Key Points
-
Mutationally activated BRAF is expressed in melanoma, glioblastoma, thyroid, lung and colon cancers and in a subset of haematological malignancies.
-
The most common BRAF mutation leads to the substitution of a glutamic acid for valine at amino acid 600 (V600E) in the kinase domain of the protein. This substitution mimics phosphorylation of the activation loop, thereby inducing constitutive BRAF protein kinase activity.
-
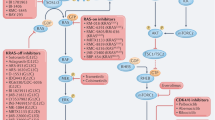
Point mutations in the related ARAF and CRAF protein kinases, although very rare, have been reported as oncogenic drivers in some human cancers. In addition to point mutation, gene fusion events are reported to activate BRAF and CRAF.
-
Numerous non-V600E alterations in BRAF have been reported in cancer and in a rare developmental disorder. Many of these promote kinase activity by relieving autoinhibitory mechanisms or promote activation of other RAF isoforms in a RAS-dependent manner.
-
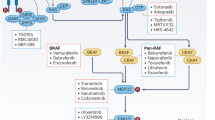
ATP-competitive BRAF kinase inhibitors are currently under investigation for the treatment of BRAF-mutated cancers. However, to date, efficacy is limited to a subset of melanomas owing to primary or adaptive resistance mechanisms in colorectal and thyroid cancers that reactivate signalling downstream of receptor tyrosine kinases.
-
In clinical trials of BRAF-mutated melanoma, BRAF inhibitors tend to induce high rates of response that show transient durability due to the onset of drug-resistant disease.
-
Acquired resistance to BRAF-V600E inhibitors is strikingly complex but frequently involves reactivation of MEK–ERK MAP kinase signalling. Drug resistance due to overexpression of oncogenic BRAF-V600E leads to 'oncogene overdose' following cessation of drug administration — a phenomenon that could be clinically exploitable to forestall the onset of drug resistance.
-
The efficacy of RAF inhibitors in tumours with other RAF mutations is mostly unknown, although preclinical studies indicate varied responses that are inhibitor-specific and depend on the biochemical mechanism of oncogene activation.
Abstract
The identification of mutationally activated BRAF in many cancers altered our conception of the part played by the RAF family of protein kinases in oncogenesis. In this Review, we describe the development of BRAF inhibitors and the results that have emerged from their analysis in both the laboratory and the clinic. We discuss the spectrum of RAF mutations in human cancer and the complex interplay between the tissue of origin and the response to RAF inhibition. Finally, we enumerate mechanisms of resistance to BRAF inhibition that have been characterized and postulate how strategies of RAF pathway inhibition may be extended in scope to benefit not only the thousands of patients who are diagnosed annually with BRAF-mutated metastatic melanoma but also the larger patient population with malignancies harbouring mutationally activated RAF genes that are ineffectively treated with the current generation of BRAF kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rapp, U. R. et al. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc. Natl Acad. Sci. USA 80, 4218–4222 (1983).
Jansen, H. W., Ruckert, B., Lurz, R. & Bister, K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 2, 1969–1975 (1983).
Moelling, K., Heimann, B., Beimling, P., Rapp, U. R. & Sander, T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature 312, 558–561 (1984).
Bonner, T. I. et al. Structure and biological activity of human homologs of the raf/mil oncogene. Mol. Cell. Biol. 5, 1400–1407 (1985).
Bonner, T. et al. The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4. Science 223, 71–74 (1984).
Mark, G. E., MacIntyre, R. J., Digan, M. E., Ambrosio, L. & Perrimon, N. Drosophila melanogaster homologs of the raf oncogene. Mol. Cell. Biol. 7, 2134–2140 (1987).
Han, M., Golden, A., Han, Y. & Sternberg, P. W. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature 363, 133–140 (1993).
Kozak, C., Gunnell, M. A. & Rapp, U. R. A new oncogene, c-raf, is located on mouse chromosome 6. J. Virol. 49, 297–299 (1984).
Jansen, H. W., Trachmann, C. & Bister, K. Structural relationship between the chicken protooncogene c-mil and the retroviral oncogene v-mil. Virology 137, 217–224 (1984).
Jansen, H. W. et al. Homologous cell-derived oncogenes in avian carcinoma virus MH2 and murine sarcoma virus 3611. Nature 307, 281–284 (1984).
Kyriakis, J. M. et al. Raf-1 activates MAP kinase-kinase. Nature 358, 417–421 (1992).
Moodie, S. A., Willumsen, B. M., Weber, M. J. & Wolfman, A. Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260, 1658–1661 (1993).
Van Aelst, L., Barr, M., Marcus, S., Polverino, A. & Wigler, M. Complex formation between RAS and RAF and other protein kinases. Proc. Natl Acad. Sci. USA 90, 6213–6217 (1993).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002). This is the first report of BRAF mutations in human cancers.
Forbes, S. A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011).
Garnett, M. J. & Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6, 313–319 (2004).
Wan, P. T. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004). This is the first paper to describe the crystal structure of the BRAF-V600E kinase domain and the biochemical mechanism of oncogene activation.
Brastianos, P. K. et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nature Genet. 46, 161–165 (2014).
Cerami, E. et al. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Tiacci, E. et al. BRAF mutations in hairy-cell leukemia. New Engl. J. Med. 364, 2305–2315 (2011).
Choueiri, T. K. et al. BRAF mutations in metanephric adenoma of the kidney. Eur. Urol. 62, 917–922 (2012).
Cancer Statistics Working Group, U.S. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. (U.S. Department of Health and Human Services, 2013).
Garnett, M. J., Rana, S., Paterson, H., Barford, D. & Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20, 963–969 (2005).
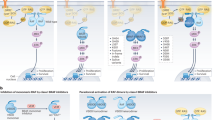
Heidorn, S. J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010). This is a demonstration of paradoxical activation of the MAP kinase pathway by RAF inhibitors or a Braf allele with impaired catalytic activity.
Holderfield, M. et al. RAF inhibitors activate the MAPK pathway by relieving inhibitory autophosphorylation. Cancer Cell 23, 594–602 (2013).
Dankort, D. et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 21, 379–384 (2007).
Dankort, D. et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nature Genet. 41, 544–552 (2009). This paper demonstrates the oncogenic potential for BRAF-V600E-driven melanoma and synergy with Pten loss to promote metastasis in a mouse model.
Dhomen, N. et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 (2009).
McFadden, D. G. et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc. Natl Acad. Sci. USA 111, E1600–E1609 (2014).
Knauf, J. A. et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 65, 4238–4245 (2005).
Charles, R. P., Iezza, G., Amendola, E., Dankort, D. & McMahon, M. Mutationally activated BRAFV600E elicits papillary thyroid cancer in the adult mouse. Cancer Res. 71, 3863–3871 (2011).
Charles, R. P., Silva, J., Iezza, G., Phillips, W. A. & McMahon, M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol. Cancer Res. http://dx.doi.org/10.1158/1541-7786.mcr-14-0158-t (2014).
Carragher, L. A. et al. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol. Med. 2, 458–471 (2010).
Rad, R. et al. A genetic progression model of BrafV600E-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 24, 15–29 (2013).
Collisson, E. A. et al. A central role for RAF→MEK→ ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2, 685–693 (2012).
Wang, J. et al. B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer. Cancer Res. 72, 4765–4776 (2012).
Huillard, E. et al. Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc. Natl Acad. Sci. USA 109, 8710–8715 (2012).
Mito, J. K. et al. Oncogene-dependent control of miRNA biogenesis and metastatic progression in a model of undifferentiated pleomorphic sarcoma. J. Pathol. 229, 132–140 (2013).
Kamata, T. et al. Hematopoietic expression of oncogenic BRAF promotes aberrant growth of monocyte-lineage cells resistant to PLX4720. Mol. Cancer Res. 11, 1530–1541 (2013).
Imielinski, M. et al. Oncogenic and sorafenib-sensitive ARAF mutations in lung adenocarcinoma. J. Clin. Invest. 124, 1582–1586 (2014).
Pandit, B. et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature Genet. 39, 1007–1012 (2007).
Razzaque, M. A. et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nature Genet. 39, 1013–1017 (2007).
Roberts, A. E., Allanson, J. E., Tartaglia, M. & Gelb, B. D. Noonan syndrome. Lancet 381, 333–342 (2013).
Pollock, P. M. et al. High frequency of BRAF mutations in nevi. Nature Genet. 33, 19–20 (2003).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Emuss, V., Garnett, M., Mason, C. & Marais, R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 65, 9719–9726 (2005).
Light, Y., Paterson, H. & Marais, R. 14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol. 22, 4984–4996 (2002).
Dhillon, A. S., Meikle, S., Yazici, Z., Eulitz, M. & Kolch, W. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 21, 64–71 (2002).
Kubicek, M. et al. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J. Biol. Chem. 277, 7913–7919 (2002).
Dumaz, N. et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 66, 9483–9491 (2006).
Simon, R. et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 61, 4514–4519 (2001).
Nowell, P. C. & Hungerford, D. A. A minute chromosome in human chronic granulocytic leukemia. Science 142, 1497 (1960).
Palanisamy, N. et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature Med. 16, 793–798 (2010).
Ciampi, R. et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115, 94–101 (2005).
Cin, H. et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 121, 763–774 (2011).
Badiali, M. et al. KIAA1549-BRAF fusions and IDH mutations can coexist in diffuse gliomas of adults. Brain Pathol. 22, 841–847 (2012).
Jones, D. T. et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature Genet. 45, 927–932 (2013).
Jones, D. T. et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 28, 2119–2123 (2009).
Ikawa, S. et al. B-raf, a new member of the raf family, is activated by DNA rearrangement. Mol. Cell. Biol. 8, 2651–2654 (1988).
Stanton, V. P. Jr., Nichols, D. W., Laudano, A. P. & Cooper, G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol. Cell. Biol. 9, 639–647 (1989).
Kerkhoff, E. & Rapp, U. R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol. Cell. Biol. 17, 2576–2586 (1997).
Botton, T. et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell. Melanoma Res. 26, 845–851 (2013).
Hutchinson, K. E. et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin. Cancer Res. 19, 6696–6702 (2013).
Jones, D. T. et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68, 8673–8677 (2008).
Pfister, S. et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Invest. 118, 1739–1749 (2008).
Heidecker, G. et al. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol. Cell. Biol. 10, 2503–2512 (1990).
Rushworth, L. K., Hindley, A. D., O'Neill, E. & Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26, 2262–2272 (2006). This paper describes the biochemical characterization and function of RAF dimerization.
Rajakulendran, T., Sahmi, M., Lefrancois, M., Sicheri, F. & Therrien, M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461, 542–545 (2009).
Sievert, A. J. et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl Acad. Sci. USA 110, 5957–5962 (2013).
Lyons, J. F., Wilhelm, S., Hibner, B. & Bollag, G. Discovery of a novel Raf kinase inhibitor. Endocr. Relat. Cancer 8, 219–225 (2001).
Wilhelm, S. M. et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Abou-Alfa, G. K. et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 24, 4293–4300 (2006).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA 105, 3041–3046 (2008).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. New Engl. J. Med. 363, 809–819 (2010).
Joseph, E. W. et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc. Natl Acad. Sci. USA 107, 14903–14908 (2010).
Bollag, G. et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nature Rev. Drug Discov. 11, 873–886 (2012). This is a review of the discovery, development and initial clinical results of vemurafenib.
Hatzivassiliou, G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 (2010).
Poulikakos, P. I., Zhang, C., Bollag, G., Shokat, K. M. & Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 (2010).
Doma, E. et al. Skin tumorigenesis stimulated by Raf inhibitors relies upon Raf functions that are dependent and independent of ERK. Cancer Res. 73, 6926–6937 (2013).
McKay, M. M., Ritt, D. A. & Morrison, D. K. RAF inhibitor-induced KSR1/B-RAF binding and its effects on ERK cascade signaling. Curr. Biol. 21, 563–568 (2011).
Brennan, D. F. et al. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 472, 366–369 (2011).
Long, G. V. et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 29, 1239–1246 (2011).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. New Engl. J. Med. 366, 707–714 (2012).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl. J. Med. 364, 2507–2516 (2011).
McArthur, G. A. et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 15, 323–332 (2014).
Ascierto, P. A. et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J. Clin. Oncol. 31, 3205–3211 (2013).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365 (2012).
Flaherty, K. T. et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. New Engl. J. Med. 367, 107–114 (2012).
Dietrich, S. et al. Continued response off treatment after BRAF inhibition in refractory hairy cell leukemia. J. Clin. Oncol. 31, e300–e303 (2013).
Anforth, R., Fernandez-Penas, P. & Long, G. V. Cutaneous toxicities of RAF inhibitors. Lancet Oncol. 14, e11–e18 (2013).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. New Engl. J. Med. 366, 207–215 (2012). This paper documents the ability of BRAF inhibitors to promote skin tumorigenesis from initiated, HRAS-mutated keratinocytes.
Oberholzer, P. A. et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J. Clin. Oncol. 30, 316–321 (2012).
Lacouture, M. E. et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist 18, 314–322 (2013).
Holderfield, M. et al. Vemurafenib cooperates with HPV to promote initiation of cutaneous tumors. Cancer Res, 74, 2238–2245 (2014).
Gibney, G. T., Messina, J. L., Fedorenko, I. V., Sondak, V. K. & Smalley, K. S. Paradoxical oncogenesis—the long-term effects of BRAF inhibition in melanoma. Nat. Rev. Clin. Oncol. 10, 390–399 (2013).
Abdel-Wahab, O. et al. Efficacy of Intermittent Combined RAF and MEK Inhibition in a Patient with Concurrent BRAF- and NRAS-Mutant Malignancies. Cancer Discov. http://dx.doi.org/10.1158/2159-8290.cd-13-1038 (2014). This paper shows the clinical usefulness of intermittent RAF inhibitor therapy in a patient with simultaneous NRAS-mutated leukaemia and BRAF-mutated melanoma.
Callahan, M. K. et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. New Engl. J. Med. 367, 2316–2321 (2012).
Andrews, M. C. et al. BRAF inhibitor-driven tumor proliferation in a KRAS-mutated colon carcinoma is not overcome by MEK1/2 inhibition. J. Clin. Oncol. 31, e448–e451 (2013).
Lito, P. et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22, 668–682 (2012).
Kopetz, S. et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J. Clin. Oncol. 28, 3534 (2010).
Corcoran, R. B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012). This paper shows that a loss of feedback from ERK MAP kinase to EGFR signalling can lead to rapid adaptive resistance to the cytotoxic effects of vemurafenib in BRAF-mutated colorectal cancer cells.
Samuels, M. L. & McMahon, M. Inhibition of platelet-derived growth factor- and epidermal growth factor-mediated mitogenesis and signaling in 3T3 cells expressing delta Raf-1:ER, an estradiol-regulated form of Raf-1. Mol. Cell. Biol. 14, 7855–7866 (1994).
Montero-Conde, C. et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 3, 520–533 (2013).
Trejo, C. L., Juan, J., Vicent, S., Sweet-Cordero, A. & McMahon, M. MEK1/2 inhibition elicits regression of autochthonous lung tumors induced by KRASG12D or BRAFV600E. Cancer Res. 72, 3048–3059 (2012).
Trejo, C. L. et al. Mutationally activated PIK3CAH1047R cooperates with BRAFV600E to promote lung cancer progression. Cancer Res. 73, 6448–6461 (2013).
Azam, M., Seeliger, M. A., Gray, N. S., Kuriyan, J. & Daley, G. Q. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nature Struct. Mol. Biol. 15, 1109–1118 (2008).
Whittaker, S. et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci. Transl. Med. 2, 35ra41 (2010).
Nazarian, R. et al. Melanomas acquire resistance to B-RAFV600E inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Emery, C. M. et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl Acad. Sci. USA 106, 20411–20416 (2009).
Van Allen, E. M. et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 4, 94–109 (2014).
Montagut, C. et al. Elevated CRAF potential mechanism acquired resistance BRAF inhibition in melanoma. Cancer Res. 68, 4853–4861 (2008).
Su, F. et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 72, 969–978 (2012).
Karreth, F. A., DeNicola, G. M., Winter, S. P. & Tuveson, D. A. C-Raf inhibits MAPK activation and transformation by B-RafV600E. Mol. Cell 36, 477–486 (2009).
Johannessen, C. M. et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468, 968–972 (2010).
Schimke, R. T., Alt, F. W., Kellems, R. E., Kaufman, R. J. & Bertino, J. R. Amplification of dihydrofolate reductase genes in methotrexate-resistant cultured mouse cells. Cold Spring Harb. Symp. Quant. Biol. 42, (Pt. 2) 649–657 (1978).
Shi, H. et al. Melanoma whole-exome sequencing identifies V600EB-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nature Commun. 3, 724 (2012).
Poulikakos, P. I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 (2011). Of the multiple mechanisms of vemurafenib resistance, this paper demonstrates the clinical relevance of altered patterns of oncogene mRNA splicing as a mechanism for drug resistance.
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Villanueva, J. et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 (2010).
Abel, E. V. et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J. Clin. Invest. 123, 2155–2168 (2013).
Haq, R. et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc. Natl Acad. Sci. USA 110, 4321–4326 (2013).
Smith, M. P. et al. Effect of SMURF2 targeting on susceptibility to MEK inhibitors in melanoma. J. Natl Cancer Inst. 105, 33–46 (2013).
Johannessen, C. M. et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 504, 138–142 (2013).
Straussman, R. et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 (2012).
Wilson, T. R. et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487, 505–509 (2012).
Flaherty, K. T. et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New Engl. J. Med. 367, 1694–1703 (2012).
Kim, K. B. et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 31, 482–489 (2013).
Wagle, N. et al. MAP Kinase Pathway Alterations in BRAF-Mutant Melanoma Patients with Acquired Resistance to Combined RAF/MEK Inhibition. Cancer Discov. 4, 61–68 (2014).
Morris, E. J. et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 3, 742–750 (2013).
Woods, D. et al. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17, 5598–5611 (1997).
Das Thakur, M. et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494, 251–255 (2013). This paper demonstrates the phenomenon of oncogene overdose and the usefulness of intermittent dosing of vemurafenib in the preclinical setting to forestall the onset of drug resistance in patient-derived xenografts of melanoma.
Zhu, J., Woods, D., McMahon, M. & Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998).
Marshall, C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995). This highly influential review succinctly surveys how differences in the magnitude and duration of RTK-mediated activation of RAF–MEK–ERK signalling could be integrated by cells to elicit profoundly different biological responses.
Old, W. M. et al. Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma. Mol. Cell 34, 115–131 (2009).
Blasco, R. B. et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell 19, 652–663 (2011).
Karreth, F. A., Frese, K. K., DeNicola, G. M., Baccarini, M. & Tuveson, D. A. C-Raf is required for the initiation of lung cancer by K-RasG12D. Cancer Discov. 1, 128–136 (2011).
Ehrenreiter, K. et al. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell 16, 149–160 (2009).
Freeman, A. K., Ritt, D. A. & Morrison, D. K. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol. Cell 49, 751–758 (2013).
Ascierto, P. A. et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 14, 249–256 (2013).
Krauthammer, M. et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature Genet. 44, 1006–1014 (2012).
Hodis, E. et al. A landscape of driver mutations in melanoma. Cell 150, 251–263 (2012).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Seshagiri, S. et al. Recurrent R-spondin fusions in colon cancer. Nature 488, 660–664 (2012).
Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075 (2008).
The Cancer Genome Atlas Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Solit, D. B. et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 (2006). This is a survey of BRAF-mutated and RAS-mutated cancer cell lines treated with MEK inhibitors.
Holderfield, M., Nagel, T. E. & Stuart, D. D. Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. Br. J. Cancer http://dx.doi.org/10.1038/bjc.2014.139 (2014).
Deuker, M. M. & McMahon, M. Oncogene addiction and overdose: intermittent treatment in models of drug-resistant braf-mutated melanoma. The Melanoma Letter, 32, No. 1 (2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Holderfield, M., Deuker, M., McCormick, F. et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 14, 455–467 (2014). https://doi.org/10.1038/nrc3760
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3760
This article is cited by
-
Therapeutic Strategies in BRAF V600 Wild-Type Cutaneous Melanoma
American Journal of Clinical Dermatology (2024)
-
Logic-based modeling and drug repurposing for the prediction of novel therapeutic targets and combination regimens against E2F1-driven melanoma progression
BMC Chemistry (2023)
-
Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies
Signal Transduction and Targeted Therapy (2023)
-
The molecular subtypes and clinical prognosis characteristic of tertiary lymphoid structures-related gene of cutaneous melanoma
Scientific Reports (2023)
-
Harnessing TRAIL-induced cell death for cancer therapy: a long walk with thrilling discoveries
Cell Death & Differentiation (2023)