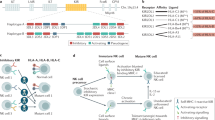
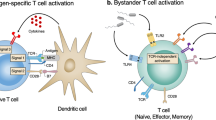
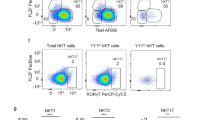
Abstract
Recent years have seen so-called natural killer T (NKT) cells emerge as important regulators of the immune response. The existence of NKT-cell subsets, and other types of T cell that resemble NKT cells, is an ongoing source of confusion in the literature. This perspective article seeks to clarify which cells fall under the NKT-cell umbrella, and which might be best considered as separate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Makino, Y., Kanno, R., Ito, T., Higashino, K. & Taniguchi, M. Predominant expression of invariant Vα14+ TCR α-chain in NK1.1+ T cell populations. Int. Immunol. 7, 1157–1161 (1995).
Budd, R. C. et al. Developmentally regulated expression of T cell receptor β-chain variable domains in immature thymocytes. J. Exp. Med. 166, 577–582 (1987).
Fowlkes, B. J. et al. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ-gene family. Nature 329, 251–254 (1987).
Ceredig, R., Lynch, F. & Newman, P. Phenotypic properties, interleukin 2 production, and developmental origin of a 'mature' subpopulation of Lyt-2−L3T4− mouse thymocytes. Proc. Natl Acad. Sci. USA 84, 8578–8582 (1987).
Zlotnik, A., Godfrey, D. I., Fischer, M. & Suda, T. Cytokine production by mature and immature CD4−CD8− T cells. αβ-T cell receptor+ CD4−CD8− T cells produce IL-4. J. Immunol. 149, 1211–1215 (1992).
Sykes, M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4−CD8− and αβ TCR+NK1.1+ cells. J. Immunol. 145, 3209–3215 (1990).
Levitsky, H. I., Golumbek, P. T. & Pardoll, D. M. The fate of CD4−8− T cell receptor-αβ+ thymocytes. J. Immunol. 146, 1113–1117 (1991).
Arase, H., Arase, N., Nakagawa, K., Good, R. A. & Onoé, K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 23, 307–310 (1993).
Yoshimoto, T. & Paul, W. E. CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179, 1285–1295 (1994).
Bendelac, A. & Schwartz, R. H. CD4+ and CD8+ T cells acquire specific lymphokine secretion potentials during thymic maturation. Nature 353, 68–71 (1991).
Bendelac, A., Matzinger, P., Seder, R. A., Paul, W. E. & Schwartz, R. H. Activation events during thymic selection. J. Exp. Med. 175, 731–742 (1992).
Hayakawa, K., Lin, B. T. & Hardy, R. R. Murine thymic CD4+ T cell subsets: a subset (Thy0) that secretes diverse cytokines and overexpresses the Vβ8 T cell receptor gene family. J. Exp. Med. 176, 269–274 (1992).
Ohteki, T. & MacDonald, H. R. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of NK1.1+ T cell receptor αβ cells in the liver of mice. J. Exp. Med. 180, 699–704 (1994).
Bix, M., Coles, M. & Raulet, D. Positive selection of Vβ8+CD4−8− thymocytes by class I molecules expressed by hematopoietic cells. J. Exp. Med. 178, 901–908 (1993).
Bendelac, A., Killeen, N., Littman, D. R. & Schwartz, R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263, 1774–1778 (1994).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995). This was the first study to show that NKT cells are restricted to CD1d.
Lantz, O. & Bendelac, A. An invariant T cell receptor α chain is used by a unique subset of MHC class I specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180, 1097–1106 (1994). Type I NKT cells express an invariant T-cell receptor (TCR) α-chain, for example, Vα14-Jα281 (now known as Vα14-Jα18) in mice and Vα24-JαQ (now known as Vα24-Jα18) in humans.
Imai, K. et al. Sequence and expression of transcripts of the T-cell antigen receptor α-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc. Natl Acad. Sci. USA 83, 8708–8712 (1986).
Koseki, H., Imai, K., Ichikawa, T., Hayata, I. & Taniguchi, M. Predominant use of a particular α-chain in suppressor T cell hybridomas specific for keyhole limpet hemocyanin. Int. Immunol. 1, 557–564 (1989).
Koseki, H. et al. Homogenous junctional sequence of the V14+ T-cell antigen receptor α-chain expanded in unprimed mice. Proc. Natl Acad. Sci. USA 87, 5248–5252 (1990).
Chen, Y. H., Chiu, N. M., Mandal, M., Wang, N. & Wang, C. R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity 6, 459–467 (1997).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Mendiratta, S. K. et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 (1997).
Smiley, S. T., Kaplan, M. H. & Grusby, M. J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275, 977–979 (1997).
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691–694 (1994).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). This paper shows that type I NKT cells recognize and are activated by α-galactosylceramide (α-GalCer) associated with CD1d.
Elewaut, D. & Kronenberg, M. Molecular biology of NK T cell specificity and development. Semin. Immunol. 12, 561–568 (2000).
Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. NKT cells: facts, functions and fallacies. Immunol. Today 21, 573–583 (2000).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nature Rev. Immunol. 2, 557–568 (2002).
MacDonald, H. R. Development and selection of NKT cells. Curr. Opin. Immunol. 14, 250–254 (2002).
Coles, M. C. & Raulet, D. H. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 180, 395–399 (1994).
Adachi, Y., Koseki, H., Zijlstra, M. & Taniguchi, M. Positive selection of invariant Vα14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow derived cells. Proc. Natl Acad. Sci. USA 92, 1200–1204 (1995).
Coles, M. C. & Raulet, D. H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
Dellabona, P., Padovan, E., Casorati, M., Brockhaus, M. & Lanzavecchia, A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180, 1171–1176 (1994).
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− αβ T cells demonstrates preferential use of several Vβ genes and an invariant TCR α-chain. J. Exp. Med. 178, 1–16 (1993).
Kashiwase, K. et al. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics 54, 776–781 (2003).
Motsinger, A. et al. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J. Virol. 77, 8153–8158 (2003).
Matsuura, A. et al. NKT cells in the rat: organ-specific distribution of NK T cells expressing distinct Vα14 chains. J. Immunol. 164, 3140–3148 (2000).
Gombert, J. M. et al. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 26, 2989–2998 (1996).
Joyce, S. et al. Expansion of natural (NK1+) T cells that express αβ T cell receptors in transporters associated with antigen presentation-1 null and thymus leukemia antigen positive mice. J. Exp. Med. 184, 1579–1584 (1996).
Bix, M. & Locksley, R. M. Natural T cells — cells that co-express NKRP-1 and TCR. J. Immunol. 155, 1020–1022 (1995).
Sato, N. et al. Functional characterization of NK1.1+ Ly-6c+ cells. Immunol. Lett. 54, 5–9 (1996).
Naumov, Y. N. et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc. Natl Acad. Sci. USA 98, 13838–13843 (2001).
Smyth, M. J., Crowe, N. Y., Takeda, K., Yagita, H. & Godfrey, D. I. NKT cells — conductors of tumor immunity? Curr. Opin. Immunol. 14, 165–171 (2002).
Hammond, K. J. L. et al. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29, 3768–3781 (1999). Together with reference 48, this paper shows that some NK1.1+ T cells are not CD1d or Vα14-Jα18 dependent.
Hammond, K. J. et al. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167, 1164–1173 (2001).
Eberl, G. et al. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162, 6410–6419 (1999).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000). This report, together with reference 56, indicates that CD1d tetramers loaded with α-GalCer can specifically bind to NKT cells in mice and humans. These studies also directly showed the existence of NK1.1− NKT cells in C57BL/6 mice.
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunol. 1, 515–520 (2000).
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Gadola, S. D., Dulphy, N., Salio, M. & Cerundolo, V. Vα24-JαQ-independent, CD1d-restricted recognition of α-galactosylceramide by human CD4+ and CD8 αβ+ T lymphocytes. J. Immunol. 168, 5514–5520 (2002).
Takahashi, T. et al. Cutting edge: analysis of human Vα24+CD8+ NK T cells activated by α-galactosylceramide-pulsed monocyte-derived dendritic cells. J. Immunol. 168, 3140–3144 (2002).
Shao, H., Van Kaer, L., Sun, S. L., Kaplan, H. J. & Sun, D. Infiltration of the inflamed eye by NKT cells in a rat model of experimental autoimmune uveitis. J. Autoimmun. 21, 37–45 (2003).
Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 195, 637–641 (2002).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Hameg, A. et al. IL-7 upregulates IL-4 production by splenic NK1.1+ and NK1.1− MHC class I-like/CD1-dependent CD4+ T cells. J. Immunol. 162, 7067–7074 (1999). The authors describe that some CD1d-dependent Vα14-Jα18 NKT cells do not express the NK1.1 marker.
Hameg, A. et al. A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the α-galactosylceramide antigen. J. Immunol. 165, 4917–4926 (2000).
Pellicci, D. G. et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195, 835–844 (2002).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Gadue, P. & Stein, P. L. NK T cell precursors exhibit differential cytokine regulation and require ITK for efficient maturation. J. Immunol. 169, 2397–2406 (2002). References 59–61 report that classical NKT cells develop through an immature NK1.1− stage that produces high levels of interleukin-4 (IL-4) and low levels of interferon-γ (IFN-γ). Most NKT cells migrate from the thymus at the immature NK1.1− stage (references 59 and 60 only).
Chen, H. J., Huang, H. & Paul, W. E. NK1.1+CD4+ T cells lose NK1.1 expression upon in vitro activation. J. Immunol. 158, 5112–5119 (1997).
Eberl, G. & Macdonald, H. R. Rapid death and regeneration of NKT cells in anti-CD3-ε- or IL-12-treated mice — a major role for bone marrow in NKT cell homeostasis. Immunity 9, 345–353 (1998).
Eberl, G. & MacDonald, H. R. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30, 985–992 (2000).
Leite-de-Moraes, M. C. et al. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J. Immunol. 165, 4367–4371 (2000).
Nakagawa, R. et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-galactosylceramide in mice. J. Immunol. 166, 6578–6584 (2001).
Osman, Y. et al. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur. J. Immunol. 30, 1919–1928 (2000).
Hayakawa, Y. et al. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of α-galactosylceramide. Eur. J. Immunol. 31, 1720–1727 (2001).
Crowe, N. Y. et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NKT cells. J. Immunol. 171, 4020–4027 (2003).
Wilson, M. T. et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl Acad. Sci. USA 100, 10913–10918 (2003). Following stimulation in vivo , references 69 and 70 show that most NKT cells do not die within hours due to activation-induced cell death (as previously thought). Instead, they downregulate NK1.1 expression and undergo marked but transient clonal expansion to ten times their steady-state levels. NK1.1 expression remains reduced on antigen-experienced NKT cells for at least 12 days.
Park, S. H., Kyin, T., Bendelac, A. & Carnaud, C. The contribution of NKT cells, NK cells, and other γ-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J. Immunol. 170, 1197–1201 (2003).
Lizuka, K., Naidenko, O. V., Plougastel, B. F., Fremont, D. H. & Yokoyama, W. M. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nature Immunol. 4, 801–807 (2003).
Behar, S. M., Podrebarac, T. A., Roy, C. J., Wang, C. R. & Brenner, M. B. Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 (1999).
Chiu, Y. H. et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189, 103–110 (1999).
Cardell, S. et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182, 993–1004 (1995). The first study to show the existence of CD1d-dependent T cells in mice that are not Vα14-Jα18+NK1.1+.
Park, S. H. et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193, 893–904 (2001).
Skold, M., Faizunnessa, N. N., Wang, C. R. & Cardell, S. CD1d-specific NK1.1+ T cells with a transgenic variant TCR. J. Immunol. 165, 168–174 (2000).
Huber, S., Sartini, D. & Exley, M. Role of CD1d in coxsackievirus B3-induced myocarditis. J. Immunol. 170, 3147–3153 (2003).
Chiu, Y. H. et al. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nature Immunol. 3, 55–60 (2002).
Baron, J. L. et al. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16, 583–594 (2002).
Exley, M. A. et al. Cutting edge: a major fraction of human bone marrow lymphocytes are TH2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J. Immunol. 167, 5531–5534 (2001).
Heiken, H., Schulz, R. J., Ravetch, J. V., Reinherz, E. L. & Koyasu, S. T lymphocyte development in the absence of Fc-ε receptor I-γ subunit — analysis of thymic-dependent and independent αβ and γδ pathways. Eur. J. Immunol. 26, 1935–1943 (1996).
Legendre, V. et al. Selection of phenotypically distinct NK1.1+ T cells upon antigen expression in the thymus or in the liver. Eur. J. Immunol. 29, 2330–2343 (1999).
Iwabuchi, C. et al. Intrathymic selection of NK1.1+αβ T cell antigen receptor TCR+ cells in transgenic mice bearing TCR specific for chicken ovalbumin and restricted to I-Ad. Proc. Natl Acad. Sci. USA 95, 8199–8204 (1998).
Slifka, M. K., Pagarigan, R. R. & Whitton, J. L. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164, 2009–2015 (2000).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Shimamura, M. & Huang, Y. Y. Presence of a novel subset of NKT cells bearing an invariant Vα19.1-Jα26 TCR α-chain. FEBS Letters 516, 97–100 (2002).
Hammond, K. J. L., Cain, W. E., Van Driel, I. R. & Godfrey, D. I. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int. Immunol. 10, 1491–1499 (1998).
Kikly, K. & Dennert, G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J. Immunol. 149, 403–412 (1992).
Kim, C. H., Johnston, B. & Butcher, E. C. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood 100, 11–16 (2002).
Thomas, S. Y. et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of TH1-type inflammatory homing cells. J. Immunol. 171, 2571–2580 (2003).
Park, S. H., Benlagha, K., Lee, D., Balish, E. & Bendelac, A. Unaltered phenotype, tissue distribution and function of Vα14+ NKT cells in germ-free mice. Eur. J. Immunol. 30, 620–625 (2000).
Lees, R. K., Ferrero, I. & MacDonald, H. R. Tissue-specific segregation of TCR γδ+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR αβ+ NKT cells. Eur. J. Immunol. 31, 2901–2909 (2001).
Carding, S. R. & Egan, P. J. γδ T cells: functional plasticity and heterogeneity. Nature Rev. Immunol. 2, 336–345 (2002).
Arase, H. et al. Developmental arrest of NK1.1+ T cell antigen receptor (TCR)-αβ+ T cells and expansion of NK1.1+ TCR-γδ+ T cell development in CD3-ζ-deficient mice. J. Exp. Med. 182, 891–895 (1995).
Vicari, A. P., Mocci, S., Openshaw, P., O'Garra, A. & Zlotnik, A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+NK1.1+ thymocytes. Eur. J. Immunol. 26, 1424–1429 (1996).
Schumann, J., Voyle, R. B., Wei, B. Y. & MacDonald, H. R. Cutting edge: influence of the TCR Vβ domain on the avidity of CD1d–α-galactosylceramide binding by invariant Vα14 NKT cells. J. Immunol. 170, 5815–5819 (2003).
Exley, M. A. et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69, 713–718 (2001).
Dao, T. et al. Involvement of CD1 in peripheral deletion of T lymphocytes is independent of NK T cells. J. Immunol. 166, 3090–3097 (2001).
Gapin, L., Matsuda, J. L., Surh, C. D. & Kronenberg, M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nature Immunol. 2, 971–978 (2001).
Hayakawa, Y., Godfrey, D. I. & Smyth, M. J. α-Galactosylceramide: potential immunomodulatory activity and future application. Curr. Med. Chem. 11, 241–252 (2004).
Acknowledgements
We thank many colleagues in the NKT cell and related fields for stimulating discussions that were helpful in writing this opinion article. This work was supported by the National Health and Medical Research Council of Australia, The Human Frontiers Science Program and The National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Glossary
- α-GALACTOSYLCERAMIDE
-
(α-GalCer). A synthetic or marine-sponge-derived glycolipid containing α-anomeric glycosidic linkage of the galactose residue to the sphingosine base. This, and structurally related lipids, potently activate CD1d-restricted natural killer T (NKT) cells that express the semi-invariant Vα14-Jα18 T-cell receptor (TCR) in mice (and the Vα24-Jα18+ equivalent cells in humans)101.
- α-GALCER-LOADED CD1D TETRAMERS
-
A complex of four CD1d molecules loaded with α-GalCer that has sufficient affinity to detect cell-surface expression of the semi-invariant TCR expressed by NKT cells in mice (Vα14-Jα18+) and humans (Vα24-Jα18+) using flow cytometry.
- CORTICAL THYMIC EPITHELIAL CELLS
-
Epithelial cells found in the outer region or cortex of thymic lobules. These cells are crucial for the positive selection of conventional MHC class-I- and class-II-restricted T cells, but not for the positive selection of CD1d-restricted NKT cells.
- DOUBLE-POSITIVE THYMOCYTES
-
Immature T cells in the thymus that are characterized by the expression of both the CD4 and CD8 co-receptor proteins. They represent the majority (80–90%) of thymocytes. These cells express CD1d molecules and have been implicated in the positive selection of NKT cells.
Rights and permissions
About this article
Cite this article
Godfrey, D., MacDonald, H., Kronenberg, M. et al. NKT cells: what's in a name?. Nat Rev Immunol 4, 231–237 (2004). https://doi.org/10.1038/nri1309
Issue Date:
DOI: https://doi.org/10.1038/nri1309
This article is cited by
-
The causality between CD8+NKT cells and CD16−CD56 on NK cells with hepatocellular carcinoma: a Mendelian randomization study
Infectious Agents and Cancer (2024)
-
CAR-NKT cell therapy: a new promising paradigm of cancer immunotherapy
Cancer Cell International (2023)
-
Markers and makers of NKT17 cells
Experimental & Molecular Medicine (2023)
-
Invariant natural killer T cells in lung diseases
Experimental & Molecular Medicine (2023)
-
Chimeric antigen receptor T cells march into T cell malignancies
Journal of Cancer Research and Clinical Oncology (2023)