Abstract
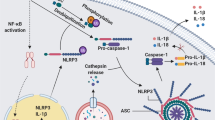
Inflammasomes are large, multimeric protein complexes that link the sensing of microbial products and metabolic stress to the proteolytic processing of prointerleukin (pro-IL)-1β to its active form. NALP1 and NALP2 are founding members of the Nod-like receptor family. Other Nod-like receptors, including NALP3 and NOD2, which are associated with inflammatory disorders, have also been described. The NALP1 and NALP3 inflammasomes are located in the cytoplasm and can, therefore, detect intracellular infection through recognition of microbial pathogen-associated molecular patterns. The inflammasome pathways cooperate with Toll-like receptor pathways to mediate a rapid and appropriate response to pathogens and genotoxic stress. Mutations in both pyrin and NALP3 components of inflammasomes are associated with innate-immune-mediated diseases (familial Mediterranean fever and the 'cryopyrinopathies'), and aberrant IL-1β processing has been reported in several autoinflammatory conditions, including Muckle–Wells syndrome, chronic infantile neurologic, cutaneous and articular syndrome/neonatal onset multisystem inflammatory disease, and gout. The effectiveness of IL-1β blockade in treating many of these conditions has transformed the understanding and management of these disorders and also highlighted the role of aberrant IL-1β signaling in other conditions, such as adult-onset Still's disease and systemic juvenile idiopathic arthritis.
Key Points
-
The NALP1 and NALP3 inflammasomes, both located in the cytoplasm, detect intracellular infection and cooperate with Toll-like receptor-mediated pathways to induce a rapid and appropriate response to invading pathogens and genotoxic stress
-
Mutations in either pyrin or NALP3 are associated with susceptibility to innate immune-mediated diseases; aberrant processing of interleukin (IL)-1β occurs in all the 'cryopyrinopathies', as well as in gout and pseudogout
-
IL-1β blockade is remarkably effective for many of these conditions; however, IL-1β antagonists with higher affinities and longer half-lives than anakinra are required
-
Further investigations into the role of inflammasomes in the pathogenesis of several autoimmune conditions, such as type 1 diabetes and neoplastic conditions, can be expected
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fritz JH et al. (2005) How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res 11: 390–394
Martinon F et al. (2005) NLRs join TLRs as innate sensors of pathogens. Trends Immunol 26: 447–454
Auron PE et al. (1984) Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci USA 81: 7907–7911
Mandrup-Poulsen T et al. (1986) Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia 29: 63–67
Martinon F et al. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241
Church LD et al. (2006) Hereditary auto-inflammatory disorders and biologics. Springer Semin Immunopathol 27: 494–508
O'Neill LA (2006) How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol 18: 3–9
Anderson KV et al. (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42: 791–798
Medzhitov R et al. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397
Matzinger P (2002) The danger model: a renewed sense of self. Science 296: 301–305
Shi Y et al. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521
Ting JP et al. (2006) CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol 6: 183–195
Fritz JH et al. (2006) Nod-like proteins in immunity, inflammation and disease. Nat Immunol 7: 1250–1257
Mariathasan S et al. (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7: 31–40
Inohara N et al. (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278: 5509–5512
Ogura Y et al. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603–606
Maeda S et al. (2005) Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science 307: 734–738
Tromp G et al. (1996) Genetic linkage of familial granulomatous inflammatory arthritis, skin rash, and uveitis to chromosome 16. Am J Hum Genet 59: 1097–1107
Miceli-Richard C et al. (2001) CARD15 mutations in Blau syndrome. Nat Genet 29: 19–20
van Duist MM et al. (2005) A new CARD15 mutation in Blau syndrome. Eur J Hum Genet 13: 742–747
Kanazawa N et al. (2005) Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105: 1195–1197
Okamura H et al. (1998) Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol 70: 281–312
Schmitz J et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490
Carriere V et al. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA 104: 282–287
Agostini L et al. (2004) NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20: 319–325
Dowds TA et al. (2004) Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem 279: 21924–21928
Martinon F et al. (2007) Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14: 10–22
Dinarello CA (1998) Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci 856: 1–11
Martinon F et al. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426
Martinon F et al. (2004) Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol 14: 1929–1934
Kanneganti TD et al. (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440: 233–236
Mariathasan S et al. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232
Petrilli V et al. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14: 1583–1589
Feldmeyer L et al. (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17: 1140–1145
O'Connor W Jr et al. (2003) Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol 171: 6329–6333
Yu JW et al. (2006) Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ 13: 236–249
Chae JJ et al. (2006) The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci USA 103: 9982–9987
Samuels J et al. (1998) Familial Mediterranean fever at the millennium. Clinical spectrum, ancient mutations, and a survey of 100 American referrals to the National Institutes of Health. Medicine (Baltimore) 77: 268–297
Watanabe H et al. (2007) Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 127: 1956–1963
Sutterwala FS et al. (2006) Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24: 317–327
Pizarro TT et al. (2007) Cloning IL-1 and the birth of a new era in cytokine biology. J Immunol 178: 5411–5412
Dinarello CA (2007) Mutations in cryopyrin: bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum 56: 2817–2822
Lee SH et al. (2001) Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 276: 34495–34500
Humke EW et al. (2000) ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell 103: 99–111
Annand RR et al. (1999) Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem J 342: 655–665
Stehlik C et al. (2003) The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J 373: 101–113
Bedoya F et al. (2007) Pyrin-only protein 2 modulates NF-kappaB and disrupts ASC:CLR interactions. J Immunol 178: 3837–3845
Dinarello CA (2005) Blocking IL-1 in systemic inflammation. J Exp Med 201: 1355–1359
Kastner DL (2005) Hereditary periodic fever syndromes. Hematology Am Soc Hematol Educ Program 1: 74–81
McDermott MF (1999) Autosomal dominant recurrent fevers. Clinical and genetic aspects. Rev Rheum Engl Ed 66: 484–491
Hoffman HM et al. (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat Genet 29: 301–305
Feldmann J et al. (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71: 198–203
Aganna E et al. (2002) Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum 46: 2445–2452
Gattorno M et al. (2007) Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum 56: 3138–3148
Hawkins PN et al. (2004) Spectrum of clinical features in Muckle–Wells syndrome and response to anakinra. Arthritis Rheum 50: 607–612
Hoffman HM et al. (2004) Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364: 1779–1785
Brydges S et al. (2006) The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr Top Microbiol Immunol 305: 127–160
Shoham NG et al. (2003) Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci USA 100: 13501–13506
McDermott MF (2004) A common pathway in periodic fever syndromes. Trends Immunol 25: 457–460
So A et al. (2007) A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 9: R28
McGonagle D et al.: Successful treatment of resistant pseudogout with anakinra. Arthritis Rheum, in press
McGonagle D et al. (2006) A proposed classification of the immunological diseases. PLoS Med 3: e297
Pascual V et al. (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 201: 1479–1486
Verbsky JW et al. (2004) Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J Rheumatol 31: 2071–2075
Colotta F et al. (1994) The type II 'decoy' receptor: a novel regulatory pathway for interleukin 1. Immunol Today 15: 562–566
Smith DE et al. (2003) The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity 18: 87–96
Lotito AP et al. (2007) Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. J Rheumatol 34: 823–830
Maeno N et al. (2002) Highly elevated serum levels of interleukin-18 in systemic juvenile idiopathic arthritis but not in other juvenile idiopathic arthritis subtypes or in Kawasaki disease: comment on the article by Kawashima et al. Arthritis Rheum 46: 2539–2541
Kawashima M et al. (2001) Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset Still's disease. Arthritis Rheum 44: 550–560
Choi JH et al. (2003) Serum cytokine profiles in patients with adult onset Still's disease. J Rheumatol 30: 2422–2427
Bywaters EG (1971) Still's disease in the adult. Ann Rheum Dis 30: 121–133
Fitzgerald AA et al. (2005) Rapid responses to anakinra in patients with refractory adult-onset Still's disease. Arthritis Rheum 52: 1794–1803
Rudinskaya A et al. (2003) Successful treatment of a patient with refractory adult-onset still disease with anakinra. J Clin Rheumatol 9: 330–332
Kotter I et al.: Anakinra in patients with treatment-resistant adult-onset Still's disease: four case reports with serial cytokine measurements and a review of the literature. Semin Arthritis Rheum, in press
de Koning HD et al.: Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin Arthritis Rheum, in press
Evans CH et al. (2005) Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci USA 102: 8698–8703
Stack JH et al. (2005) IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol 175: 2630–2634
Wannamaker W et al. (2007) (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl- butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther 321: 509–516
Settas LD et al. (2007) Reactivation of pulmonary tuberculosis in a patient with rheumatoid arthritis during treatment with IL-1 receptor antagonists (anakinra). J Clin Rheumatol 13: 219–220
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Church, L., Cook, G. & McDermott, M. Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat Rev Rheumatol 4, 34–42 (2008). https://doi.org/10.1038/ncprheum0681
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0681
This article is cited by
-
Efficacy of Baricitinib in Patients with Refractory Adult-Onset Still’s Disease
Drugs in R&D (2023)
-
Two flares of Still’s disease after two doses of the ChAdOx1 vaccine
Clinical Rheumatology (2022)
-
Interleukin-1 Blockade in Systemic Juvenile Idiopathic Arthritis
Pediatric Drugs (2020)
-
Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches
Drug Delivery and Translational Research (2020)
-
The role of inflammation in diabetic eye disease
Seminars in Immunopathology (2019)