Abstract
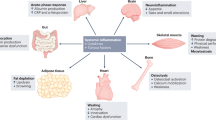
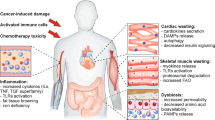
Cancer-associated cachexia is a disorder characterized by loss of body weight with specific losses of skeletal muscle and adipose tissue. Cachexia is driven by a variable combination of reduced food intake and metabolic changes, including elevated energy expenditure, excess catabolism and inflammation. Cachexia is highly associated with cancers of the pancreas, oesophagus, stomach, lung, liver and bowel; this group of malignancies is responsible for half of all cancer deaths worldwide. Cachexia involves diverse mediators derived from the cancer cells and cells within the tumour microenvironment, including inflammatory and immune cells. In addition, endocrine, metabolic and central nervous system perturbations combine with these mediators to elicit catabolic changes in skeletal and cardiac muscle and adipose tissue. At the tissue level, mechanisms include activation of inflammation, proteolysis, autophagy and lipolysis. Cachexia associates with a multitude of morbidities encompassing functional, metabolic and immune disorders as well as aggravated toxicity and complications of cancer therapy. Patients experience impaired quality of life, reduced physical, emotional and social well-being and increased use of healthcare resources. To date, no effective medical intervention completely reverses cachexia and there are no approved drug therapies. Adequate nutritional support remains a mainstay of cachexia therapy, whereas drugs that target overactivation of catabolic processes, cell injury and inflammation are currently under investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
DeWys, W. D. Pathophysiology of cancer cachexia: current understanding and areas for future research. Cancer Res. 42 (Suppl.), 721s–726s (1982).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).This Delphi consensus process provides a definition, provisional diagnostic criteria and a roadmap for future development of clinical cachexia research.
Kazemi-Bajestani, S. M. R., Mazurak, V. C. & Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 54, 2–10 (2016).
World Health Organization. Cancer fact sheet. WHOhttp://www.who.int/mediacentre/factsheets/fs297/en/ (2017).
Amano, K. et al. C-Reactive protein, symptoms and activity of daily living in patients with advanced cancer receiving palliative care. J. Cachexia Sarcopenia Muscle 8, 457–465 (2017).
Prado, C. M. et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am. J. Clin. Nutr. 98, 1012–1019 (2013).
Prado, C. M. M. et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br. J. Cancer 106, 1583–1586 (2012).
Baracos, V. E. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol. 16, 13–14 (2015).
van Dijk, D. P. et al. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J. Cachexia. Sarcopenia Muscle 6, 212–221 (2015).
Johns, N. et al. New genetic signatures associated with cancer cachexia as defined by low skeletal muscle index and weight loss. J. Cachexia Sarcopenia Muscle 8, 122–130 (2017).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by actriib antagonism leads to prolonged survival. Cell 142, 531–543 (2010).This experimental study shows a survival benefit in tumour-bearing mice from an agent for which essentially the sole action is to prevent loss of skeletal muscle mass.
Tseng, Y.-C. et al. Preclinical Investigation of the novel histone deacetylase inhibitor AR-42 in the treatment of cancer-induced cachexia. J. Natl. Cancer Inst. 107, djv274 (2015).
Arends, J. et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 36, 11–48 (2017).This paper describes evidence-based guidelines for nutritional screening and intervention in patients with cancer.
Pressoir, M. et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 102, 966–971 (2010).
Bozzetti, F. & SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support. Care Cancer 17, 279–284 (2009).
Segura, A. et al. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin. Nutr. 24, 801–814 (2005).
Hébuterne, X. et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN. J. Parenter. Enteral Nutr. 38, 196–204 (2014).
Kazemi-Bajestani, S. M. R., Becher, H., Fassbender, K., Chu, Q. & Baracos, V. E. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J. Cachexia Sarcopenia Muscle 5, 95–104 (2014).
Antoun, S. et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J. Clin. Oncol. 28, 1054–1060 (2010).
World Health Organization. BMI classification. WHOhttp://apps.who.int/bmi/index.jsp?introPage=intro_3.html (2017).
World Health Organization. Obesity and overweight fact sheet. WHOhttp://www.who.int/mediacentre/factsheets/fs311/en/ (2016).
Martin, L. et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 33, 90–99 (2015).This paper describes the risk of mortality in an international cachexia data set stratified by BMI and weight loss, providing a prognostic grading scheme for cancer-associated weight loss.
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).This paper describes the risk of mortality in patients with solid tumours, which is associated with reduced skeletal muscle mass.
Demark-Wahnefried, W., Campbell, K. L. & Hayes, S. C. Weight management and its role in breast cancer rehabilitation. Cancer 118, 2277–2287 (2012).
Kubrak, C. et al. Clinical determinants of weight loss in patients receiving radiation and chemoirradiation for head and neck cancer: a prospective longitudinal view. Head Neck 35, 695–703 (2012).
Silver, H. J., Dietrich, M. S. & Murphy, B. A. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 29, 893–900 (2007).
Engelen, M. P. K. J., Klimberg, V. S., Allasia, A. & Deutz, N. E. P. Presence of early stage cancer does not impair the early protein metabolic response to major surgery. J. Cachexia Sarcopenia Muscle 8, 447–456 (2017).
Engelen, M. P. K. J., Safar, A. M., Bartter, T., Koeman, F. & Deutz, N. E. P. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann. Oncol. 26, 1960–1966 (2015).Together with reference 9, this study demonstrates robust protein synthetic responses to amino acid feeding in patients with advanced-stage cancer.
Hall, K. D. & Baracos, V. E. Computational modeling of cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 11, 214–221 (2008).
Friesen, D. E., Baracos, V. E. & Tuszynski, J. A. Modeling the energetic cost of cancer as a result of altered energy metabolism: implications for cachexia. Theor. Biol. Med. Model. 12, 17 (2015).
Beck, S. A. & Tisdale, M. J. Effect of cancer cachexia on triacylglycerol/fatty acid substrate cycling in white adipose tissue. Lipids 39, 1187–1189 (2004).
Kir, S. & Spiegelman, B. M. Cachexia and brown fat: a burning issue in cancer. Trends Cancer 2, 461–463 (2016).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
VanderVeen, B. N., Fix, D. K. & Carson, J. A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid. Med. Cell. Longev. 2017, 3292087 (2017).
Zhang, G. et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 8, 589 (2017).
Murphy, K. T. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol. 310, H466–H477 (2016).
Tashjian, A. H. Role of prostaglandins in the production of hypercalcemia by tumors. Cancer Res. 38, 4138–4141 (1978).
Fearon, K. C. H., Glass, D. J. & Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16, 153–166 (2012).
Loomans, H. A. & Andl, C. D. Intertwining of activin A and TGFβ signaling: dual roles in cancer progression and cancer cell invasion. Cancers 7, 70–91 (2014).
Loumaye, A. et al. Role of activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 100, 2030–2038 (2015).
Togashi, Y. et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer Lett. 356, 819–827 (2015).
Chen, J. L. et al. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Res. 76, 5372–5382 (2016).
Johnston, A. J. et al. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell 162, 1365–1378 (2015).
Mittal, A. et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J. Cell Biol. 188, 833–849 (2010).This is an early study demonstrating the requirement of the TWEAK ligand and TNFRSF12A in regulating muscle wasting due to disuse atrophy.
Sato, S., Ogura, Y., Tajrishi, M. M. & Kumar, A. Elevated levels of TWEAK in skeletal muscle promote visceral obesity, insulin resistance, and metabolic dysfunction. FASEB J. 29, 988–1002 (2015).
Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001).
Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A. & Goldberg, A. L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl Acad. Sci. USA 98, 14440–14445 (2001).
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004).
Sandri, M. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).Together with reference 47, this study identifies FOXO transcription factors as regulators of the ubiquitin–proteasome system.
Acharyya, S. et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 (2004).
Clarke, B. A. et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 6, 376–385 (2007).
Sandri, M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 45, 2121–2129 (2013).
Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 54, 11–19 (2016).
Milan, G. et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 6, 6670 (2015).
Song, Y.-H. et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Invest. 115, 451–458 (2005).
Sartori, R. et al. BMP signaling controls muscle mass. Nat. Genet. 45, 1309–1318 (2013).
Johns, N. et al. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PLoS ONE 9, e83618 (2014).
Guttridge, D. C., Mayo, M. W., Madrid, L. V., Wang, C. Y. & Baldwin, A. S. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289, 2363–2366 (2000).
Cai, D. et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).This study provides in vivo proof of concept that NF-κB signalling regulates skeletal muscle atrophy.
Mourkioti, F. et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Invest. 116, 2945–2954 (2006).
Silva, K. A. S. et al. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J. Biol. Chem. 290, 11177–11187 (2015).
Zimmers, T. A., Fishel, M. L. & Bonetto, A. STAT3 in the systemic inflammation of cancer cachexia. Semin. Cell Dev. Biol. 54, 28–41 (2016).
Ma, J. F. et al. STAT3 promotes IFNγ/TNFα-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol. Med. 9, 622–637 (2017).
Guo, D., Wang, C., Wang, Q., Qiao, Z. & Tang, H. Pantoprazole blocks the JAK2/STAT3 pathway to alleviate skeletal muscle wasting in cancer cachexia by inhibiting inflammatory response. Oncotarget 8, 39640–39648 (2017).
Zhang, G., Lin, R.-K., Kwon, Y. T. & Li, Y.-P. Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2. FASEB J. 27, 2893–2901 (2013).
Marchildon, F., Lamarche, É., Lala-Tabbert, N., St-Louis, C. & Wiper-Bergeron, N. Expression of CCAAT/enhancer binding protein beta in muscle satellite cells inhibits myogenesis in cancer cachexia. PLoS ONE 10, e0145583 (2015).
Sun, R. et al. Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia. Am. J. Physiol. Cell Physiol. 311, C101–C115 (2016).
Mueller, T. C., Bachmann, J., Prokopchuk, O., Friess, H. & Martignoni, M. E. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia — can findings from animal models be translated to humans? BMC Cancer 16, 75 (2016).
Bennegård, K., Lindmark, L., Edén, E., Svaninger, G. & Lundholm, K. Flux of amino acids across the leg in weight-losing cancer patients. Cancer Res. 44, 386–393 (1984).
DeJong, C. H. C. et al. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol. Rep. 14, 257–263 (2005).
Gallagher, I. J. et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clin. Cancer Res. 18, 2817–2827 (2012).
Tisdale, M. J. Cachexia in cancer patients. Nat. Rev. Cancer 2, 862–871 (2002).
MacDonald, A. J. et al. Habitual myofibrillar protein synthesis is normal in patients with upper GI cancer cachexia. Clin. Cancer Res. 21, 1734–1740 (2015).
Schmitt, T. L. et al. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 85, 647–654 (2007).
Acharyya, S. et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8, 421–432 (2005).
Penna, F. et al. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int. J. Cancer 127, 1706–1717 (2010).
Stephens, N. A. et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 6, 53–61 (2015).
Burfeind, K. G., Michaelis, K. A. & Marks, D. L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 54, 42–52 (2016).
Grossberg, A. J. et al. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology 151, 606–616 (2010).
Bodnar, R. J. et al. Mediation of anorexia by human recombinant tumor necrosis factor through a peripheral action in the rat. Cancer Res. 49, 6280–6284 (1989).
Braun, T. P. et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J. Exp. Med. 208, 2449–2463 (2011).
DeBoer, M. D. Ghrelin and cachexia: will treatment with GHSR-1a agonists make a difference for patients suffering from chronic wasting syndromes? Mol. Cell. Endocrinol. 340, 97–105 (2011).
Fouladiun, M. et al. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care — correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 103, 2189–2198 (2005).
Zuijdgeest-van Leeuwen, S. D. et al. Lipolysis and lipid oxidation in weight-losing cancer patients and healthy subjects. Metabolism 49, 931–936 (2000).
Rydén, M. & Arner, P. Fat loss in cachexia — is there a role for adipocyte lipolysis? Clin. Nutr. 26, 1–6 (2007).
Agustsson, T. et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 67, 5531–5537 (2007).
Das, S. K. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).This paper provides evidence that lipolysis in cancer predisposes skeletal muscle to undergo wasting.
Kir, S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104 (2014).
Neves, R. X. et al. White adipose tissue cells and the progression of cachexia: inflammatory pathways. J. Cachexia Sarcopenia Muscle 7, 193–203 (2015).
Camargo, R. et al. NF-κBp65 and expression of its pro-inflammatory target genes are upregulated in the subcutaneous adipose tissue of cachectic cancer patients. Nutrients 7, 4465–4479 (2015).
Zimmermann, R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004).
Rohm, M. et al. An AMP-activated protein kinase–stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 22, 1120–1130 (2016).
Martin, L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr. Opin. Clin. Nutr. Metab. Care 19, 188–198 (2016).
Vagnildhaug, O. M. et al. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J. Cachexia Sarcopenia Muscle 8, 789–797 (2017).
Vazeille, C. et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am. J. Clin. Nutr. 105, 1139–1147 (2017).
Read, J. A. et al. Nutritional assessment in cancer: comparing the Mini-Nutritional Assessment (MNA) with the Scored Patient-Generated Subjective Global Assessment (PGSGA). Nutr. Cancer 53, 51–56 (2005).
Hubbard, J. M., Cohen, H. J. & Muss, H. B. Incorporating biomarkers into cancer and aging research. J. Clin. Oncol. 32, 2611–2616 (2014).
Fujiwara, N. et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 63, 131–140 (2015).This paper shows an association of muscle depletion with mortality in an Asian population.
Epstein, F. H., Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 (1999).
Douglas, E. & McMillan, D. C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 40, 685–691 (2014).
Laird, B. J. A. et al. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J. Clin. Oncol. 34, 2769–2775 (2016).
Wei, B. et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco. Targets. Ther. 9, 5567–5575 (2016).
Dolan, R. D., McSorley, S. T., Horgan, P. G., Laird, B. & McMillan, D. C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 116, 134–146 (2017).
Hong, N. et al. Serum PTHrP predicts weight loss in cancer patients independent of hypercalcemia, inflammation, and tumor burden. J. Clin. Endocrinol. Metab. 101, 1207–1214 (2016).
Lerner, L. et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 6, 317–324 (2015).
Macciò, A. et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol. Oncol. 124, 417–425 (2012).
Isenring, E. & Elia, M. Which screening method is appropriate for older cancer patients at risk for malnutrition? Nutrition 31, 594–597 (2015).
Scott, D., Reid, J., Hudson, P., Martin, P. & Porter, S. Health care professionals' experience, understanding and perception of need of advanced cancer patients with cachexia and their families: The benefits of a dedicated clinic. BMC Palliat. Care 15, 100 (2016).
Dev, R. et al. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J. Cachexia Sarcopenia Muscle 6, 95–98 (2015).
Vigano, A., Del Fabbro, E., Bruera, E. & Borod, M. The cachexia clinic: from staging to managing nutritional and functional problems in advanced cancer patients. Crit. Rev. Oncog. 17, 293–304 (2012).
Blum, D., Hess, J., Omlin, A., Jurt, G. & Strasser, A. B. H. P. M. Comprehensive cancer cachexia staging and its impact in the outpatient oncology setting: a phase II study. J. Clin. Oncol. 27, e20530–e20530 (2009).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531 (2016).This paper describes two phase III clinical studies of a low-molecular-mass growth hormone secretagogue receptor type 1 agonist, demonstrating lean tissue gain in patients with non-small-cell lung cancer.
Crawford, J. et al. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr. Oncol. Rep. 18, 37 (2016).
Fearon, K. C. H. et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J. Clin. Oncol. 24, 3401–3407 (2006).
Bruera, E. et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J. Clin. Oncol. 21, 129–134 (2003).
Strasser, F. et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 24, 3394–3400 (2006).
Awad, S. et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin. Nutr. 31, 74–77 (2012).
Fearon, K. C. H. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 52, 1479–1486 (2003).
Baracos, V. E., Watanbe, S. & Fearon, K. in The Oxford Textbook of Palliative Medicine 5th edn (eds Cherny, N., Fallon, M., Kaasa, S., Portenoy, R. K. & Currow, D. C.) 702–712 (2015).
Dallmann, R. et al. The orally active melanocortin-4 receptor antagonist BL-6020/979: a promising candidate for the treatment of cancer cachexia. J. Cachexia Sarcopenia Muscle 2, 163–174 (2011).
MacDonald, N., Easson, A. M., Mazurak, V. C., Dunn, G. P. & Baracos, V. E. Understanding and managing cancer cachexia. J. Am. Coll. Surg. 197, 143–161 (2003).
Garvey, C. et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. Prev. 36, 75–83 (2016).
Stene, G. B. et al. Effect of physical exercise on muscle mass and strength in cancer patients during treatment — a systematic review. Crit. Rev. Oncol. Hematol. 88, 573–593 (2013).
Wasley, D. et al. Patients with established cancer cachexia lack the motivation and self-efficacy to undertake regular structured exercise. Psychooncology https://doi.org/10.1002/pon.4512 (2017).
Mayo, N. E. et al. Pedometer-facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial. Clin. Rehabil. 28, 1198–1209 (2014).
Shragge, J. E., Wismer, W. V., Olson, K. L. & Baracos, V. E. Shifting to conscious control: psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat. Med. 21, 227–233 (2007).
Reilly, C. M. et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support. Care Cancer 21, 1525–1550 (2013).
Pakhomov, S. V., Jacobsen, S. J., Chute, C. G. & Roger, V. L. Agreement between patient-reported symptoms and their documentation in the medical record. Am. J. Manag. Care 14, 530–539 (2008).
Gagnon, B. et al. A prospective evaluation of an interdisciplinary nutrition–rehabilitation program for patients with advanced cancer. Curr. Oncol. 20, 310 (2013).
Chasen, M. R., Feldstain, A., Gravelle, D., MacDonald, N. & Pereira, J. An interprofessional palliative care oncology rehabilitation program: effects on function and predictors of program completion. Curr. Oncol. 20, 301 (2013).
Smith, T. J. et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J. Clin. Oncol. 30, 880–887 (2012).
Hesketh, P. J. et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 35, 3240–3261 (2017).
Prado, C. M. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 9, 629–635 (2008).
Sjøblom, B. et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin-doublet chemotherapy in advanced non-small-cell lung cancer. Clin. Lung Cancer 18, e129–e136 (2017).
Fearon, K. C. H. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 44, 1124–1132 (2008).
Maddocks, M. et al. Practical multimodal care for cancer cachexia. Curr. Opin. Support. Palliat. Care 10, 298–305 (2016).
Fearon, K., Arends, J. & Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 10, 90–99 (2012).
Aapro, M. et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann. Oncol. 25, 1492–1499 (2014).
Lassen, K. et al. Pancreaticoduodenectomy: ERAS recommendations. Clin. Nutr. 32, 870–871 (2013).
Nelson, G. et al. Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: the ERAS Alberta Colorectal Surgery Experience. World J. Surg. 40, 1092–1103 (2016).
Palmela, C. et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J. Gastr. Cancer 17, 74 (2017).
Daly, L. E. et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 116, 310–317 (2017).
Lieffers, J. R. et al. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 89, 1173–1179 (2009).
Bozzetti, F. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin. Nutr. 35, 1577 (2016).
Bozzetti, F. et al. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann. Oncol. 26, 2335–2340 (2015).
Kalantar-Zadeh, K. et al. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J. Cachexia Sarcopenia Muscle 4, 89–94 (2013).
Tarricone, R., Ricca, G., Nyanzi-Wakholi, B. & Medina-Lara, A. Impact of cancer anorexia-cachexia syndrome on health-related quality of life and resource utilisation: a systematic review. Crit. Rev. Oncol. Hematol. 99, 49–62 (2016).
Wheelwright, S. et al. A systematic review of health-related quality of life instruments in patients with cancer cachexia. Support. Care Cancer 21, 2625–2636 (2013).
Wheelwright, S. J. et al. Development of the EORTC QLQ-CAX24, a questionnaire for cancer patients with cachexia. J. Pain Symptom Manage. 53, 232–242 (2017).
Hopkinson, J. B. Psychosocial impact of cancer cachexia. J. Cachexia Sarcopenia Muscle 5, 89–94 (2014).
Oberholzer, R. et al. Psychosocial effects of cancer cachexia: a systematic literature search and qualitative analysis. J. Pain Symptom Manage. 46, 77–95 (2013).
Maschke, J. et al. Nutritional care of cancer patients: a survey on patients' needs and medical care in reality. Int. J. Clin. Oncol. 22, 200–206 (2016).
Hopkinson, J. B. Food connections: A qualitative exploratory study of weight- and eating-related distress in families affected by advanced cancer. Eur. J. Oncol. Nurs. 20, 87–96 (2016).
Wheelwright, S., Darlington, A.-S., Hopkinson, J. B., Fitzsimmons, D. & Johnson, C. A systematic review and thematic synthesis of quality of life in the informal carers of cancer patients with cachexia. Palliat. Med. 30, 149–160 (2016).
Hopkinson, J. B. & Richardson, A. A mixed-methods qualitative research study to develop a complex intervention for weight loss and anorexia in advanced cancer: The Family Approach to Weight and Eating. Palliat. Med. 29, 164–176 (2014).
von Haehling, S. & Anker, S. D. Cachexia as major underestimated unmet medical need: Facts and numbers. Int. J. Cardiol. 161, 121–123 (2012).
Journal of Cachexia, Sarcopenia and Muscle. http://onlinelibrary.wiley.com/journal/10.1007/13539.2190-6009 (2017).
He, W. A. et al. NF-kB mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Invest. 123, 4821–4835 (2013).This study provides evidence that dysfunctional regeneration is a causal event in cancer-induced muscle wasting.
Viguie, C. A., Lu, D.-X., Huang, S.-K., Rengen, H. & Carlson, B. M. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat. Rec. 248, 346–354 (1997).
Seale, P. et al. Pax7 Is Required for the Specification of myogenic satellite cells. Cell 102, 777–786 (2000).
Waning, D. L. et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21, 1262–1271 (2015).
Sheen-Chen, S.-M. Serum levels of transforming growth factor β1 in patients with breast cancer. Arch. Surg. 136, 937 (2001).
Huang, A. et al. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin. Exp. Immunol. 134, 270–278 (2003).
Li, X. et al. Elevated serum level and gene polymorphisms of TGF-β1 in gastric cancer. J. Clin. Lab. Anal. 22, 164–171 (2008).
Poch, B. et al. Systemic immune dysfunction in pancreatic cancer patients. Langenbecks Arch. Surg. 392, 353–358 (2007).
Craven, K. E., Gore, J., Wilson, J. L. & Korc, M. Angiogenic gene signature in human pancreatic cancer correlates with TGF-beta and inflammatory transcriptomes. Oncotarget 1, 323–341 (2010).This study establishes an association between increased TGFβ signalling in pancreatic ductal adenocarcinoma, increased expression of pro-inflammatory genes and increased tyrosine kinase JAK signalling, which may combine to worsen muscle loss.
Morrison, S. D. Feeding response to change in absorbable food fraction during growth of Walker 256 carcinosarcoma. Cancer Res. 32, 968–972 (1972).
Baccino, F. M., Tessitore, L., Bonelli, G. & Isidoro, C. Protein turnover states of tumour cells and host tissues in an experimental model. Biomed. Biochim. Acta 45, 1585–1590 (1986).
Ohe, Y. et al. Interleukin-6 cDNA transfected Lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shortened survival in syngeneic mice. Br. J. Cancer 67, 939–944 (1993).
Tanaka, Y. et al. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 50, 2290–2295 (1990).
Michaelis, K. A. et al. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J. Cachexia Sarcopenia Muscle 8, 824–838 (2017).
Go, K. L. et al. Orthotopic patient-derived pancreatic cancer xenografts engraft into the pancreatic parenchyma, metastasize, and induce muscle wasting to recapitulate the human disease. Pancreas 46, 813–819 (2017).
Wiedenmann, B. et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J. Support. Oncol. 6, 18–25 (2008).
Tan, B. H. L. et al. P-selectin genotype is associated with the development of cancer cachexia. EMBO Mol. Med. 4, 462–471 (2012).
Johns, N. et al. Genetic basis of interindividual susceptibility to cancer cachexia: selection of potential candidate gene polymorphisms for association studies. J. Genet. 93, 893–916 (2014).
Narasimhan, A. et al. Small RNAome profiling from human skeletal muscle: novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 8, 405–416 (2017).
Twelkmeyer, B., Tardif, N. & Rooyackers, O. Omics and cachexia. Curr. Opin. Clin. Nutr. Metab. Care 20, 181–185 (2017).
Gallagher, I. J., Jacobi, C., Tardif, N., Rooyackers, O. & Fearon, K. Omics/systems biology and cancer cachexia. Semin. Cell Dev. Biol. 54, 92–103 (2016).
Ebhardt, H. A. et al. Comprehensive proteome analysis of human skeletal muscle in cachexia and sarcopenia: a pilot study. J. Cachexia Sarcopenia Muscle 8, 567–582 (2017).
Sagar, G. et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 65, 1165–1174 (2015).This is a clinical study of a novel mechanism of tumour-induced lipolysis.
Sidler, B. et al. Amplification of the parathyroid hormone-related peptide gene in a colonic carcinoma. J. Clin. Endocrinol. Metab. 81, 2841–2847 (1996).
Washam, C. L. et al. Identification of PTHrP(12–48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol. Biomarkers Prev. 22, 972–983 (2013).
He, W. A. et al. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl Acad. Sci. USA 111, 4525–4529 (2014).
Samatar, A. A. & Poulikakos, P. I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat. Rev. Drug Discov. 13, 928–942 (2014).
Baracos, V. E. Mitogen-activated protein kinases inhibitors: potential therapeutic agents for cancer cachexia. Mol. Cancer Ther. 16, 263–264 (2017).
Fearon, K. C. H. et al. Request for regulatory guidance for cancer cachexia intervention trials. J. Cachexia Sarcopenia Muscle 6, 272–274 (2015).
Hall, K. D. et al. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 95, 989–994 (2012).
Purcell, S. A., Elliott, S. A., Baracos, V. E., Chu, Q. S. C. & Prado, C. M. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur. J. Clin. Nutr. 70, 1230–1238 (2016).
Jager-Wittenaar, H. & Ottery, F. D. Assessing nutritional status in cancer: role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 20, 322–329 (2017).
Vellas, B. et al. Overview of the MNA — its history and challenges. J. Nutr. Health Aging 10, 456–463; discussion 463–465 (2006).
Rubenstein, L. Z., Harker, J. O., Salvà, A., Guigoz, Y. & Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A. Biol. Sci. Med. Sci. 56, M366–372 (2001).
Ferguson, M., Capra, S., Bauer, J. & Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 15, 458–464 (1999).
Stratton, R. J. et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 92, 799–808 (2004).
Kruizenga, H. M., Seidell, J. C., de Vet, H. C. W., Wierdsma, N. J. & van Bokhorst-de van der Schueren, M. A. E. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 24, 75–82 (2005).
Kondrup, J. & Rasmussen, H. H., Hamberg, O., Stanga, Z. & Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin. Nutr. 22, 321–336 (2003).
Bourdel-Marchasson, I. et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS ONE 9, e108687 (2014).
Sánchez-Lara, K. et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin. Nutr. 33, 1017–1023 (2014).
Madeddu, C. et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin. Nutr. 31, 176–182 (2012).
Acknowledgements
The authors thank R.J.E. Skipworth (University of Edinburgh) for his valuable input.
Author information
Authors and Affiliations
Contributions
Introduction (V.E.B.); Epidemiology (L.M.); Mechanisms/pathophysiology (M.K. and D.C.G.); Diagnosis, screening and prevention (V.E.B.); Management (K.C.H.F.); Quality of life (V.E.B.); Outlook (V.E.B.); and Overview of the Primer (V.E.B.).
Corresponding author
Ethics declarations
Competing interests
V.E.B. receives financial support from the Canadian Institutes of Health Research and The Alberta Cancer Foundation. L.M. is funded by Alberta Innovates, the Izaak Walton Killam Foundation and the American Society of Parenteral and Enteral Nutrition. M.K. is partially funded by National Cancer Institute grant CA-075059 and by the consortium for the study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (U01 DK108323). D.C.G. receives funding support from the US NIH and from the Ohio State University Comprehensive Cancer Center.
Rights and permissions
About this article
Cite this article
Baracos, V., Martin, L., Korc, M. et al. Cancer-associated cachexia. Nat Rev Dis Primers 4, 17105 (2018). https://doi.org/10.1038/nrdp.2017.105
Published:
DOI: https://doi.org/10.1038/nrdp.2017.105
This article is cited by
-
Comprehensive evaluation of serum hepatic proteins in predicting prognosis among cancer patients with cachexia: an observational cohort study
BMC Cancer (2024)
-
Assessment of lipolysis biomarkers in adipose tissue of patients with gastrointestinal cancer
Cancer & Metabolism (2024)
-
Optimization of a mouse model of pancreatic cancer to simulate the human phenotypes of metastasis and cachexia
BMC Cancer (2024)
-
Systemic inflammation and insulin resistance-related indicator predicts poor outcome in patients with cancer cachexia
Cancer & Metabolism (2024)
-
A new bio imagery user-friendly tool for automatic morphometry measurement on muscle cell cultures and histological sections
Scientific Reports (2024)