Abstract
Neutrophils are key innate immune effector cells that are essential to fighting bacterial and fungal pathogens. Here we report that mice carrying a hematopoietic lineage–specific deletion of Jagn1 (encoding Jagunal homolog 1) cannot mount an efficient neutrophil-dependent immune response to the human fungal pathogen Candida albicans. Global glycobiome analysis identified marked alterations in the glycosylation of proteins involved in cell adhesion and cytotoxicity in Jagn1-deficient neutrophils. Functional analysis confirmed marked defects in neutrophil migration in response to Candida albicans infection and impaired formation of cytotoxic granules, as well as defective myeloperoxidase release and killing of Candida albicans. Treatment with granulocyte/macrophage colony-stimulating factor (GM-CSF) protected mutant mice from increased weight loss and accelerated mortality after Candida albicans challenge. Notably, GM-CSF also restored the defective fungicidal activity of bone marrow cells from humans with JAGN1 mutations. These data directly identify Jagn1 (JAGN1 in humans) as a new regulator of neutrophil function in microbial pathogenesis and uncover a potential treatment option for humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Borregaard, N. Neutrophils, from marrow to microbes. Immunity 33, 657–670 (2010).
Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210, 1283–1299 (2013).
Klein, C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu. Rev. Immunol. 29, 399–413 (2011).
Boztug, K. et al. JAGN1 deficiency causes aberrant myeloid cell homeostasis and congenital neutropenia. Nat. Genet. 10.1038/ng.3069 (17 August 2014).
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003).
Fulurija, A., Ashman, R.B. & Papadimitriou, J.M. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 142, 3487–3496 (1996).
Brown, G.D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Lionakis, M.S., Lim, J.K., Lee, C.C. & Murphy, P.M. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun. 3, 180–199 (2011).
Lionakis, M.S. & Netea, M.G. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog. 9, e1003079 (2013).
Vonk, A.G., Netea, M.G. & Kullberg, B.J. Phagocytosis and intracellular killing of Candida albicans by murine polymorphonuclear neutrophils. Methods Mol. Biol. 845, 277–287 (2012).
Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9, 737–748 (2011).
Sachs, U.J. et al. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J. Biol. Chem. 282, 23603–23612 (2007).
Wang, L. et al. Surface receptor CD177/NB1 does not confer a recruitment advantage to neutrophilic granulocytes during human peritonitis. Eur. J. Haematol. 90, 436–437 (2013).
Hentzen, E.R. et al. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood 95, 911–920 (2000).
Borjesson, D.L. et al. Roles of neutrophil β2 integrins in kinetics of bacteremia, extravasation, and tick acquisition of Anaplasma phagocytophila in mice. Blood 101, 3257–3264 (2003).
Anderson, D.C. & Springer, T.A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38, 175–194 (1987).
Borregaard, N. & Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521 (1997).
Borregaard, N., Sorensen, O.E. & Theilgaard-Monch, K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28, 340–345 (2007).
Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223 (2005).
Aratani, Y. et al. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun. 67, 1828–1836 (1999).
Aratani, Y. et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J. Infect. Dis. 185, 1833–1837 (2002).
Homme, M., Tateno, N., Miura, N., Ohno, N. & Aratani, Y. Myeloperoxidase deficiency in mice exacerbates lung inflammation induced by nonviable Candida albicans. Inflamm. Res. 62, 981–990 (2013).
Welte, K., Zeidler, C. & Dale, D.C. Severe congenital neutropenia. Semin. Hematol. 43, 189–195 (2006).
Kullberg, B.J., Oude Lashof, A.M. & Netea, M.G. Design of efficacy trials of cytokines in combination with antifungal drugs. Clin. Infect. Dis. 39 (suppl. 4), S218–S223 (2004).
Vonk, A.G. et al. Treatment of intra-abdominal abscesses caused by Candida albicans with antifungal agents and recombinant murine granulocyte colony-stimulating factor. Antimicrob. Agents Chemother. 47, 3688–3693 (2003).
Gu, J. et al. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 29, 599–607 (2012).
Janik, M.E., Litynska, A. & Vereecken, P. Cell migration—the role of integrin glycosylation. Biochim. Biophys. Acta 1800, 545–555 (2010).
Gu, J. et al. A mutual regulation between cell-cell adhesion and N-glycosylation: implication of the bisecting GlcNAc for biological functions. J. Proteome Res. 8, 431–435 (2009).
Barboza, M. et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria–host interactions. Mol. Cell Proteomics 11, M111.015248 (2012).
van Veen, H.A., Geerts, M.E., van Berkel, P.H. & Nuijens, J.H. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur. J. Biochem. 271, 678–684 (2004).
Van Antwerpen, P. et al. Glycosylation pattern of mature dimeric leukocyte and recombinant monomeric myeloperoxidase: glycosylation is required for optimal enzymatic activity. J. Biol. Chem. 285, 16351–16359 (2010).
Antonopoulos, A., North, S.J., Haslam, S.M. & Dell, A. Glycosylation of mouse and human immune cells: insights emerging from N-glycomics analyses. Biochem. Soc. Trans. 39, 1334–1340 (2011).
Vizcaíno, J.A. et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 (2013).
Boxio, R., Bossenmeyer-Pourie, C., Steinckwich, N., Dournon, C. & Nusse, O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75, 604–611 (2004).
Bourgeois, C., Majer, O., Frohner, I. & Kuchler, K. In vitro systems for studying the interaction of fungal pathogens with primary cells from the mammalian innate immune system. Methods Mol. Biol. 470, 125–139 (2009).
Frohner, I.E., Bourgeois, C., Yatsyk, K., Majer, O. & Kuchler, K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 71, 240–252 (2009).
Nielsen, M.L., Savitski, M.M. & Zubarev, R.A. Improving protein identification using complementary fragmentation techniques in Fourier transform mass spectrometry. Mol. Cell. Proteomics 4, 835–845 (2005).
Acknowledgements
We thank all members of the Penninger laboratory for helpful discussions and technical support. We thank all members of the IMP-IMBA Biooptics service facility for assistance in cell sorting and image quantification. K.M. is supported by the European Community's Seventh Framework Programme PRIME-XS (262067) and MeioSys (222883-2) projects and by the Austrian Science Fund (SFB F3402, P24685-B24 and TRP 308-N15). K.K. was supported by a grant from the Medical University of Vienna and in part by a grant from the Austrian Science Fund (FWF) (FWF-P-25333-B08). C.K. is supported by the European Research Council, an EU ERARE program (NEUTRO-NET), the German Research Foundation (DFG) Gottfried-Wilhelm-Leibniz program, DFG-SFB914 (Migration of Immune Cells) and the DZIF (German Center for Infection Research). J.M.P. is supported by grants from IMBA, the Austrian National Foundation, the Austrian Academy of Sciences, NEUTRO-NET and a European Union European Research Council Advanced Grant.
Author information
Authors and Affiliations
Contributions
Immune phenotyping and flow cytometry analyses were performed by G.W. and L.T. Neutrophil characterization by flow cytometry, including cytokine signaling analysis, was performed by G.W. U.E. created Jagn1 conditional knockout mice and the antibody specifically recognizing Jagn1. Jagn1 full-body knockout mice were generated by K.B. F.Z. and K.K. carried out the infection experiments with Candida albicans. J.S., G.D. and K.M. performed the mass spectrometry analysis of the neutrophil glycoproteome. S.W.L. helped with immunophenotyping. I.K. performed protein blot analysis and chemotaxis assays. T.P. and L.T. performed immunoglobulin isotyping. R.S. performed quantitative PCR analysis. A.D.M. analyzed histology and cytology and the electron microscopy data. G.W., C.K. and J.M.P. designed the experiments, and G.W. and J.M.P. wrote the manuscript. P.J. helped in designing experiments with human patient cells.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Generation of Jagn1fl/fl mice and immune phenotyping.
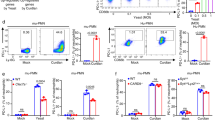
(a) Schematic depiction of the wild-type (wt), floxed (fl) and deleted (Δ) Jagn1 locus. loxP sites were introduced up- and downstream of exon 2. Exons, the transcriptional start site (ATG), and the positions and orientations of the primers used to assess deletion of exon 2 of Jagn1 are indicated. (b) Splenic genomic DNA was isolated from mice of the indicated genotypes and analyzed by PCR to test for deletion of exon 2. The positions and amplicon sizes of the respective alleles are indicated. (c) Loss of Jagn1 protein as assessed by protein blot for both the spleens and mesenteric lymph nodes (mLNs) of mice of the indicated genotypes. Gapdh is shown as a loading control. (d) Expression and deletion of Jagn1 in peripheral blood neutrophils as assessed by intracellular staining and flow cytometry. The histogram depicts the overlay of cells of the indicated genotypes. Unstained cells are shown as a background control. (e) Expression and deletion efficiency of Jagn1 in the indicated splenic hematopoietic lineages as assessed by intracellular staining. The histograms depict the overlays of Jagn1fl/fl and Jagn1Δhem cells for the indicated cell types. (f) Cellularity of spleen, thymus, bone marrow (BM) and mesenteric lymph nodes (mLNs) of Jagn1fl/fl and Jagn1Δhem mice. n = 4 mice for each genotype; mean values ± s.d. are shown. (g–i) Cellular composition of the hematopoietic cells isolated from the (g) spleen, (h) thymus and (i) bone marrow as assessed by flow cytometry. Plots depict the percentage of live cells of the indicated lineages in the respective organs. (j) White blood cell (WBC) and red blood cell (RBC) counts (left) and the cellular composition of WBCs (right) of Jagn1fl/fl and Jagn1Δhem mice. For g–j, mean values ± s.d. are shown. Each data point represents an individual mouse analyzed.
Supplementary Figure 2 Characterization of effector lymphocyte lineages and bone marrow chimeric mice.
(a) Proportions of naive CD62LhighCD44– and memory CD62L–CD44high T cells among total splenic CD4+ or CD8+ T cells as determined by immunostaining and FACS analysis. (b) Proportion of FoxP3+CD4+ regulatory T cells among total CD4+ cells in the indicated organs. (c) Proportion of cytokine-producing lymphocytes of the spleen as assessed by phorbol myristate acetate (PMA)/ionomycin stimulation (100 ng/ml and 1 μg/ml, respectively) and subsequent intracellular staining for the cytokines IL-17A and Ifnγ. Plotted is the proportion of cytokine-producing cells among total splenocytes, or TCRβ+CD4+ T cells, TCRβ+CD8+ T cells and TCRgd+ T cell subpopulations. In a–c, each data point represents an individual mouse analyzed. (d,e) Proportion of class-switched B cells in the mLNs (d) and Peyer’s patches (e) of Jagn1fl/fl and Jagn1Δhem mice. n = 3 mice per genotype. (f) Immunoglobulin concentrations in sera from Jagn1fl/fl and Jagn1Δhem mice as detected by an isotype-multiplexing assay. n = 4 mice per genotype. (g) White blood cell composition of mixed–bone marrow chimeric mice generated by reconstitution of irradiated Rag1-deficient mice with one-third Jagn1Δhem (CD45.2) bone marrow plus two-thirds CD45.1+ C57BL/6 bone marrow or one-third Jagn1fl/fl (CD45.2) bone marrow plus two-thirds C57BL/6 CD45.1 bone marrow as a control group. The contribution of CD45.2+ donor cells to the respective lineage was assessed 10 weeks after reconstitution. n = 7–8 mice per group. (h) Contribution of Jagn1fl/fl or Jagn1Δhem cells (CD45.2) to the indicated B cell populations normalized to their contribution to the total B cell pool. (i) Contribution of Jagn1fl/fl or Jagn1Δhem cells (CD45.2) to the indicated effector T cell population normalized to their contribution to the total T cell pool. For a–I, mean values ± s.d. are shown.
Supplementary Figure 3 Histological analysis of organs from Candida albicans–infected mice.
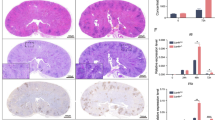
Representative histology of the (a) kidney (arrowheads indicate inflammatory infiltrates), (b) lung, (c) liver and (d) spleen of Jagn1fl/fl and Jagn1Δhem littermate mice infected intravenously with Candida albicans (1 × 105 CFU/21.5 g body weight). Mice were sacrificed on day 3 after infection, and sections were stained with hematoxylin and eosin. Magnification: kidney and lung: 50×, insets 400×; liver and spleen: 100×, insets 400×
Supplementary Figure 4 Comparative glycoproteomic profiles of bone marrow neutrophils and peritoneal neutrophils recruited after Candida albicans challenge.
Comparative glycoproteomic profiles of neutrophils isolated from (a) the bone marrow of Jagn1Δhem and Jagn1fl/fl mice or (b) the peritoneum of Jagn1Δhem and Jagn1fl/fl mice 24 h after intraperitoneal Candida albicans challenge. The plot depicts the median ratios for all glycopeptides found to bear the indicated N-glycan structures in Jagn1Δhem versus Jagn1fl/fl neutrophils. Ratios below 1 indicate downregulation in knockout neutrophils, and ratios above 1 indicate upregulation in Jagn1Δhem knockout cells. Sugar structures shown in Figure 2a are highlighted in bold. In all panels, data are shown as box-and-whisker plots. Horizontal bars indicate the median; boxes span the respective interquantile range; whiskers extend to 1.5 times the interquantile range; and outliers are indicated by circles. n = 3 samples isolated from 3 different mice for each genotype.
Supplementary Figure 5 Accumulation of Ly6G+ neutrophils and F4/80+ macrophages in Candida albicans–infected tissues.
Representative immunohistochemistry to detect Ly6G+ neutrophils in the (a) kidney, (b) lung, (c) liver and (d) spleen or F4/80+ macrophages in the (e) kidney, (f) lung and (g) spleen of Jagn1fl/fl and Jagn1Δhem littermate mice infected intravenously with Candida albicans (1 × 105 CFU/21.5 g body weight). Mice were sacrificed at day 3 after infection, and sections were immunostained with antibody to Ly6G or F4/80. Magnification: lung: 50×, insets 400×; liver: 100×, insets 400×; spleen: 200×, insets 400×.
Supplementary Figure 6 Granule proteins and signaling of G-CSF and GM-CSF receptors in Jagn1-mutant neutrophils and flow cytometry analysis of JAGN1-mutant human bone marrow.
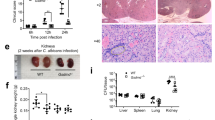
(a) MPO levels in blood neutrophils were assessed by staining with an MPO-specific antibody. Representative staining for Jagn1fl/fl and Jagn1Δhem blood neutrophils is shown. (b) Expression of secondary granule proteins Mmp8 and Mmp9 as assessed by intracellular staining and flow cytometry. Left, MFI ratios of either Jagn1fl/fl/C57BL/6 or Jagn1Δhem/C57BL/6 blood neutrophils as distinguished by staining for CD45.2 and CD45.1 in bone marrow chimeric mice. n = 4 for each genotype. Right, histogram overlays from representative MMP8 and MMP9 staining of the indicated populations of blood neutrophils of bone marrow chimeric mice. (c) MPO release from purified bone marrow neutrophils co-cultured with Candida albicans for 24 h as measured by ELISA in triplicate. (d) Jagn1fl/fl and Jagn1Δhem blood neutrophils were stimulated with G-CSF for 20 min and assayed for phosphorylation of STAT3 (pSTAT3) by intracellular staining. Representative histogram overlays are shown for neutrophils stimulated with the indicated concentrations of recombinant mouse G-CSF or left unstimulated. (e) Cell surface (left) and intracellular (right) expression of G-CSF-Rα in neutrophils isolated from the peripheral blood of Jagn1fl/fl and Jagn1Δhem mice. Plots depict average mean fluorescence intensity (MFI) ± s.d. Each data point represents an individual mouse. (f) Levels of phosphorylated STAT3 (pSTAT3) in peripheral blood neutrophils from Jagn1fl/fl and Jagn1Δhem mice infected intraperitoneally with Candida albicans 24 h before analysis. Representative histogram overlays are shown for mice infected with Candida albicans or left uninfected. Of note, baseline STAT3 phosphorylation was comparable between Jagn1fl/fl and Jagn1Δhem neutrophils in uninfected mice. (g) Peripheral blood neutrophils were stimulated with the indicated concentrations of recombinant GM-CSF for 20 min and then assayed for phosphorylation of STAT5 (pSTAT5) by intracellular staining. Histogram overlays are shown for cells stimulated with the indicated concentrations of recombinant mouse GM-CSF or left unstimulated. (h) Cell surface expression of GM-CSF-Rα on neutrophils isolated from the peripheral blood of Jagn1fl/fl and Jagn1Δhem mice. The plot depicts average mean fluorescence intensity (MFI) ± s.d. Each data point represents an individual mouse. **P < 0.01, ***P < 0.001, Student’s test. (i) CD34– bone marrow cells from the indicated JAGN1-mutated patients and healthy control subjects were analyzed by flow cytometry. Granulocytes were identified on the basis of forward and side scatter characteristics. (j) CD34– bone marrow cells were stained with the granulocyte marker CD66b. Histograms depict CD66b expression on granulocytes as gated in i.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 2. (PDF 2579 kb)
Supplementary Table 1
Complete list of peptides and glycopeptides identified by mass spectrometry for both bone marrow and peritoneal neutrophils (XLSX 4524 kb)
Rights and permissions
About this article
Cite this article
Wirnsberger, G., Zwolanek, F., Stadlmann, J. et al. Jagunal homolog 1 is a critical regulator of neutrophil function in fungal host defense. Nat Genet 46, 1028–1033 (2014). https://doi.org/10.1038/ng.3070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3070
This article is cited by
-
JAGN1 mutation with distinct clinical features; two case reports and literature review
BMC Pediatrics (2023)
-
The δ subunit of F1Fo-ATP synthase is required for pathogenicity of Candida albicans
Nature Communications (2021)
-
The Fungal Histone Acetyl Transferase Gcn5 Controls Virulence of the Human Pathogen Candida albicans through Multiple Pathways
Scientific Reports (2019)
-
Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor
Nature Communications (2019)
-
The Role of Neutrophils in Host Defense Against Invasive Fungal Infections
Current Clinical Microbiology Reports (2018)