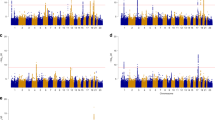
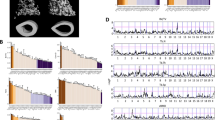
Abstract
Bone mineral density (BMD) is the most widely used predictor of fracture risk. We performed the largest meta-analysis to date on lumbar spine and femoral neck BMD, including 17 genome-wide association studies and 32,961 individuals of European and east Asian ancestry. We tested the top BMD-associated markers for replication in 50,933 independent subjects and for association with risk of low-trauma fracture in 31,016 individuals with a history of fracture (cases) and 102,444 controls. We identified 56 loci (32 new) associated with BMD at genome-wide significance (P < 5 × 10−8). Several of these factors cluster within the RANK-RANKL-OPG, mesenchymal stem cell differentiation, endochondral ossification and Wnt signaling pathways. However, we also discovered loci that were localized to genes not known to have a role in bone biology. Fourteen BMD-associated loci were also associated with fracture risk (P < 5 × 10−4, Bonferroni corrected), of which six reached P < 5 × 10−8, including at 18p11.21 (FAM210A), 7q21.3 (SLC25A13), 11q13.2 (LRP5), 4q22.1 (MEPE), 2p16.2 (SPTBN1) and 10q21.1 (DKK1). These findings shed light on the genetic architecture and pathophysiological mechanisms underlying BMD variation and fracture susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 22, 465–475 (2007).
Johnell, O. et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 20, 1185–1194 (2005).
Kanis, J.A. et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 18, 1033–1046 (2007).
Peacock, M., Turner, C.H., Econs, M.J. & Foroud, T. Genetics of osteoporosis. Endocr. Rev. 23, 303–326 (2002).
Ralston, S.H. & Uitterlinden, A.G. Genetics of osteoporosis. Endocr. Rev. 31, 629–662 (2010).
Hardy, J. & Singleton, A. Genomewide association studies and human disease. N. Engl. J. Med. 360, 1759–1768 (2009).
Manolio, T.A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 363, 166–176 (2010).
Richards, J.B. et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371, 1505–1512 (2008).
Styrkarsdottir, U. et al. Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 358, 2355–2365 (2008).
Rivadeneira, F. et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 41, 1199–1206 (2009).
Styrkarsdottir, U. et al. New sequence variants associated with bone mineral density. Nat. Genet. 41, 15–17 (2009).
Hsu, Y.H. et al. An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility loci for osteoporosis-related traits. PLoS Genet. 6, e1000977 (2010).
Kung, A.W. et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am. J. Hum. Genet. 86, 229–239 (2010).
Duncan, E.L. et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 7, e1001372 (2011).
Richards, J.B. et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann. Intern. Med. 151, 528–537 (2009).
van Meurs, J.B. et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. J. Am. Med. Assoc. 299, 1277–1290 (2008).
Bagger, Y.Z. et al. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos. Int. 18, 505–512 (2007).
Kiel, D.P. et al. Genetic variation at the low-density lipoprotein receptor–related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone 40, 587–596 (2007).
Raychaudhuri, S. et al. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 5, e1000534 (2009).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Brunkow, M.E. et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot–containing protein. Am. J. Hum. Genet. 68, 577–589 (2001).
Guo, Y.F. et al. Polymorphisms of the low-density lipoprotein receptor–related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J. Med. Genet. 43, 798–803 (2006).
Kornak, U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001).
Panagiotou, O.A., Evangelou, E. & Ioannidis, J.P. Genome-wide significant associations for variants with minor allele frequency of 5% or less—an overview: a HuGE review. Am. J. Epidemiol. 172, 869–889 (2010).
Jin, W. et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J. Clin. Invest. 118, 1858–1866 (2008).
Sundaram, K., Shanmugarajan, S., Rao, D.S. & Reddy, S.V. Mutant p62P392L stimulation of osteoclast differentiation in Paget's disease of bone. Endocrinology 152, 4180–4189 (2011).
Grundberg, E. et al. Population genomics in a disease targeted primary cell model. Genome Res. 19, 1942–1952 (2009).
Duan, Y., Beck, T.J., Wang, X.F. & Seeman, E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J. Bone Miner. Res. 18, 1766–1774 (2003).
Karasik, D. & Ferrari, S.L. Contribution of gender-specific genetic factors to osteoporosis risk. Ann. Hum. Genet. 72, 696–714 (2008).
Ohlsson, C. et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 7, e1002313 (2011).
Styrkarsdottir, U. et al. European bone mineral density loci are also associated with BMD in East-Asian populations. PLoS ONE 5, e13217 (2010).
Wood, A.R. et al. Allelic heterogeneity and more detailed analyses of known loci explain additional phenotypic variation and reveal complex patterns of association. Hum. Mol. Genet. 20, 4082–4092 (2011).
Mosekilde, L., Torring, O. & Rejnmark, L. Emerging anabolic treatments in osteoporosis. Curr. Drug Saf. 6, 62–74 (2011).
Hasselblad, V. & Hedges, L.V. Meta-analysis of screening and diagnostic tests. Psychol. Bull. 117, 167–178 (1995).
Harris, S.T. et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. J. Am. Med. Assoc. 282, 1344–1352 (1999).
Jackson, R.D. et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 354, 669–683 (2006).
Frazer, K.A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Servin, B. & Stephens, M. Imputation-based analysis of association studies: candidate regions and quantitative traits. PLoS Genet. 3, e114 (2007).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Li, Y., Willer, C., Sanna, S. & Abecasis, G. Genotype imputation. Annu. Rev. Genomics Hum. Genet. 10, 387–406 (2009).
Estrada, K. et al. GRIMP: a web- and grid-based tool for high-speed analysis of large-scale genome-wide association using imputed data. Bioinformatics 25, 2750–2752 (2009).
Abecasis, G.R., Cherny, S.S., Cookson, W.O. & Cardon, L.R. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 30, 97–101 (2002).
Aulchenko, Y.S., Struchalin, M.V. & van Duijn, C.M. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics 11, 134 (2010).
Devlin, B., Roeder, K. & Wasserman, L. Genomic control, a new approach to genetic-based association studies. Theor. Popul. Biol. 60, 155–166 (2001).
Ioannidis, J.P., Thomas, G. & Daly, M.J. Validating, augmenting and refining genome-wide association signals. Nat. Rev. Genet. 10, 318–329 (2009).
Pereira, T.V., Patsopoulos, N.A., Salanti, G. & Ioannidis, J.P. Discovery properties of genome-wide association signals from cumulatively combined data sets. Am. J. Epidemiol. 170, 1197–1206 (2009).
Yang, J. et al. Genomic inflation factors under polygenic inheritance. Eur. J. Hum. Genet. 19, 807–812 (2011).
Pe'er, I., Yelensky, R., Altshuler, D. & Daly, M.J. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet. Epidemiol. 32, 381–385 (2008).
Reppe, S. et al. Eight genes are highly associated with BMD variation in postmenopausal Caucasian women. Bone 46, 604–612 (2010).
Ge, B. et al. Global patterns of cis variation in human cells revealed by high-density allelic expression analysis. Nat. Genet. 41, 1216–1222 (2009).
Montgomery, S.B. et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464, 773–777 (2010).
Stranger, B.E. et al. Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 (2007).
Grundberg, E. et al. Global analysis of the impact of environmental perturbation on cis-regulation of gene expression. PLoS Genet. 7, e1001279 (2011).
Emilsson, V. et al. Genetics of gene expression and its effect on disease. Nature 452, 423–428 (2008).
Zeller, T. et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5, e10693 (2010).
Acknowledgements
We thank all study participants for making this work possible. This research and the Genetic Factors for Osteoporosis (GEFOS) consortium have been funded by the European Commission (HEALTH-F2-2008-201865-GEFOS). We acknowledge funding from the following organizations: the US National Institutes of Health (NIH; R01 AG18728, R01 HL088119, R01AR046838, U01 HL084756, P30 DK072488, T32 AG000262, F32 AR059469, P01 AG-18397, R01 AG041517, M01 RR-00750 and N01-AG-12100), the NIA Intramural Research Program (AG-023629, AG-15928, AG-20098 and AG-027058), Hjartavernd (the Icelandic Heart Association), the Althingi (the Icelandic Parliament), the Australian National Health and Medical Research Council (511132), the Australian Cancer Research Foundation and the Rebecca Cooper Foundation, the Australian National Health and Medical Research Council Career Development Award (569807 to E.L.D.), an MRC New Investigator Award (MRC G0800582 to D.M.E.), the Health Research Council of New Zealand, Sanofi-Aventis, Eli Lilly, Pfizer, Proctor & Gamble Pharmaceuticals, Roche, the Medical Benefits Fund (MBF) Living Well Foundation, the Ernst Heine Family Foundation, Arthritis Research UK (17539 and 15389), The Victorian Health Promotion Foundation, Geelong Region Medical Research Foundation, Australia (628582), Action Research UK, the European Commission (QLRT-2001-02629), the UK Food Standards Agency, BioPersMed (COMET K-project 825329), the Austrian Federal Ministry of Transport, Innovation and Technology (BMVIT), the Austrian Federal Ministry of Economics and Labour (BMWA), the Austrian Federal Ministry of Economy, Family and Youth (BMWFJ), the Styrian Business Promotion Agency (SFG), the Red de Envejecimiento y Fragilidad (RETICEF), Instituto Carlos III, the Spanish Ministry of Education and Science (SAF2010-15707), the Government of Catalonia (2009SGR971 and 2009SGR818), Instituto de Salud Carlos III–Fondo de Investigaciones Sanitarias (PI 06/0034 and PI08/0183), Healthway Health Promotion Foundation of Western Australia, Australasian Menopause Society and the Australian National Health and MRC Project (254627, 303169 and 572604), the Finnish Ministry of Education, Merck Frosst Canada, Eli Lilly Canada, Novartis Pharmaceuticals, Procter & Gamble Pharmaceuticals Canada, Servier Canada, Amgen Canada, The Dairy Farmers of Canada, The Arthritis Society, the US National Heart, Lung, and Blood Institute (NHLBI; N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, HL080295, HL075366, HL087652, HL105756 NINDS, HL 043851 and HL69757, CA 047988, and the Framingham Heart Study (N01-HC-25195) and its contract with Affymetrix, Inc, for genotyping services (N02-HL-6-4278)). Untied Educational Grants were provided by Amgen, Eli Lilly International, GE-Lunar, Merck Australia, Sanofi-Aventis Australia and Servier. Additional support was provided by the US National Center for Research Resources (M01-RR00425 to the Cedars-Sinai General Clinical Research Center Genotyping Core), the US National Institute of Diabetes and Digestive and Kidney Diseases (DK063491 to the Southern California Diabetes Endocrinology Research Center), deCODE Genetics, The UK National Institute for Medical Research (NIMR) Biomedical Research Centre, the Cancer Research Campaign, the Stroke Association, the British Heart Foundation, the UK Department of Health, the Europe Against Cancer Programme Commission of the European Union, the Ministry of Agriculture, Fisheries and Food, EU Biomed 1 (BMHICT920182, CIPDCT925012, ERBC1PDCT 940229 and ERBC1PDCT930105), the UK MRC (G9321536 and G9800062), the Wellcome Trust Collaborative Research Initiative 1995, MAFF AN0523, EU Framework Programme 5 (FP5; QLK6-CT-2002-02629), the Food Standards Agency (N05046), the Netherlands Organization for Scientific Research (NWO), Erasmus University Medical Center, the Centre for Medical Systems Biology (CMSB1 and CMSB2) of the Netherlands Genomics Initiative (NGI), the F.I.R.M.O. Fondazione Raffaella Becagli, the National Institute for Arthritis, Musculoskeletal and Skin Diseases, the National Institute on Aging (R01 AR/AG 41398, N01AG62101, N01AG62103, N01AG62106, 1R01AG032098 and R01 AR 050066), the Canadian Institutes for Health Research (86748), Federal Program of the Ministry of Education and Science of the Russian Federation Scientific and Pedagogical Staff of Innovative Russia in 2009–2013 (P-601), the Federal Program Research and Development of Prior Directions of Scientific-Technological Complex of Russia in 2007–2012 (16.512.11.2032), the Swedish Research Council (K2010-54X-09894-19-3, 2006-3832, K2010-52X-20229-05-3 and K20006-72X-20155013) the Swedish Foundation for Strategic Research, the ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, the Västra Götaland Foundation, the Göteborg Medical Society, the Novo Nordisk foundation, University of Athens, Greece (Kapodistrias 2009), the UK NIHR Musculoskeletal BRU Oxford, the UK NIHR Nutrition BRU Southampton, The Center for Inherited Disease Research (CIDR), National Institutes of Health (HHSN268200782096C), the Hong Kong Research Grant Council (HKU 768610M), The Bone Health Fund of the HKU Foundation, The KC Wong Education Foundation, Small Project Funding (201007176237), Matching Grant, Committee of Research and Conference Grants (CRCG) Grant, the Osteoporosis and Endocrine Research Fund, the Genomics Strategic Research Theme of The University of Hong Kong, Chinese University of Hong Kong, the Korea Health 21 Research & Development Project, the Korean Ministry of Health & Welfare, Republic of Korea (A010252), the Korea Healthcare Technology Research & Development Project, the Ministry for Health, Welfare and Family Affairs (A110536), The Netherlands Ministry of Health, Welfare and Sports Directorate of Long-Term Care, the World Anti-Doping Agency, the Danish Ministry of Culture, the Institute of Clinical Research of the University of Southern Denmark, the Chief Scientists Office of the Scottish Government (CZB/4/276), a Royal Society University Research Fellowship (to J.F.W.), the European Union Framework Program 6 EUROSPAN project (LSHG-CT-2006-018947), the European Union's Seventh Framework Programme (FP7/2007-2013; HEALTH-F2-2009-223004 PHASE), the Netherlands Organization of Scientific Research NWO Investments (175.010.2005.011 and 911-03-012), the Research Institute for Diseases in the Elderly (RIDE2; 014-93-015), the Netherlands Genomics Initiative/Netherlands Consortium for Healthy Aging (050-060-810), the German Bundesministerium fuer Forschung und Technology (01 AK 803 A-H and 01 IG 07015 G), the NIHR Biomedical Research Centre (grant to Guys' and St. Thomas' Hospitals and King's College London), the Chronic Disease Research Foundation, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the Fonds de la Recherche en Santé Québec, The Lady Davis Institute, the Jewish General Hospital, the Ministère du Développement Economique, de l'Innovation et de l'Exportation du Quebec, the Swedish Sports Research Council (87/06), the Swedish Society of Medicine, the Kempe Foundation (JCK-1021), the Medical Faculty of Umeå University (ALFVLL:968:22-2005, ALFVL:-937-2006, ALFVLL:223:11-2007 and ALFVLL:78151-2009), the County Council of Västerbotten (Spjutspetsanslag; VLL:159:33-2007), the US National Cancer Institute, the Donald W. Reynolds Foundation, the Fondation Leducq, the Academy of Finland (126925, 121584, 124282, 129378 (Salve), 117787 (Gendi) and 41071 (Skidi)), the Social Insurance Institution of Finland, Kuopio, Tampere and Turku University Hospital Medical Funds (9M048 for TeLeht), the Juho Vainio Foundation, the Paavo Nurmi Foundation, the Finnish Foundation of Cardiovascular Research, the Finnish Cultural Foundation, the Tampere Tuberculosis Foundation and the Emil Aaltonen Foundation (K08AR055688 to T.L.). A detailed list of acknowledgments by study is given in the Supplementary Note. The members of the GEFOS Consortium mourn the passing of co-author Philip Neil Sambrook, a good friend, respected colleague and outstanding research scientist in the prevention, treatment, epidemiology and genetics of osteoporosis.
Author information
Authors and Affiliations
Contributions
This work was done under the auspices of the European Commission–sponsored Genetic Factors for Osteoporosis (GEFOS) consortium.
Study-specific design and management were performed by U.S., M.A., L.M., J.P., S.B., M.L.B., B.M.B., C. Christiansen, C. Cooper, G.D., I.F., M.F., D.G., J.G.-M., M. Kähönen, M. Karlsson, J.-M.K., P.K., B.L.L., W.D.L., P.L., Ö.L., R.S.L., J.M., D.M., J.M.O., U.P.-K., J.A.R., P.M.R., F. Rousseau, P.E.S., N.L.S.T., R.U., W.V.H., J.V., M.T.Z., K.M.G., T.P., D.I.C., S.R.C., R.E., J.A.E., V.G., A.H., R.D.J., G.J., J.W.J., K.-T.K., T.L., M. Lorentzon, E.M., B.D.M., G.C.N., M.P., H.A.P.P., R.L.P., O.R., I.R.R., P.N.S., P.C.S., A.R.S., F.A.T., C.M.v.D., N.J.W., L.A.C., M.J.E., T.B.H., A.W.C.K., B.M.P., J. Reeve, T.D.S., E.A.S., M.C.Z., U.T., C.O., J.B.R., M.A.B., K. Stefansson, A.G.U., S.H.R., J.P.A.I., D.P.K. and F. Rivadeneira. Study-specific genotyping was performed by K.E., U.S., E.L.D., L.O., L.V., S.-M.X., A.K.A., D.J.D., S.G., R.K., C.K., A.Z.L., J.R.L., S.M., S.M.-B., S.S., S.T., O.T., S.C., E.K., J.M., B.O.-P., Y.S.A., E.G., L.H., H.J., T. Kwan, R. Luben, C.M.-G., S.T.P., S. Reppe, J.I.R., J.B.J.v.M., D.V., K.M.G., D.I.C., G.R.C., P.D., R.D.J., T.L., Y.L., M. Lorentzon, R.L.P., N.J.W., L.A.C., C.O., M.A.B., A.G.U. and F. Rivadeneira. Study-specific phenotyping was performed by U.S., E.L.D., O.M.E.A., A.M., S.-M.X., N. Alonso, S.K.K., S.G.W., A.K.A., T.A., J.R.C., Z.D., N.G.-G., S.G., G.H., L.B.H., K.A.J., G.K., G.S.K., C.K., T. Koromila, M. Kruk, M. Laaksonen, A.Z.L., S.H.L., P.C.L., L.M., X.N., J.P., L.M.R., K. Siggeirsdottir, O.S., N.M.v.S., J.W., K.Z., M.L.B., C. Christiansen, M.F., M. Kähönen, M. Karlsson, J.-M.K., Ö.L., J.M., D.M., B.O.-P., J.M.O., U.P.-K., D.M.R., J.A.R., P.M.R., F. Rousseau, W.V.H., J.V., M.C.-B., E.G., T.I., R. Luben, S. Reppe, G.S., J.B.J.v.M., D.V., F.M.K.W., K.M.G., J.A.C., D.I.C., E.M.D., R.E., J.A.E., V.G., A.H., R.D.J., G.J., Y.L., M. Lorentzon, E.M., G.C.N., B.A.O., M.P., H.A.P.P., R.L.P., O.R., I.R.R., J. Robbins, P.N.S., C.M.v.D., M.J.E., J. Reeve, E.A.S., M.C.Z., C.O., M.A.B., A.G.U., D.P.K. and F. Rivadeneira. Study-specific data analysis were performed by K.E., U.S., E.E., Y.-H.H., E.L.D., E.E.N., L.O., O.M.E.A., N. Amin, J.P.K., D.L.K., G.L., C.L., R.L.M., A.M., L.V., D.W., S.-M.X., L.M.Y.-A., H.-F.Z., J.E., C.M.K., S.K.K., P.J.L., G.T., J.F.W., V.A., A.K.A., T.A., J.R.C., G.H., L.J.H., C.K., T. Koromila, A.Z.L., S.M.-B., T.V.N., M.S.P., J.P., L.M.R., A.V.S., O.S., S.T., S.C., J.M., B.O.-P., U.P.-K., R. Li, R. Luben, S. Reppe, J.I.R., A.R.W., Y.Z., S. Raychaudhuri, D.I.C., J.A.E., R.D.J., T.L., K.N., O.R., D.M.E., D.K., J.B.R., M.A.B., J.P.A.I., D.P.K. and F. Rivadeneira. Analysis plan design was performed by K.E., E.E., U.S., D.K., D.P.K., J.P.A.I. and F. Rivadeneira. K.E., E.E., Y.-H.H. and E.E.N. carried out meta-analyses. K.E., E.E. and A.R.W. determined gene-by-gene interaction. Risk modeling and analysis of secondary signals were performed by K.E. and F. Rivadeneira. Expression QTLs were analyzed by U.S., G.T., E.G., S. Reppe, K.M.G. and T.P. Y.-H.H. performed functional SNP prediction. GRAIL was carried out by K.E., E.L.D., D.W. and S. Raychaudhuri. Standardization of phenotype and genotype replication data sets was performed by K.E., U.S., E.E., E.L.D., L.O., G.T., L.H. and C.M.-G. Interpretation of results was carried out by K.E., U.S., E.E., Y.-H.H., E.L.D., E.E.N., L.O., O.M.E.A., N. Amin, D.L.K., C.-T.L., R.L.M., A.M., L.V., D.W., S.-M.X., L.M.Y.-A., J.E., C.M.K., S.K.K., A.W.C.K., J. Reeve, M.C.Z., C.O., D.K., J.B.R., M.A.B., A.G.U., S.H.R., J.P.A.I., D.P.K. and F. Rivadeneira. The manuscript draft was prepared by K.E., U.S., E.E., Y.-H.H., E.L.D., E.E.N., L.O., O.M.E.A., A.M., C.O., D.K., J.B.R., M.A.B., A.G.U., S.H.R., J.P.A.I., D.P.K. and F. Rivadeneira. The steering committee for GEFOS includes U.S., E.E., U.T., A.G.U., S.H.R., J.P.A.I. and F. Rivadeneira.
Corresponding author
Ethics declarations
Competing interests
The coauthors affiliated with deCODE genetics in Reykjavik, Iceland, hold stock options in that company.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–20, Supplementary Figures 1–10 and Supplementary Note (PDF 6849 kb)
Rights and permissions
About this article
Cite this article
Estrada, K., Styrkarsdottir, U., Evangelou, E. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44, 491–501 (2012). https://doi.org/10.1038/ng.2249
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2249
This article is cited by
-
Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing
Reviews in Endocrine and Metabolic Disorders (2024)
-
Association between AXIN1 gene polymorphism (rs9921222) of WNT signaling pathway and susceptibility to osteoporosis in Egyptian patients: a case-control study
BMC Musculoskeletal Disorders (2023)
-
Osteoporosis and osteoarthritis: a bi-directional Mendelian randomization study
Arthritis Research & Therapy (2023)
-
Bone fragility and osteoporosis in children and young adults
Journal of Endocrinological Investigation (2023)
-
A genome-wide genomic score added to standard recommended stratification tools does not improve the identification of patients with very low bone mineral density
Osteoporosis International (2023)