing features of acute coronary syndromes.
van Lammeren, G. W. et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation 129, 2269–2276 (2014).
Amento, E. P., Ehsani, N., Palmer, H. & Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. 11, 1223–1230 (1991).
Ovchinnikova, O. A. et al. The collagen cross-linking enzyme lysyl oxidase is associated with the healing of human atherosclerotic lesions. J. Intern. Med. 276, 525–536 (2014).
Hansson, G. K., Hellstrand, M., Rymo, L., Rubbia, L. & Gabbiani, G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J. Exp. Med. 170, 1595–1608 (1989).
Ovchinnikova, O. et al. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe−/− mice. Am. J. Pathol. 174, 693–700 (2009).
Back, M., Ketelhuth, D. F. & Agewall, S. Matrix metalloproteinases in atherothrombosis. Prog. Cardiovasc. Dis. 52, 410–428 (2010).
Kovanen, P. T., Kaartinen, M. & Paavonen, T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92, 1084–1088 (1995).
Ehara, S. et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110, 3424–3429 (2004).
Edfeldt, K., Swedenborg, J., Hansson, G. K. & Yan, Z. Q. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105, 1158–1161 (2002). Discovery of Toll-like receptors in human atherosclerosis.
Miller, Y. I. et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568 (2003).
West, X. Z. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467, 972–976 (2010).
Lundberg, A. M. & Hansson, G. K. Innate immune signals in atherosclerosis. Clin. Immunol. 134, 5–24 (2010).
Bjorkbacka, H. et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 10, 416–421 (2004). Study demonstrating an effect of innate immune activation on atherosclerosis.
Hurt-Camejo, E., Camejo, G., Peilot, H., Oorni, K. & Kovanen, P. Phospholipase A2 in vascular disease. Circ. Res. 89, 298–304 (2001).
Lonn, E. et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293, 1338–1347 (2005).
Mann, J. F. et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease — results of the renal Hope-2 study. Nephrol. Dial. Transplant. 23, 645–653 (2008).
White, H. D. et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 370, 1702–1711 (2014).
O'Donoghue, M. L. et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 312, 1006–1015 (2014).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). Study showing that cholesterol crystals activate the inflammasome in early atherogenesis.
Rajamaki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Ridker, P. M., Thuren, T., Zalewski, A. & Libby, P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 (2011).
Loppnow, H. & Libby, P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J. Clin. Invest. 85, 731–738 (1990).
Maier, W. et al. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation 111, 1355–1361 (2005).
Biasucci, L. M. et al. Elevated levels of interleukin-6 in unstable angina. Circulation 94, 874–877 (1996).
Drechsler, M., Megens, R. T., van Zandvoort, M., Weber, C. & Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010).
Tupin, E. et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 199, 417–422 (2004). Study demonstrating a role of lipid-recognizing natural killer T cells in atherosclerosis.
Whitman, S. C., Rateri, D. L., Szilvassy, S. J., Yokoyama, W. & Daugherty, A. Depletion of natural killer cell function decreases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler. Thromb. Vasc. Biol. 24, 1049–1054 (2004).
van Puijvelde, G. H. et al. Effect of natural killer T cell activation on the initiation of atherosclerosis. Thromb. Haemost. 102, 223–230 (2009).
Cheng, H. Y., Wu, R. & Hedrick, C. C. Gammadelta (γδ) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis 234, 265–269 (2014).
Elhage, R. et al. Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am. J. Pathol. 165, 2013–2018 (2004).
Jonasson, L., Holm, J., Skalli, O., Gabbiani, G. & Hansson, G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J. Clin. Invest. 76, 125–131 (1985).
Bobryshev, Y. V. & Lord, R. S. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc. Res. 37, 799–810 (1998).
Llodra, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA 101, 11779–11784 (2004).
Palinski, W. et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl Acad. Sci. USA 86, 1372–1376 (1989).
Bui, M. N. et al. Autoantibody titers to oxidized low-density lipoprotein in patients with coronary atherosclerosis. Am. Heart J. 131, 663–667 (1996).
Wu, R., Huang, Y. H., Elinder, L. S. & Frostegard, J. Lysophosphatidylcholine is involved in the antigenicity of oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 18, 626–630 (1998).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740 (2000).
Fredrikson, G. N. et al. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 23, 872–878 (2003).
Xu, Q., Kleindienst, R., Waitz, W., Dietrich, H. & Wick, G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J. Clin. Invest. 91, 2693–2702 (1993).
Benagiano, M. et al. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J. Immunol. 174, 6509–6517 (2005).
George, J., Afek, A., Gilburd, B., Shoenfeld, Y. & Harats, D. Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in LDL-receptor deficient mice. J. Am. Coll. Cardiol. 38, 900–905 (2001).
Schett, G. et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J. Clin. Invest. 96, 2569–2577 (1995).
Wick, G., Jakic, B., Buszko, M., Wick, M. C. & Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 11, 516–529 (2014).
Maron, R. et al. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation 106, 1708–1715 (2002).
Klingenberg, R., Ketelhuth, D. F., Strodthoff, D., Gregori, S. & Hansson, G. K. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe−/− mice. Immunobiology 217, 540–547 (2012).
George, J. et al. Adoptive transfer of beta2-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation 102, 1822–1827 (2000).
Duner, P. et al. Immunization of apoE−/− mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovasc. Res. 91, 528–536 (2011).
Grabner, R. et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J. Exp. Med. 206, 233–248 (2009).
Martel, C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123, 1571–1579 (2013).
Cuffy, M. C. et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J. Immunol. 179, 5246–5254 (2007).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Watanabe, M. et al. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J. Atheroscler. Thromb. 14, 325–331 (2007).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity 42, 1100–1115 (2015). Study describing the formation of tertiary lymphoid organs around atherosclerotic arteries.
Clement, M. et al. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 560–570 (2015).
Hansson, G. K., Holm, J. & Jonasson, L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am. J. Pathol. 135, 169–175 (1989).
Zhou, X., Stemme, S. & Hansson, G. K. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am. J. Pathol. 149, 359–366 (1996).
Stemme, S., Holm, J. & Hansson, G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler. Thromb. 12, 206–211 (1992).
Paulsson, G., Zhou, X., Tornquist, E. & Hansson, G. K. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20, 10–17 (2000).
Zhou, X., Nicoletti, A., Elhage, R. & Hansson, G. K. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102, 2919–2922 (2000).
Zhou, X., Robertson, A. K., Hjerpe, C. & Hansson, G. K. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26, 864–870 (2006).
Dansky, H. M., Charlton, S. A., Harper, M. M. & Smith, J. D. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA 94, 4642–4646 (1997).
Song, L., Leung, C. & Schindler, C. Lymphocytes are important in early atherosclerosis. J. Clin. Invest. 108, 251–259 (2001).
Daugherty, A. et al. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J. Clin. Invest. 100, 1575–1580 (1997).
Emeson, E. E., Shen, M. L., Bell, C. G. & Qureshi, A. Inhibition of atherosclerosis in CD4 T-cell-ablated and nude (nu/nu) C57BL/6 hyperlipidemic mice. Am. J. Pathol. 149, 675–685 (1996).
Olofsson, P. S. et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 117, 1292–1301 (2008).
Hermansson, A. et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 207, 1081–1093 (2010). Study showing that T cells recognize ApoB peptides of LDL.
Uyemura, K. et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Invest. 97, 2130–2138 (1996).
Gupta, S. et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997). Study showing that the T H 1 cytokine IFN γ promotes atherosclerosis.
Whitman, S. C., Ravisankar, P. & Daugherty, A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J. Interferon Cytokine Res. 22, 661–670 (2002).
Buono, C. et al. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 23, 454–460 (2003).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102, 1596–1601 (2005). Study showing that the T H 1 pathway is proatherogenic.
Pober, J. S. et al. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J. Exp. Med. 157, 1339–1353 (1983).
Hansson, G. K., Jonasson, L., Holm, J., Clowes, M. M. & Clowes, A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ. Res. 63, 712–719 (1988).
Perisic, L. et al. Gene expression signatures, pathways and networks in carotid atherosclerosis. J. Intern. Med. 279, 293–308 (2016).
Zhou, X., Paulsson, G., Stemme, S. & Hansson, G. K. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Invest. 101, 1717–1725 (1998).
Davenport, P. & Tipping, P. G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163, 1117–1125 (2003).
King, V. L., Szilvassy, S. J. & Daugherty, A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 22, 456–461 (2002).
Binder, C. J. et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 (2004).
Cardilo-Reis, L. et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 (2012).
Miller, A. M. et al. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 205, 339–346 (2008).
IBC 50K CAD Consortium. Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 7, e1002260 (2011).
Lim, H. et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40, 153–165 (2014).
Eid, R. E. et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119, 1424–1432 (2009).
de Boer, O. J. et al. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 220, 499–508 (2010).
Danzaki, K. et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 273–280 (2012).
Usui, F. et al. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem. Biophys. Res. Commun. 420, 72–77 (2012).
Madhur, M. S. et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1565–1572 (2011).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Gao, Q. et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 185, 5820–5827 (2010).
Erbel, C. et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 183, 8167–8175 (2009).
Smith, E. et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755 (2010).
Taleb, S. et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206, 2067–2077 (2009).
Cheng, X. et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis 215, 471–474 (2011).
Gistera, A. et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl Med. 5, 196ra100 (2013). Study of the plaque-stabilizing role of T H 17 cells.
Cheng, X. et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 127, 89–97 (2008).
Simon, T. et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 34, 570–577 (2013).
de Boer, O. J., van der Meer, J. J., Teeling, P., van der Loos, C. M. & van der Wal, A. C. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE 2, e779 (2007).
Maganto-Garcia, E., Tarrio, M. L., Grabie, N., Bu, D. X. & Lichtman, A. H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124, 185–195 (2011).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 (2006). Study showing an atheroprotective role of T reg cells.
Klingenberg, R. et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 123, 1323–1334 (2013). Definitive evidence for an atheroprotective role of FOXP3+ T reg cells.
Mor, A. et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 893–900 (2007).
Dinh, T. N. et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 126, 1256–1266 (2012).
Takeda, M. et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler. Thromb. Vasc. Biol. 30, 2495–2503 (2010).
Sasaki, N. et al. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation 120, 1996–2005 (2009).
Kita, T. et al. Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice. Cardiovasc. Res. 102, 107–117 (2014).
Foks, A. C. et al. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis 218, 53–60 (2011).
Klingenberg, R. et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 946–952 (2010). Study showing that mucosal vaccination protects against atherosclerosis.
Wigren, M. et al. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Intern. Med. 269, 546–556 (2011).
Herbin, O. et al. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32, 605–612 (2012).
Mallat, Z. et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 85, e17–e24 (1999). Discovery of the atheroprotective role of IL-10.
Pinderski Oslund, L. J. et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 19, 2847–2853 (1999).
Caligiuri, G. et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 9, 10–17 (2003).
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. & Flavell, R. A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006).
Robertson, A. K. et al. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 112, 1342–1350 (2003). Study demonstrating that the immunomodulatory action of TGFβ controls atherosclerosis.
Mallat, Z. et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89, 930–934 (2001).
Lutgens, E. et al. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 22, 975–982 (2002).
Frutkin, A. D. et al. TGF-β1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 29, 1251–1257 (2009).
Reifenberg, K. et al. Overexpression of TGF-beta1 in macrophages reduces and stabilizes atherosclerotic plaques in ApoE-deficient mice. PLoS ONE 7, e40990 (2012).
Buday, A. et al. Elevated systemic TGF-beta impairs aortic vasomotor function through activation of NADPH oxidase-driven superoxide production and leads to hypertension, myocardial remodeling, and increased plaque formation in apoE−/− mice. Am. J. Physiol. Heart Circ. Physiol. 299, H386–H395 (2010).
Zhou, X. & Hansson, G. K. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand. J. Immunol. 50, 25–30 (1999).
Caligiuri, G., Nicoletti, A., Poirier, B. & Hansson, G. K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109, 745–753 (2002). Discovery of atheroprotective immunity carried by B cells.
Major, A. S., Fazio, S. & Linton, M. F. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 22, 1892–1898 (2002).
Robinette, C. D. & Fraumeni, J. F. Jr. Splenectomy and subsequent mortality in veterans of the 1939–1945 war. Lancet 2, 127–129 (1977).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 207, 1579–1587 (2010).
Kyaw, T. et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 185, 4410–4419 (2010).
Kerekes, G. et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin. Rheumatol. 28, 705–710 (2009).
Kyaw, T. et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 109, 830–840 (2011).
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 (2003). Report that molecular mimicry with pneumococci is involved in LDL reactivity and atherosclerosis.
Centa, M. et al. Atherosclerosis susceptibility in mice is independent of the V1 immunoglobulin heavy chain gene. Arterioscler. Thromb. Vasc. Biol. 36, 25–36 (2016).
Chang, M. K. et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 200, 1359–1370 (2004).
Grasset, E. K. et al. Sterile inflammation in the spleen during atherosclerosis provides oxidation-specific epitopes that induce a protective B-cell response. Proc. Natl Acad. Sci. USA 112, E2030–E2038 (2015).
Isomaa, B. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689 (2001).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006). Expert review on the role of inflammation in metabolic disorders.
Sammalkorpi, K., Valtonen, V., Kerttula, Y., Nikkila, E. & Taskinen, M. R. Changes in serum lipoprotein pattern induced by acute infections. Metab. Clin. Exp. 37, 859–865 (1988).
Tracey, K. J. et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J. Exp. Med. 167, 1211–1227 (1988).
Beutler, B. & Cerami, A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320, 584–588 (1986). Discovery of TNF as a metabolic cytokine — a pioneering study of immunometabolism.
Khovidhunkit, W. et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45, 1169–1196 (2004).
Bensinger, S. J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008).
Holmqvist, M. E. et al. Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J. Intern. Med. 268, 578–585 (2010). Study demonstrating an increased risk of cardiovascular disease in patients with rheumatoid arthritis.
Kerekes, G. et al. Effects of biologics on vascular function and atherosclerosis associated with rheumatoid arthritis. Ann. NY Acad. Sci. 1173, 814–821 (2009).
Jacobsson, L. T. et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 32, 1213–1218 (2005). Study demonstrating that TNF blockade protects against cardiovascular disease in patients with rheumatoid arthritis.
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). Study demonstrating that gut microbiota control regulatory immunity.
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Sommer, F. & Backhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Jonasson, L., Holm, J. & Hansson, G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc. Natl Acad. Sci. USA 85, 2303–2306 (1988).
Marx, S. O. & Marks, A. R. Bench to bedside: the development of rapamycin and its application to stent restenosis. Circulation 104, 852–855 (2001).
Back, M. & Hansson, G. K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 12, 199–211 (2015).
Palinski, W., Miller, E. & Witztum, J. L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl Acad. Sci. USA 92, 821–825 (1995). First report of protective immunity against atherosclerosis induced by vaccination.
Ameli, S. et al. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 16, 1074–1079 (1996).
George, J. et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 138, 147–152 (1998).
Zhou, X., Caligiuri, G., Hamsten, A., Lefvert, A. K. & Hansson, G. K. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21, 108–114 (2001).
Fredrikson, G. N. et al. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler. Thromb. Vasc. Biol. 23, 879–884 (2003). Study showing that ApoB vaccination reduces atherosclerosis in mice.
Tse, K. et al. Atheroprotective vaccination with MHC-II restricted peptides from ApoB-100. Front. Immunol. 4, 493 (2013).
Gistera, A. et al. Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. J. Intern. Med. 281, 383–397 (2017).
van Puijvelde, G. H. et al. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation 114, 1968–1976 (2006).
Hermansson, A. et al. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 123, 1083–1091 (2011). Study of cellular immunotherapy targeting LDL in mice.
Ketelhuth, D. F., Gistera, A., Johansson, D. K. & Hansson, G. K. T cell-based therapies for atherosclerosis. Curr. Pharm. Des. 19, 5850–5858 (2013).
Tsimikas, S. et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48, 425–433 (2007).
Lehrer-Graiwer, J. et al. FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc. Imaging 8, 493–494 (2015).
Hartvigsen, K. et al. The role of innate immunity in atherogenesis. J. Lipid Res. 50 (Suppl.), S388–S393 (2009).
Shoji, T. et al. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis 148, 171–177 (2000).
Habets, K. L. et al. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc. Res. 85, 622–630 (2010).
Freigang, S., Horkko, S., Miller, E., Witztum, J. L. & Palinski, W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler. Thromb. Vasc. Biol. 18, 1972–1982 (1998).
Schiopu, A. et al. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation 110, 2047–2052 (2004).
Ketelhuth, D. F. et al. Identification of a danger-associated peptide from apolipoprotein B100 (ApoBDS-1) that triggers innate proatherogenic responses. Circulation 124, 2433–2443 (2011). Study showing that ApoB activates innate immunity.
Steinberg, D. The LDL modification hypothesis of atherogenesis: an update. J. Lipid Res. 50 (Suppl.), S376–S381 (2009).
Dongre, A. R. et al. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur. J. Immunol. 31, 1485–1494 (2001).
Acknowledgements
The authors' work is supported by project grant 02738 and Linnaeus support 349-2007-8703 from the Swedish Research Council, and by grants from the Swedish Heart-Lung Foundation, the Foundation for Strategic Research (SSF), Vinnova, Stockholm County Council, King Gustav V and Queen Victoria Foundation, Prof Nanna Svartz foundation, and the European Union's Seventh Framework Programme [FP7/2007-2013] under grant agreement Athero-Flux (no 602222) and VIA (no 603131).
Author information
Authors and Affiliations
Contributions
Both authors researched the data, discussed the content, wrote the manuscript and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G.K.H has intellectual property rights in the area of immunotherapy for cardiovascular disease. A.G. declares no competing interests.
Glossary
- Acute phase response
-
The systemic inflammatory reaction to an infection or injury, including fever, tachycardia, leukocytosis and changes in circulating acute phase proteins, such as C-reactive protein.
- Foam cells
-
Lipid-laden macrophages with a 'foamy' appearance.
- Scavenger receptors
-
Receptors that mediate uptake of macromolecules with a negative charge, such as oxidized LDL.
- Pinocytosis
-
A mechanism for uptake of extracellular fluids and small particles through an invagination of the cell membrane.
- Germinal centres
-
Sites in lymphoid organs where mature B cells proliferate, differentiate, mutate their antibody genes and switch antibody classes.
- Apoptotic cell immunization
-
A vaccination approach in which cells undergoing programmed cell death are used to evoke an immune response against oxidation-specific epitopes.
Rights and permissions
About this article
Cite this article
Gisterå, A., Hansson, G. The immunology of atherosclerosis. Nat Rev Nephrol 13, 368–380 (2017). https://doi.org/10.1038/nrneph.2017.51
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.51
This article is cited by
-
Tissue-plasminogen activator effects on the phenotype of splenic myeloid cells in acute inflammation
Journal of Inflammation (2024)
-
Vascular damage in systemic lupus erythematosus
Nature Reviews Nephrology (2024)
-
Dysregulation of micro-RNA 143-3p as a Biomarker of Carotid Atherosclerosis and the Associated Immune Reactions During Disease Progression
Journal of Cardiovascular Translational Research (2024)
-
Porphyromonas gingivalis infection in the oral cavity is associated with elevated galactose-deficient IgA1 and increased nephritis severity in IgA nephropathy
Clinical and Experimental Nephrology (2024)
-
Electronic cigarettes and cardiovascular disease: epidemiological and biological links
Pflügers Archiv - European Journal of Physiology (2024)