Key Points
-
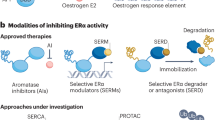
Aromatase inhibitors (AIs) are frequently prescribed for patients with oestrogen receptor-positive (ER+) breast cancer to control advanced disease and to prevent relapse after treatment with localized breast cancer (adjuvant therapy). However, resistance to AI therapy is common, occurring in over 20% of patients with early-stage disease and is inevitable in patients with metastatic disease.
-
Resistance to AI therapy can be detected in primary tumours by measuring on-treatment tumour Ki67 expression. The idea of monitoring tumour Ki67 expression as a clinical tool is being prospectively evaluated for individualized treatment approaches that de-escalate therapy for responsive tumours (Ki67low after AI treatment) and escalate therapy for unresponsive tumours (Ki67high after treatment).
-
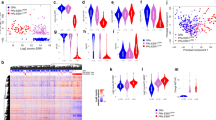
Genomic analyses of ER+ tumours have identified more than 30 significantly mutated genes the role of which in AI therapy responsiveness is under investigation. To date, TP53 has been associated with high levels of Ki67 both before and after therapy, and therefore with more aggressive disease, and MAP3K1 has the opposite pattern, and is therefore associated with more indolent disease. GATA3 mutation was associated with a greater fall in Ki67 expression with treatment, suggesting that GATA3-mutant tumours are more dependent on oestrogen than GATA3 wild-type tumours.
-
ERα ligand-binding domain mutations emerge after prolonged periods of AI therapy and are therefore an acquired resistance mechanism to AI therapy. Other genomic aberrations in the ESR1 locus have also been identified, including translocations, amplifications and localized gene rearrangements within the long arm of chromosome 6. The frequency of these findings in AI-resistant tumours and their role in AI resistance is under investigation.
-
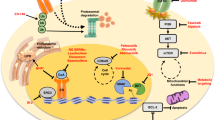
Cancer cell-intrinsic mechanisms for AI resistance include loss of ER expression, upregulation of growth factor receptor pathways including the ERBB family of receptors, fibroblast growth factor receptor (FGFR), insulin-like growth factor 1 receptor (IGF1R) and their downstream signalling including MAPK and PI3K–AKT–mTOR, deregulation of apoptosis and cell cycle machinery.
-
Cancer cell-extrinsic mechanisms depend on interactions with other cell types within the tumour microenvironment (fibroblasts, immune cells, adipose cells and mesenchymal stem cells) that collectively orchestrate the development and maintenance of AI resistance.
-
Mechanism-based inhibitors against cyclin-dependent kinase 4 (CDK4) and CDK6, PI3K and histone deacetylases are among the most promising strategies being tested to overcome AI resistance in Phase III clinical trials.
-
Further advances in our understanding of AI-resistance mechanisms rely on prospective longitudinal studies of tumour samples collected at multiple disease time points and also on preclinical models that capture the full spectrum and biology of AI-resistance mechanisms.
Abstract
Oestrogen receptor-positive (ER+) breast cancer is a major cause of cancer death in women. Although aromatase inhibitors suppress the function of ER and reduce the risk of recurrence, therapeutic resistance is common and essentially inevitable in advanced disease. This Review considers both genomic and cell biological explanations as to why ER+ breast cancer cells persist, progress and cause an incurable, lethal, systemic disease. The design and outcomes of clinical trials are considered with the perspective that resistance mechanisms are heterogeneous, and therefore biomarker and somatic mutation-based stratification and eligibility will be essential for improvements in patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Beatson, G. T. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet 33, 108–121 (1983). This paper is the starting place for any discussion of endocrine therapy and reminds us how instructive case reports can be: the genomic analysis of extreme responders to targeted therapy is a modern iteration of this theme.
Lin, N. U. & Winer, E. P. Advances in adjuvant endocrine therapy for postmenopausal women. J. Clin. Oncol. 26, 798–805 (2008).
Pagani, O. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. New Engl. J. Med. 371, 107–118 (2014). This is a well-powered study showing a benefit for the AI exemestane versus tamoxifen in combination with ovarian suppression. Further follow-up will be required to demonstrate the full magnitude of the advantage of AIs in this setting.
Di Leo, A. et al. Final overall survival: fulvestrant 500 mg versus 250 mg in the randomized CONFIRM trial. J. Natl Cancer Inst. 106, djt337 (2014).
Robertson, J. F. R. et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II 'first' study. Abstracts from the 37th Annual SABCS S6–04 (2014).
Early Breast Cancer Trialists' Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 (2011).
Mauri, D., Pavlidis, N., Polyzos, N. P. & Ioannidis, J. P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J. Natl Cancer Inst. 98, 1285–1291 (2006).
Ellis, M. Overcoming endocrine therapy resistance by signal transduction inhibition. Oncologist 9, 20–26 (2004).
Ellis, M. J. et al. Lower-dose versus high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA 302, 774–780 (2009). This is a study in the modern era of oestradiol for AI-resistant breast cancer showing that the lower dose (6 mg) is safer, better tolerated and as effective as the traditional high-dose approach.
Ellis, M. J. et al. Z1031B neoadjuvant aromatase inhibitor trial: a phase 2 study of triage to chemotherapy based on 2 to 4 week Ki67 level >10%. Cancer Res. 72, PD07-01 (2012).
Zanotti, L., Bottini, A., Rossi, C., Generali, D. & Cappelletti, M. R. Diagnostic tests based on gene expression profile in breast cancer: from background to clinical use. Tumor Biol. 35, 8461–8470 (2014).
Kiyotani, K., Mushiroda, T., Nakamura, Y. & Zembutsu, H. Pharmacogenomics of tamoxifen: roles of drug metabolizing enzymes and transporters. Drug Metab. Pharmacokinet. 27, 122–131 (2012).
Ding, L. et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010).
Shah, S. P. et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461, 809–813 (2009). This is the first paper to document, using next-generation DNA sequencing, the evolution of mutations over time as ER+ breast cancer progresses.
Eirew, P. et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518, 422–426 (2015).
Goncalves, R., Ma, C., Luo, J., Suman, V. & Ellis, M. J. Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nature Rev. Clin. Oncol. 9, 223–229 (2012).
Ellis, M. J. et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl Cancer Inst. 100, 1380–1388 (2008). This paper established a prognostic model for ER+ breast cancer treated with neoadjuvant endocrine therapy based on the biological characteristics and pathological stage of the surgical specimen after completion of preoperative treatment.
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J. Natl Cancer Inst. 103, 1656–1664 (2011).
Dowsett, M. et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J. Natl Cancer Inst. 99, 167–170 (2007).
Ellis, M. J. et al. Tumor Ki67 proliferation index within 4 weeks of initiating neoadjuvant endocrine therapy for early identification of non-responders. Cancer Res. 69, 78 (2009).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353–360 (2012). This study applied whole-genome sequencing to clinical trial samples from a neoadjuvant AI trial to determine the somatic genome of ER+ breast cancer and how it may explain variable responsiveness to targeted therapy.
Goncalves, R. et al. A Ki67-based clinical trial assay for neoadjuvant endocrine therapy response monitoring in breast cancer. J. Pharmacogenomics Pharmacoproteomics 5, 5 (2014).
Ellis, M. J. et al. Randomized Phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype — ACOSOG Z1031. J. Clin. Oncol. 29, 2342–2349 (2011).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Med. 20, 548–554 (2014).
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Goldstein, T. C., Paull, E. O., Ellis, M. J. & Stuart, J. M. Molecular pathways: extracting medical knowledge from high-throughput genomic data. Clin. Cancer Res. 19, 3114–3120 (2013).
Ghazoui, Z. et al. Close and stable relationship between proliferation and a hypoxia metagene in aromatase inhibitor-treated ER-positive breast cancer. Clin. Cancer Res. 17, 3005–3012 (2011).
Veeraraghavan, J. et al. Recurrent ESR1–CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nature Commun. 5, 4577 (2014).
Robinson, D. R. et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature Med. 17, 1646–1651 (2011).
Sakarya, O. et al. RNA-seq mapping and detection of gene fusions with a suffix array algorithm. PLoS Comput. Biol. 8, e1002464 (2012).
Li, S. et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 4, 1116–1130 (2013). This paper demonstrates the value of PDXs from patients with endocrine therapy-resistant disease by revealing how gene fusions, amplifications and point mutations activate ESR1 in advanced disease.
Fuqua, S. A. W. et al. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 51, 105–109 (1991).
Roodi, N. et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J. Natl Cancer Inst. 87, 446–451 (1995).
Toy, W. et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nature Genet. 45, 1439–1445 (2013).
Robinson, D. R. et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nature Genet. 45, 1446–1451 (2013). References 34 and 35 describe LBD mutations in ESR1 as a clinically important mechanism of endocrine therapy resistance.
Zhang, Q. X., Borg, A., Wolf, D. M., Oesterreich, S. & Fuqua, S. A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 57, 1244–1249 (1997).
Merenbakh-Lamin, K. et al. D538G mutation in estrogen receptor-α: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 73, 6856–6864 (2013).
Ma, Y. et al. Fusion transcript discovery in formalin-fixed paraffin-embedded human breast cancer tissues reveals a link to tumor progression. PLoS ONE 9, e94202 (2014).
Fan, P. et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur. J. Cancer 50, 457–468 (2014).
Paul, D. et al. Letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor-positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor (AI) therapy. Cancer Res. 73, S3-07 (2013).
Adelaide, J. et al. Absence of ESR1 amplification in a series of breast cancers. Int. J. Cancer 123, 2970–2972 (2008).
Holst, F. et al. Estrogen receptor α (ESR1) gene amplification is frequent in breast cancer. Nature Genet. 39, 655–660 (2007).
Vincent-Salomon, A., Raynal, V., Lucchesi, C., Gruel, N. & Delattre, O. ESR1 gene amplification in breast cancer: a common phenomenon? Nature Genet. 40, 809 (2008).
Reis-Filho, J. S. et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nature Genet. 40, 809–810 (2008).
Horlings, H. M. et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nature Genet. 40, 807–808 (2008).
Brown, L. A. et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nature Genet. 40, 806–807 (2008).
Ooi, A. et al. Gene amplification of ESR1 in breast cancers — fact or fiction? A fluorescence in situ hybridization and multiplex ligation-dependent probe amplification study. J. Pathol. 227, 8–16 (2012).
Albertson, D. G. ESR1 amplification in breast cancer: controversy resolved? J. Pathol. 227, 1–3 (2012).
Holst, F. et al. On the evidence for ESR1 amplification in breast cancer. Nature Rev. Cancer 12, 149 (2012).
Tomita, S. et al. Estrogen receptor α gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 100, 1012–1017 (2009).
Moelans, C. B. et al. ESR1 amplification is rare in breast cancer and is associated with high grade and high proliferation: a multiplex ligation-dependent probe amplification study. Anal. Cell. Pathol. (Amst.) 33, 13–18 (2010).
Moelans, C. B., Holst, F., Hellwinkel, O., Simon, R. & van Diest, P. J. ESR1 amplification in breast cancer by optimized RNase FISH: frequent but low-level and heterogeneous. PLoS ONE 8, e84189 (2013).
Esslimani-Sahla, M. et al. Estrogen receptor β (ERβ) level but not its ERβcx variant helps to predict tamoxifen resistance in breast cancer. Clin. Cancer Res. 10, 5769–5776 (2004).
Madeira, M., Mattar, A., Logullo, A., Soares, F. & Gebrim, L. Estrogen receptor α/β ratio and estrogen receptor β as predictors of endocrine therapy responsivenessa randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer 13, 425 (2013).
Hopp, T. A. et al. Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin. Cancer Res. 10, 7490–7499 (2004).
Shou, J. et al. Mechanisms of tamoxifen resistance: increased estrogen receptor–HER2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl Cancer Inst. 96, 926–935 (2004).
Arpino, G. et al. HER2 amplification, HER1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin. Cancer Res. 10, 5670–5676 (2004).
Turner, N. et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 70, 2085–2094 (2010).
Fox, E. M. et al. A kinome-wide screen identifies the insulin/IGFI receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 71, 6773–6784 (2011).
Stephen, R. L., Shaw, L. E., Larsen, C., Corcoran, D. & Darbre, P. D. Insulin-like growth factor receptor levels are regulated by cell density and by long term estrogen deprivation in MCF7 human breast cancer cells. J. Biol. Chem. 276, 40080–40086 (2001).
Martin, L. A. et al. Enhanced estrogen receptor (ER) α, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF7 cells to long term estrogen deprivation. J. Biol. Chem. 278, 30458–30468 (2003).
Jeng, M. H., Yue, W., Eischeid, A., Wang, J. P. & Santen, R. J. Role of MAP kinase in the enhanced cell proliferation of long term estrogen deprived human breast cancer cells. Breast Cancer Res. Treat. 62, 167–175 (2000).
Sanchez, C. G. et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 13, R21 (2011).
Miller, T. W. et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Invest. 120, 2406–2413 (2010).
Roop, R. P. & Ma, C. X. Endocrine resistance in breast cancer: molecular pathways and rational development of targeted therapies. Future Oncol. 8, 273–292 (2012).
Lopez-Tarruella, S. & Schiff, R. The dynamics of estrogen receptor status in breast cancer: reshaping the paradigm. Clin. Cancer Res. 13, 6921–6925 (2007).
Creighton, C. J. et al. Activation of mitogen-activated protein kinase in estrogen receptor α-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor α-negative human breast tumors. Cancer Res. 66, 3903–3911 (2006).
Oh, A. S. et al. Hyperactivation of MAPK induces loss of ERα expression in breast cancer cells. Mol. Endocrinol. 15, 1344–1359 (2001).
Bayliss, J., Hilger, A., Vishnu, P., Diehl, K. & El-Ashry, D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin. Cancer Res. 13, 7029–7036 (2007).
Font de Mora, J. & Brown, M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20, 5041–5047 (2000).
Osborne, C. K. et al. Role of the estrogen receptor coactivator AIB1 (SRC3) and HER2/neu in tamoxifen resistance in breast cancer. J. Natl Cancer Inst. 95, 353–361 (2003).
Fan, P., Wang, J., Santen, R. J. & Yue, W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor α out of the nucleus and enhances its interaction with EGFR in MCF7 breast cancer cells. Cancer Res. 67, 1352–1360 (2007).
Song, R. X. et al. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc. Natl Acad. Sci. USA 101, 2076–2081 (2004).
Chen, D. et al. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene 21, 4921–4931 (2002).
Rajbhandari, P. et al. Regulation of estrogen receptor α N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol. Cell. Biol. 32, 445–457 (2012).
Robertson, J. F. Estrogen receptor downregulators: new antihormonal therapy for advanced breast cancer. Clin. Ther. 24, A17–A30 (2002).
Crowder, R. J. et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 69, 3955–3962 (2009). This paper explores the therapeutic potential of combined ER and PI3K pathway inhibition in ER+ breast cancer.
Carroll, J. S. et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 (2005). This paper pioneered the use of new techniques to map ER-binding sites across the genome.
Massarweh, S. et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 68, 826–833 (2008).
Lupien, M. et al. Growth factor stimulation induces a distinct ERα cistrome underlying breast cancer endocrine resistance. Genes Dev. 24, 2219–2227 (2010).
Bhat-Nakshatri, P. et al. AKT alters genome-wide estrogen receptor α binding and impacts estrogen signaling in breast cancer. Mol. Cell. Biol. 28, 7487–7503 (2008).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Levin, E. R. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 20, 477–482 (2009).
Johnston, S. R. Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin. Cancer Res. 11, 889s–899s (2005).
Kaufman, B. et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J. Clin. Oncol. 27, 5529–5537 (2009).
Marcom, P. K. et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res. Treat. 102, 43–49 (2007).
Schwartzberg, L. S. et al. Lapatinib plus letrozole as first-line therapy for HER2+ hormone receptor-positive metastatic breast cancer. Oncologist 15, 122–129 (2010).
Burstein, H. J. et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance). J. Clin. Oncol. 32, 3959–3966 (2014).
Osborne, C. K. et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin. Cancer Res. 17, 1147–1159 (2011).
Kaufman, P. A. et al. A randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC). Cancer Res. 70, S1–S4 (2010).
Sergina, N. V. et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445, 437–441 (2007).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Cizkova, M. et al. PIK3CA mutation impact on survival in breast cancer patients and in ERα, PR and ERBB2-based subgroups. Breast Cancer Res. 14, R28 (2012).
Kalinsky, K. et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin. Cancer Res. 15, 5049–5059 (2009).
Creighton, C. J. et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 12, R40 (2010).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Huang, H. & Tindall, D. J. Dynamic FoxO transcription factors. J. Cell Sci. 120, 2479–2487 (2007).
Guo, S. & Sonenshein, G. E. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol. Cell. Biol. 24, 8681–8690 (2004).
deGraffenried, L. A. et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin. Cancer Res. 10, 8059–8067 (2004).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. New Engl. J. Med. 366, 520–529 (2012). This study established the clinical benefits of mTOR inhibition with everolimus in combination with the steroidal AI exemestane for the treatment of non-steroidal AI-resistant disease.
Bachelot, T. et al. Randomized Phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J. Clin. Oncol. 30, 2718–2724 (2012).
O'Reilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 (2006).
Wan, X., Harkavy, B., Shen, N., Grohar, P. & Helman, L. J. Rapamycin induces feedback activation of Akt signaling through an IGF1R-dependent mechanism. Oncogene 26, 1932–1940 (2007).
Piccart, M. 1LBA everolimus plus exemestane for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (BC): overall survival results from BOLERO-2. Eur. J. Cancer 50, S1 (2014).
Juric, D. et al. Phase I study of BYL719, an α-specific PI3K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-positive (ER+) metastatic breast cancer (MBC). Cancer Res. 72, P61007 (2012).
Hortobagyi, G. N. et al. Correlation of molecular alterations with efficacy of everolimus in hormone receptor-positive, HER2-negative advanced breast cancer: results from BOLERO-2. J. Clin. Oncol. 31, LBA509 (2013).
Thangavel, C. et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer 18, 333–345 (2011).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
Finn, R. S. et al. Results of a randomized phase 2 study of PD 0332991, a cyclin-dependent kinase (CDK) 4/6 inhibitor, in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (BC). Cancer Res. 72, S16 (2012).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA1/TRIO18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2014). This trial describes the pharmacological benefits of CDK4 and CDK6 inhibition in combination with an AI for the control of advanced ER+ breast cancer.
Shangary, S. & Wang, S. Targeting the MDM2–p53 interaction for cancer therapy. Clin. Cancer Res. 14, 5318–5324 (2008).
Ottaviano, Y. L. et al. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 54, 2552–2555 (1994).
Yan, L., Yang, X. & Davidson, N. E. Role of DNA methylation and histone acetylation in steroid receptor expression in breast cancer. J. Mammary Gland Biol. Neoplasia 6, 183–192 (2001).
Yang, X. et al. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 60, 6890–6894 (2000).
Yang, X. et al. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ERα-negative breast cancer cells. Cancer Res. 61, 7025–7029 (2001).
Widschwendter, M. et al. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 64, 3807–3813 (2004).
Sabnis, G. J. et al. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res. 71, 1893–1903 (2011).
Keen, J. C. et al. A novel histone deacetylase inhibitor, scriptaid, enhances expression of functional estrogen receptor α (ER) in ER negative human breast cancer cells in combination with 5-aza 2′-deoxycytidine. Breast Cancer Res. Treat. 81, 177–186 (2003).
Giacinti, L. et al. Scriptaid effects on breast cancer cell lines. J. Cell. Physiol. 227, 3426–3433 (2012).
Zhou, Q., Shaw, P. G. & Davidson, N. E. Inhibition of histone deacetylase suppresses EGF signaling pathways by destabilizing EGFR mRNA in ER-negative human breast cancer cells. Breast Cancer Res. Treat. 117, 443–451 (2009).
Hirokawa, Y., Arnold, M., Nakajima, H., Zalcberg, J. & Maruta, H. Signal therapy of breast cancers by the HDAC inhibitor FK228 that blocks the activation of PAK1 and abrogates the tamoxifen-resistance. Cancer Biol. Ther. 4, 956–960 (2005).
Reid, G. et al. Multiple mechanisms induce transcriptional silencing of a subset of genes, including oestrogen receptor α, in response to deacetylase inhibition by valproic acid and trichostatin A. Oncogene 24, 4894–4907 (2005).
Marks, P. A. The mechanism of the anti-tumor activity of the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA). Cell Cycle 3, 534–535 (2004).
Munster, P. N. et al. A Phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer 104, 1828–1835 (2011).
Yardley, D. A. et al. Randomized Phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 31, 2128–2135 (2013). This paper presents early clinical trial evidence for a role for HDAC inhibition in modulating AI resistance.
Crawford, A. C., Riggins, R. B., Shajahan, A. N., Zwart, A. & Clarke, R. Co-inhibition of BCL-W and BCL-2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS ONE 5, e8604 (2010).
Schwartz-Roberts, J. L. et al. GX15070 (obatoclax) induces apoptosis and inhibits cathepsin D− and L–mediated autophagosomal lysis in antiestrogen-resistant breast cancer cells. Mol. Cancer Ther. 12, 448–459 (2013).
Signore, M., Ricci-Vitiani, L. & De Maria, R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 332, 374–382 (2013).
Wiezorek, J., Holland, P. & Graves, J. Death receptor agonists as a targeted therapy for cancer. Clin. Cancer Res. 16, 1701–1708 (2010).
Okuhira, K. et al. Development of hybrid small molecules that induce degradation of estrogen receptor-α and necrotic cell death in breast cancer cells. Cancer Sci. 104, 1492–1498 (2013).
Stanculescu, A. et al. Estrogen promotes breast cancer cell survival in an inhibitor of apoptosis (IAP)-dependent manner. Horm. Cancer 1, 127–135 (2010).
Sakariassen, P. O., Immervoll, H. & Chekenya, M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 9, 882–892 (2007).
Engelmann, K., Shen, H. & Finn, O. J. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 68, 2419–2426 (2008).
Kuhnle, M. et al. Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar. J. Med. Chem. 52, 1190–1197 (2009).
Wang, S., Yang, D. & Lippman, M. E. Targeting Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin. Oncol. 30, 133–142 (2003).
Harrison, H. et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 70, 709–718 (2010).
Harris, L. G., Samant, R. S. & Shevde, L. A. Hedgehog signaling: networking to nurture a promalignant tumor microenvironment. Mol. Cancer Res. 9, 1165–1174 (2011).
Korkaya, H. et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 7, e1000121 (2009).
Zhang, M., Atkinson, R. L. & Rosen, J. M. Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl Acad. Sci. USA 107, 3522–3527 (2010).
Liu, S. & Wicha, M. S. Targeting breast cancer stem cells. J. Clin. Oncol. 28, 4006–4012 (2010).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Ponti, D. et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 65, 5506–5511 (2005).
Hardt, O. et al. Highly sensitive profiling of CD44+/CD24− breast cancer stem cells by combining global mRNA amplification and next generation sequencing: evidence for a hyperactive PI3K pathway. Cancer Lett. 325, 165–174 (2012).
Harrison, H. et al. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res. 15, R21 (2013).
Creighton, C. J. et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl Acad. Sci. USA 106, 13820–13825 (2009).
Haughian, J. M. et al. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc. Natl Acad. Sci. USA 109, 2742–2747 (2012).
Dittmer, J. & Leyh, B. The impact of tumor stroma on drug response in breast cancer. Semin. Cancer Biol. 31, 3–15 (2014).
Polanska, U. M. & Orimo, A. Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J. Cell. Physiol. 228, 1651–1657 (2013).
Tchou, J. & Conejo-Garcia, J. in Advances in Pharmacology (ed. Keiran, S. M. S.) 45–61 (Academic Press, 2012).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF1/CXCL12 secretion. Cell 121, 335–348 (2005).
Hiscox, S. et al. Chronic exposure to fulvestrant promotes overexpression of the c-Met receptor in breast cancer cells: implications for tumour-stroma interactions. Endocr. Relat. Cancer 13, 1085–1099 (2006).
Rhodes, L. V. et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 71, 603–613 (2011).
Shi, X. P. et al. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial–mesenchymal transition features. Int. J. Mol. Sci. 14, 15655–15668 (2013).
Hiscox, S. et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of β-catenin phosphorylation. Int. J. Cancer 118, 290–301 (2006).
Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010).
Borley, A. C. et al. Anti-oestrogens but not oestrogen deprivation promote cellular invasion in intercellular adhesion-deficient breast cancer cells. Breast Cancer Res. 10, R103 (2008).
Martinez-Outschoorn, U. E. et al. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol. Ther. 12, 924–938 (2011).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007). This is a key paper documenting the role of the cellular microenvironment in the control of metastatic behaviour in breast cancer.
Rhodes, L. et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res. Treat. 121, 293–300 (2010).
Kristensen, V. N. et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc. Natl Acad. Sci. USA 109, 2802–2807 (2012).
DeNardo, D. G. et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102 (2009).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 470, 548–553 (2011).
Baratelli, F. et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175, 1483–1490 (2005).
Bates, G. J. et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 24, 5373–5380 (2006).
Generali, D. et al. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin. Cancer Res. 15, 1046–1051 (2009).
Treilleux, I. et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 10, 7466–7474 (2004).
Dunbier, A. K. et al. Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin. Cancer Res. 19, 2775–2786 (2013).
Bianchini, G. et al. A dendritic metagene that predicts prognosis and endocrine resistance in breast cancer. J. Clin. Oncol. 30, 545 (2012).
Emens, L. A. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev. Anticancer Ther. 12, 1597–1611 (2012).
Goss, P. E. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl Cancer Inst. 97, 1262–1271 (2005). This paper described the benefits of AI therapy after tamoxifen to control late relapse.
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013) This paper reports a randomized trial demonstrating the role of prolonged tamoxifen therapy beyond 5 years for the long-term control of ER+ breast cancer.
Esposito, M. & Kang, Y. Targeting tumor–stromal interactions in bone metastasis. Pharmacol. Ther. 141, 222–233 (2014).
Diel, I. J. et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J. Natl Cancer Inst. 88, 1652–1658 (1996).
Braun, S. et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N. Engl. J. Med. 342, 525–533 (2000).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nature Rev. Cancer 11, 411–425 (2011).
Tran, D. D., Corsa, C. A., Biswas, H., Aft, R. L. & Longmore, G. D. Temporal and spatial cooperation of Snail1 and Twist1 during epithelial–mesenchymal transition predicts for human breast cancer recurrence. Mol. Cancer Res. 9, 1644–1657 (2011).
Ben-Aharon, I. et al. Bisphosphonates in the adjuvant setting of breast cancer therapy — effect on survival: a systematic review and meta-analysis. PLoS ONE 8, e70044 (2013).
Piccart, M. et al. Assessment of genetic alterations in postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer from the BOLERO-2 trial by next-generation sequencing. Ann. Oncol. 24 (Suppl. 3), 25–26 (2013).
Jeselsohn, R. et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 20, 1757–1767 (2014).
Adelson, K. B., Raptis, G., Sparano, J. & Germain, D. Randomized phase II study of fulvestrant versus fulvestrant plus bortezomib in postmenopausal women with estrogen receptor (ER) positive, aromatase-inhibitor (AI) resistant metastatic breast cancer (MBC): New York Cancer Consortium trial P8457. Cancer Res. 71, OT3-01-01 (2014).
Acknowledgements
C.X.M. is supported by the National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award, the Breast Cancer Research Foundation, the Siteman Cancer Center, and the Susan G. Komen Foundation. All four authors were supported by the AVON visiting scholarship program during the development of the manuscript. M.J.E. is also supported by R01 CA095614, the Barnes-Jewish Foundation, the Breast Cancer Research Foundation, the Susan G. Komen Foundation, a McNair Scholarship, the Cancer Prevention Research Institute of Texas, Lester and Sue Smith, the Glen Smith Family and the Theresa Research Foundation for Metastatic Breast Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.J.E. receives royalties for patent licensing to Nanostring for sales of the breast cancer prognostic test “Prosigna” and has stock in Bioclassifer LLC that advises Nanostring on Prosigna development. He is also paid as an ad hoc consultant for Novartis, Pfizer and AstraZeneca. C.X.M., T.R. and I.C. declare no competing interests.
Related links
Glossary
- Aromatase inhibitors
-
(AIs). A class of drugs, including non-steroidal AIs (for example, anastrozole and letrozole), and steroidal AIs (for example, exemestane), that lower oestrogen levels by inhibiting the enzyme aromatase.
- Tamoxifen
-
A selective oestrogen receptor (ER) modulator (SERM), which antagonizes ER function in breast tissue but also acts as an ER agonist in other tissues, including the endometrium and blood coagulation system, resulting in side effects such as endometrial cancer and an increased risk of thrombosis, respectively.
- Endocrine therapy
-
Targets the oestrogen receptor (ER) pathway for the treatment of ER+ breast cancer either by lowering oestrogen level or by antagonizing ER function.
- Neoadjuvant
-
(also known as preoperative treatment). Refers to therapy administered before curative surgery of the primary cancer. This is often used to reduce tumour size and render large or locally advanced cancers operable.
- Natural logarithm
-
The natural logarithm (loge) of 2.7% is ∼ 1. A natural scale based on multiples of loge = 1 was used in the preoperative endocrine prognostic index because it creates Ki67 intervals, measured during neoadjuvant aromatase inhibitor therapy, associated with significant stepwise increased risk of relapse and death.
- Pathological complete response
-
(pCR). Commonly defined as the absence of residual invasive cancers in the breast and in the axillary lymph node following completion of neoadjuvant systemic therapy, but other definitions exist.
- Luminal B
-
One of the five intrinsic molecular subtypes of breast cancer characterized by higher expression levels of proliferation genes and lower expression levels of oestrogen receptor (ER)-regulated genes compared with the luminal A subtype, and is associated with a poor prognosis.
- Luminal A
-
One of the five intrinsic subtypes of breast cancer characterized by high expression levels of oestrogen receptor (ER) pathway genes and low expression levels of proliferation genes, and associated with a good prognosis.
- Patient-derived xenografts
-
(PDXs). Xenograft models generated by engrafting the cancerous tissue from a patient into an immunodeficient mouse to generate an individualized model of their disease.
- Synthetic lethality
-
The simultaneous perturbation of two genes or processes that results in cellular or organism death, whereas loss of either alone does not.
- Epithelial-to-mesenchymal transition
-
(EMT). A process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties.
- Plasmacytoid DCs
-
Innate immune cells that circulate in the blood and that are found in peripheral lymphoid organs, specialized in rapid and massive secretion of type I interferon (IFNα/β) in response to foreign nucleic acids.
Rights and permissions
About this article
Cite this article
Ma, C., Reinert, T., Chmielewska, I. et al. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 15, 261–275 (2015). https://doi.org/10.1038/nrc3920
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3920
This article is cited by
-
Modeling of Mouse Experiments Suggests that Optimal Anti-Hormonal Treatment for Breast Cancer is Diet-Dependent
Bulletin of Mathematical Biology (2024)
-
Immunotherapy: an emerging modality to checkmate brain metastasis
Molecular Cancer (2023)
-
SERPINA3-ANKRD11-HDAC3 pathway induced aromatase inhibitor resistance in breast cancer can be reversed by HDAC3 inhibition
Communications Biology (2023)
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy
Nature Reviews Cancer (2023)
-
Joint inference of exclusivity patterns and recurrent trajectories from tumor mutation trees
Nature Communications (2023)