Key Points
-
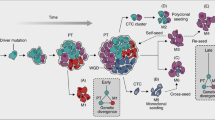
Metastasis occurs through a series of sequential steps in which tumour cells first migrate from the primary tumour, penetrate blood vessels and then colonize distant sites. It is a highly inefficient process. Indeed, very few of the tumour cells that gain access to the vasculature give rise to metastastic foci in a secondary organ.
-
Recent data indicate that the mechanisms controlling metastasis can be regulated independently from primary tumour development.
-
In vitro and in vivo, the metastatic potential of tumours is associated with an increased resistance to apoptosis. Furthermore, the experimental modulation of apoptotic or anti-apoptotic factors influences metastatic efficiency.
-
Anoikis and amorphosis are important barriers to metastasis. Anoikis is cell death induced by the disruption of cell attachment and cell–matrix interactions, whereas amorphosis is cell death stimulated by the loss of cytoskeletal architecture.
-
Early survival of tumour cells after attachment to the secondary site and the development of micrometastases are crucial steps of the metastatic process.
-
Metastasis is the most common cause of cancer death. Most patients with metastatic disease respond transiently to conventional treatments. Further elucidation of the relationship between resistance to apoptosis of metastatic cancer cells and their chemoresistance should provide important clues to improve systemic therapies.
Abstract
The metastatic process is highly inefficient — very few of the many cells that migrate from the primary tumour successfully colonize distant sites. One proposed mechanism to explain this inefficiency is provided by the cancer stem cell model, which hypothesizes that micrometastases can only be established by tumour stem cells, which are few in number. However, recent in vitro and in vivo observations indicate that apoptosis is an important process regulating metastasis. Here we stress that the inhibition of cell death, apart from its extensively described function in primary tumour development, is a crucial characteristic of metastatic cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weigelt, B., Peterse, J. L. & van 't Veer, L. J. Breast cancer metastasis: markers and models. Nature Rev. Cancer 5, 591–602 (2005).
Salvesen, G. S. & Dixit, V. M. Caspases: intracellular signaling by proteolysis. Cell 91, 443–446 (1997).
Zimmermann, K. C. & Green, D. R. How cells die: apoptosis pathways. J. Allergy Clin. Immunol. 108, S99–S103 (2001).
Salvesen, G. S. & Duckett, C. S. IAP proteins: blocking the road to death's door. Nature Rev. Mol. Cell Biol. 3, 401–410 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). A seminal review describing the main rules that govern the transformation of normal human cells into malignant cancers.
Yin, C., Knudson, C. M., Korsmeyer, S. J. & Van Dyke, T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385, 637–640 (1997).
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Fidler, I. J. & Nicolson, G. L. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J. Natl Cancer Inst. 58, 1867–1872 (1977).
Liotta, L. A., Vembu, D., Saini, R. K. & Boone, C. In vivo monitoring of the death rate of artificial murine pulmonary micrometastases. Cancer Res. 38, 1231–1236 (1978).
Varani, J., Lovett, E. J., Elgebaly, S., Lundy, J. & Ward, P. A. In vitro and in vivo adherence of tumor cell variants correlated with tumor formation. Am. J. Pathol. 101, 345–352 (1980).
Inbal, B. et al. DAP kinase links the control of apoptosis to metastasis. Nature 390, 180–184 (1997). By using lung carcinoma clones, this study shows that the inhibition of the expression of DAPK, a positive mediator of apoptosis, favours the metastatic process.
Um, J. H. et al. Relationship between antiapoptotic molecules and metastatic potency and the involvement of DNA-dependent protein kinase in the chemosensitization of metastatic human cancer cells by epidermal growth factor receptor blockade. J. Pharmacol. Exp. Ther. 311, 1062–1070 (2004).
Glinsky, G. V., Glinsky, V. V., Ivanova, A. B. & Hueser, C. J. Apoptosis and metastasis: increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett. 115, 185–193 (1997). Data showing that the metastatic potential of murine and human cancer cells is strictly associated with the increased resistance to apoptosis.
Del Bufalo, D., Biroccio, A., Leonetti, C. & Zupi, G. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 11, 947–953 (1997). An elegant study showing that the overexpression of the anti-apoptotic oncoprotein BCL2 increases the metastatic potential of human breast cancer cells.
McConkey, D. J., Greene, G. & Pettaway, C. A. Apoptosis resistance increases with metastatic potential in cells of the human LNCaP prostate carcinoma line. Cancer Res. 56, 5594–5599 (1996).
Furuya, Y., Krajewski, S., Epstein, J. I., Reed, J. C. & Isaacs, J. T. Expression of bcl-2 and the progression of human and rodent prostatic cancers. Clin. Cancer Res. 2, 389–398 (1996).
Owen-Schaub, L. B., van Golen, K. L., Hill, L. L. & Price, J. E. Fas and Fas ligand interactions suppress melanoma lung metastasis. J. Exp. Med. 188, 1717–1723 (1998).
Thompson, T. C. et al. Loss of p53 function leads to metastasis in ras+myc-initiated mouse prostate cancer. Oncogene 10, 869–879 (1995).
Silvestrini, R. et al. Validation of p53 accumulation as a predictor of distant metastasis at 10 years of follow-up in 1400 node-negative breast cancers. Clin. Cancer Res. 2, 2007–2013 (1996).
Lewis, B. C. et al. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol. Cell. Biol. 25, 1228–1237 (2005).
Ko, J. et al. Transgenic mouse model for breast cancer: induction of breast cancer in novel oncogene HCCR-2 transgenic mice. Oncogene 23, 1950–1953 (2004).
Esteller, M. Relevance of DNA methylation in the management of cancer. Lancet Oncol. 4, 351–358 (2003).
Bao, S. et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5, 329–339 (2004).
Martin, S. S. & Vuori, K. Regulation of Bcl-2 proteins during anoikis and amorphosis. Biochim. Biophys. Acta 1692, 145–157 (2004). A detailed analysis of the molecular mechanisms of apoptosis induced during anoikis and amorphosis.
Streuli, C. H. & Gilmore, A. P. Adhesion-mediated signaling in the regulation of mammary epithelial cell survival. J. Mammary Gland. Biol. Neoplasia 4, 183–191 (1999).
Martin, S. S. & Leder, P. Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Mol. Cell. Biol. 21, 6529–6536 (2001).
Puthalakath, H., Huang, D. C., O'Reilly, L. A., King, S. M. & Strasser, A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 (1999).
Puthalakath, H. et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Zhu, Z. et al. Anoikis and metastatic potential of cloudman S91 melanoma cells. Cancer Res. 61, 1707–1716 (2001).
Owens, L. V. et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 55, 2752–2755 (1995).
McLean, G. W. et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998–3003 (2004).
Gentile, A. & Comoglio, P. M. Invasive growth: a genetic program. Int. J. Dev. Biol. 48, 451–456 (2004).
Camp, E. R. et al. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann. Surg. Oncol. 12, 273–281 (2005).
Peace, B. E., Toney-Earley, K., Collins, M. H. & Waltz, S. E. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 65, 1285–1293 (2005).
Zeng, Q. et al. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFκB. J. Biol. Chem. 277, 25203–25208 (2002).
Pinkas, J., Martin, S. S. & Leder, P. Bcl-2-mediated cell survival promotes metastasis of EpH4 βMEKDD mammary epithelial cells. Mol. Cancer Res. 2, 551–556 (2004).
Martin, S. S. et al. A cytoskeleton-based functional genetic screen identifies Bcl-xL as an enhancer of metastasis, but not primary tumor growth. Oncogene 23, 4641–4645 (2004). An elegant study identifying BCL-X L as a suppressor of cytoskeleton-dependent death and that overexpression in mouse mammary epithelial cells does not induce primary tumour formation but strongly increases metastatic potential.
Olopade, O. I. et al. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J. Sci. Am. 3, 230–237 (1997).
Fernandez, Y., Gu, B., Martinez, A., Torregrosa, A. & Sierra, A. Inhibition of apoptosis in human breast cancer cells: role in tumor progression to the metastatic state. Int. J. Cancer 101, 317–326 (2002).
Berezovskaya, O. et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 65, 2378–2386 (2005). Comprehensive work showing that the apoptosis-inhibitory protein XIAP contributes to the anoikis resistance of circulating metastatic prostate cancer cells.
Shen, T. L. et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 169, 941–952 (2005).
Wyckoff, J. B., Jones, J. G., Condeelis, J. S. & Segall, J. E. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 60, 2504–2511 (2000).
Fidler, I. J. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl Cancer Inst. 45, 773–782 (1970).
Wong, C. W. et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 61, 333–338 (2001).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998). References 43, 44 and 45 examine the fate of cancer cells in the blood stream and in the site of metastasis, aiming to identify mechanisms explaining the overall metastatic inefficiency.
Jakobisiak, M., Lasek, W. & Golab, J. Natural mechanisms protecting against cancer. Immunol. Lett. 90, 103–122 (2003).
Pardoll, D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21, 807–839 (2003).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Rosenberg, S. A. Progress in human tumour immunology and immunotherapy. Nature 411, 380–384 (2001).
Kim, S., Iizuka, K., Aguila, H. L., Weissman, I. L. & Yokoyama, W. M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl Acad. Sci. USA 97, 2731–2736 (2000).
Smyth, M. J. et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J. Exp. Med. 200, 1325–1335 (2004).
Takeda, K. et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-γ-dependent suppression of subcutaneous tumor growth. Cell Immunol. 214, 194–200 (2001).
Cretney, E. et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 168, 1356–1361 (2002).
Weiss, L., Elkin, G. & Barbera-Guillem, E. The differential resistance of B16 wild-type and F10 cells to mechanical trauma in vitro. Invasion Metastasis 13, 92–101 (1993).
Ziegler, T., Silacci, P., Harrison, V. J. & Hayoz, D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension 32, 351–355 (1998).
Wang, H. H. et al. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 60, 5862–5869 (2000).
Edmiston, K. H. et al. Role of nitric oxide and superoxide anion in elimination of low metastatic human colorectal carcinomas by unstimulated hepatic sinusoidal endothelial cells. Cancer Res. 58, 1524–1531 (1998).
Jaattela, M., Wissing, D., Kokholm, K., Kallunki, T. & Egeblad, M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17, 6124–6134 (1998).
Kluger, H. M. et al. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. 65, 5578–5587 (2005).
Schmitt, C. A. et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1, 289–298 (2002).
Nikiforov, M. A. et al. p53 modulation of anchorage independent growth and experimental metastasis. Oncogene 13, 1709–1719 (1996). Data showing that the inactivation of p53 in transformed mouse embryonic fibroblasts facilitates experimental metastasis by promoting the survival of tumour cells in circulation.
Schuler, M. & Green, D. R. Transcription, apoptosis and p53: catch-22. Trends Genet. 21, 182–187 (2005).
Ponta, H., Sherman, L. & Herrlich, P. A. CD44: from adhesion molecules to signalling regulators. Nature Rev. Mol. Cell Biol. 4, 33–45 (2003).
Yu, Q., Toole, B. P. & Stamenkovic, I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J. Exp. Med. 186, 1985–1996 (1997).
O'Reilly, M. S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
O'Reilly, M. S., Holmgren, L., Chen, C. & Folkman, J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med. 2, 689–692 (1996).
O'Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Giehl, K. Oncogenic Ras in tumour progression and metastasis. Biol. Chem. 386, 193–205 (2005).
Liao, Y. et al. Modulation of apoptosis, tumorigenesity and metastatic potential with antisense H-ras oligodeoxynucleotides in a high metastatic tumor model of hepatoma: LCI-D20. Hepatogastroenterology 47, 365–370 (2000).
Varghese, H. J. et al. Activated ras regulates the proliferation/apoptosis balance and early survival of developing micrometastases. Cancer Res. 62, 887–891 (2002). By using in vivo video microscopy and detailed cell quantification, this study provides evidence for a direct role for Ras in the maintenance of metastatic growth by mediating a shift in the proliferation/apoptosis balance.
Mehlen, P. The dependence receptor notion: another way to see death. Cell Death Differ. 12, 1003 (2005).
Mehlen, P. & Bredesen, D. E. The dependence receptor hypothesis. Apoptosis 9, 37–49 (2004).
Mehlen, P. & Thibert, C. Dependence receptors: between life and death. Cell Mol. Life Sci. 61, 1854–1866 (2004).
Bredesen, D. E., Mehlen, P. & Rabizadeh, S. Receptors that mediate cellular dependence. Cell Death Differ. 12, 1031–1043 (2005).
Wang, J. J. et al. Dimerization-dependent block of the proapoptotic effect of p75(NTR). J. Neurosci. Res. 60, 587–593 (2000).
Forcet, C. et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc. Natl Acad. Sci. USA 98, 3416–3421 (2001).
Stupack, D. G., Puente, X. S., Boutsaboualoy, S., Storgard, C. M. & Cheresh, D. A. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155, 459–470 (2001).
Mehlen, P. & Furne, C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol. Life Sci. 62, 2599–2616 (2005).
Fearon, E. R. et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247, 49–56 (1990).
Mehlen, P. & Fearon, E. R. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J. Clin. Oncol. 22, 3420–3428 (2004).
Thiebault, K. et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc. Natl Acad. Sci. USA 100, 4173–4178 (2003).
Mehlen, P. et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395, 801–804 (1998).
Llambi, F., Causeret, F., Bloch-Gallego, E. & Mehlen, P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20, 2715–2722 (2001).
Mazelin, L. et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature 431, 80–84 (2004). This paper suggests for the first time, using a mouse model, that dependence receptors might regulate tumorigenesis.
Llambi, F. et al. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. EMBO J. 24, 1192–1201 (2005).
Tanikawa, C., Matsuda, K., Fukuda, S., Nakamura, Y. & Arakawa, H. p53RDL1 regulates p53-dependent apoptosis. Nature Cell Biol. 5, 216–223 (2003).
Stupack, D. G. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 12, 1021–1030 (2005).
Stupack, D. G. et al. Potentiation of neuroblastoma metastasis by loss of caspase 8. Nature 439 95–99 (2006). This paper represents the first demonstration of a role for dependence receptors in metastasis regulation.
Hofmann, U. B., Houben, R., Brocker, E. B. & Becker, J. C. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie 87, 307–314 (2005).
Abraham, R. et al. Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J. Biol. Chem. 280, 34123–34132 (2005).
Strand, S. et al. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 23, 3732–3736 (2004).
Huber, M. A. et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Chen, Z. F. & Behringer, R. R. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9, 686–699 (1995).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Maestro, R. et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 13, 2207–2217 (1999).
Valsesia-Wittmann, S. et al. Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell 6, 625–630 (2004). References 95, 96 and 97 show the oncogenic properties of H-Twist, highlighting its role as an anti-apoptotic factor (references 96 and 97), and establishing its implication in the metastatic process (reference 95).
Puisieux, A., Valsesia-Wittmann, S. & Ansieau, S. A twist for survival and cancer progression. Br. J. Cancer 94, 13–17 (2006).
Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R. & Leder, P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54, 105–115 (1988).
Greenberg, N. M., et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA 92, 3439–3443 (1995)
Sierra, A. Metastases and their microenvironments: linking pathogenesis and therapy. Drug Resist. Updat. 8, 247–257 (2005).
Fesik, S. W. Promoting apoptosis as a strategy for cancer drug discovery. Nature Rev. Cancer 5, 876–885 (2005).
Acknowledgements
We thank H. Bilak for text correction, C. Caux for critical reading of the manuscript and B. Bouchet for the development of figures. Work in Mehlen's and Puisieux's laboratories are supported by institutional funds from the Centre National de la Recherche Scientifique (P.M.), Agence Nationale de la Recherche (ANR) (P.M.), Institut national de la santé et de la recherche médicale (A.P.), and Institut National du Cancer (INCA) (P.M. and A.P.), and by grants from the Ligue Nationale Contre le Cancer (P.M. and A.P.), the Association pour la Recherche contre le Cancer (P.M. and A.P.), the Comité Départemental de l'Ain (A.P.) and the National Institute of Health (P.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Natural killer cells
-
Large granular lymphocytes that do not bear a T-cell receptor, but can recognize and destroy certain tumour cells and virally infected cells in a manner that is independent of the major histocompatibility complex.
- Perforin
-
A protein that can be released by immune cells and can form pores in the plasma membrane. These pores enable the entrance of serine proteases, such as granzyme B, which initiate caspase cleavage and activation.
- Pulsatile and cyclic circumferential stretch
-
Vascular cells are normally exposed to oscillatory distending and shearing forces owing to the pulsatility of circulating blood. This pulsatility might have an important role in regulating vessel tone and remodelling. Both shear and large-amplitude cyclic stretch have been shown to individually stimulate nitric oxide synthase.
Rights and permissions
About this article
Cite this article
Mehlen, P., Puisieux, A. Metastasis: a question of life or death. Nat Rev Cancer 6, 449–458 (2006). https://doi.org/10.1038/nrc1886
Issue Date:
DOI: https://doi.org/10.1038/nrc1886
This article is cited by
-
Farnesoid X receptor promotes non-small cell lung cancer metastasis by activating Jak2/STAT3 signaling via transactivation of IL-6ST and IL-6 genes
Cell Death & Disease (2024)
-
Aspirin and cancer treatment: systematic reviews and meta-analyses of evidence: for and against
British Journal of Cancer (2024)
-
HELLS Knockdown Inhibits the Malignant Progression of Lung Adenocarcinoma Via Blocking Akt/CREB Pathway by Downregulating KIF11
Molecular Biotechnology (2024)
-
Synergistic inter-clonal cooperation involving crosstalk, co-option and co-dependency can enhance the invasiveness of genetically distant cancer clones
BMC Ecology and Evolution (2023)
-
Correlation analysis of lung mucosa-colonizing bacteria with clinical features reveals metastasis-associated bacterial community structure in non-small cell lung cancer patients
Respiratory Research (2023)