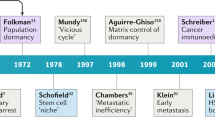
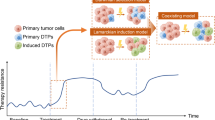
Abstract
Classical antineoplastic treatments such as chemotherapy or radiation can efficiently eradicate the majority of proliferating and genetically unstable malignant cells within neoplastic lesions. There is increasing evidence, however, that these regimens frequently fail to eliminate a minor subpopulation of resistant tumor cells that have distinct features of somatic stem cells. These serve as a reservoir for disease recurrence, and are the origin of metastatic growth. These so-called cancer stem cells (CSCs) or cancer-initiating cells represent often a rare, highly self-renewing population within the tumor mass, which is thought to be the only one required for both initiation and maintenance of disease. Tumor-cell populations enriched for CSC activity were originally identified in leukemias, but have now also been uncovered in a number of solid cancers. Their marked resistance towards classical antitumor regimens is mediated by the combination of several critical features, including relative dormancy, efficient DNA repair, high expression of multidrug-resistance-type membrane transporters and protection by a hypoxic niche environment. We review the concept of CSCs with particular emphasis on the mechanism of therapy resistance, and discuss potential future therapeutic interventions with the goal of specifically eliminating CSCs in a clinical setting.
Key Points
-
Many human neoplasms are likely to contain an often minor population of cancer cells within the tumor mass called cancer stem cells (CSCs), which are the only cells required for both initiation and maintenance of the disease
-
CSCs have unlimited self-renewal activity, and also can give rise to a large number of more-differentiated cells, which generate the bulk of the tumor
-
CSCs can either originate from normal stem cells or from more-differentiated progenitors
-
Long-lived CSCs seem to be resistant to radiation and classical chemotherapy and thus serve as an often small but highly malignant reservoir of cells
-
Due to their potential for migration and seeding in specific tissue niches, these CSCs might also drive disease recurrence and metastatic growth even after a significant latency period
-
The mechanisms that allow CSCs to escape current therapies remains to be elucidated, but are likely to be caused by several inherent features of CSCs, which include relative quiescence/dormancy, expression of multi-drug resistance transporters, localization in a protective and hypoxic niche and highly effective DNA repair mechanisms
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100: 157–168
Murphy MJ et al. (2005) More than just proliferation: Myc function in stem cells. Trends Cell Biol 15: 128–137
Blanpain C et al. (2007) Epithelial stem cells: turning over new leaves. Cell 128: 445–458
Bruce WR and Van Der Gaag H (1963) A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature 199: 79–80
Reya T et al. (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111
Wang JC and Dick JE (2005) Cancer stem cells: lessons from leukemia. Trends Cell Biol 15: 494–501
Bonnet D and Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737
Al-Hajj M et al. (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100: 3983–3988
Singh SK et al. (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401
Hope KJ et al. (2004) Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5: 738–743
Wilson A et al. (2007) Dormant and self-renewing hematopoietic stem cells and their niches. Ann NY Acad Sci 1106: 64–75
Moore KA and Lemischka IR (2006) Stem cells and their niches. Science 311: 1880–1885
Wilson A and Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6: 93–106
Tan TT and Coussens LM (2007) Humoral immunity, inflammation and cancer. Curr Opin Immunol 19: 209–216
Li F et al. (2007) Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 17: 3–14
Kaplan RN et al. (2007) Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med 13: 72–81
Dar A et al. (2006) Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol 34: 967–975
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480
Beachy PA et al. (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331
Smith MA et al. (2001) Stem cell factor: biology and relevance to clinical practice. Acta Haematol 105: 143–150
Cully M et al. (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 6: 184–192
Koch U and Radtke F (2007) Notch and cancer: a double-edged sword. Cell Mol Life Sci 64: 2746–2762
Sparmann A and van Lohuizen M (2006) Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6: 846–856
Jones PA and Baylin SB (2007) The epigenomics of cancer. Cell 128: 683–692
Huntly BJ et al. (2004) MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 6: 587–596
Krivtsov AV et al. (2006) Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442: 818–822
Kim CF et al. (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835
Lapidot T et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648
O'Brien CA et al. (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110
van Rhenen A et al. (2005) High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res 11: 6520–6527
Klein CA and Holzel D (2006) Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle 5: 1788–1798
Pantel K and Alix-Panabieres C (2007) The clinical significance of circulating tumor cells. Nat Clin Pract Oncol 4: 62–63
Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7: 834–846
Shmelkov SV et al. (2005) AC133/CD133/Prominin-1. Int J Biochem Cell Biol 37: 715–719
Singh SK et al. (2004) Cancer stem cells in nervous system tumors. Oncogene 23: 7267–7273
Dirks PB (2006) Cancer: stem cells and brain tumours. Nature 444: 687–688
Ricci-Vitiani L et al. (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445: 111–115
Collins AT et al. (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65: 10946–10951
Huttner WB and Kosodo Y (2005) Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol 17: 648–657
Dubreuil V et al. (2007) Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176: 483–495
Prince ME et al. (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104: 973–978
Li C et al. (2007) Identification of pancreatic cancer stem cells. Cancer Res 67: 1030–1037
Ponti D et al. (2005) Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65: 5506–5511
Sheridan C et al. (2006) CD44+/CD24– breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8: R59
Shackleton M et al. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88
Stingl J et al. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997
Abraham BK et al. (2005) Prevalence of CD44+/CD24–/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 11: 1154–1159
Balic M et al. (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12: 5615–5621
Schabath H et al. (2006) CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci 119: 314–325
Fang D et al. (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65: 9328–9337
Patrawala L et al. (2007) Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res 67: 6796–6805
Gibbs CP et al. (2005) Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 7: 967–976
Liu G et al. (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5: 67
Druker BJ et al. (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 344: 1038–1042
Bhatia R et al. (2003) Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 101: 4701–4707
Mauro MJ et al. (2004) Divergent clinical outcome in two CML patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res 28 (Suppl 1): S71–S73
Cortes J et al. (2004) Discontinuation of imatinib therapy after achieving a molecular response. Blood 104: 2204–2205
Diehn M and Clarke MF (2006) Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst 98: 1755–1757
Bao S et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760
Fleming WH et al. (1993) Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol 122: 897–902
Tumbar T et al. (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363
Guan Y et al. (2003) Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood 101: 3142–3149
Holyoake T et al. (1999) Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 94: 2056–2064
Graham SM et al. (2002) Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99: 319–325
Copland M et al. (2006) Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 107: 4532–4539
Jorgensen HG et al. (2007) Nilotinib exerts equipotent anti-proliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood 109: 4016–4019
Karrison TG et al. (1999) Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst 91: 80–85
Jorgensen HG et al. (2006) Intermittent exposure of primitive quiescent chronic myeloid leukemia cells to granulocyte-colony stimulating factor in vitro promotes their elimination by imatinib mesylate. Clin Cancer Res 12: 626–633
Brown JM and Giaccia AJ (1998) The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 58: 1408–1416
Green SK et al. (1999) Adhesion-dependent multicellular drug resistance. Anticancer Drug Des 14: 153–168
Ito K et al. (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451
Brown JM and Wilson WR (2004) Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4: 437–447
von Pawel J et al. (2000) Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: a report of the international CATAPULT I study group. Cisplatin and tirapazamine in subjects with advanced previously untreated non-small-cell lung tumors. J Clin Oncol 18: 1351–1359
Rischin D et al. (2005) Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol 23: 79–87
Giaccia A et al. (2003) HIF-1 as a target for drug development. Nat Rev Drug Discov 2: 803–811
Kiel MJ et al. (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121
Calabrese C et al. (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69–82
Tozer GM et al. (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5: 423–435
Kincade PW (1999) Blasting away leukemia. Nat Med 5: 619–620
Jin L et al. (2006) Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12: 1167–1174
Biedler JL and Riehm H (1970) Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res 30: 1174–1184
Gros P et al. (1986) Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci USA 83: 337–341
Roninson IB et al. (1986) Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA 83: 4538–4542
Donnenberg VS and Donnenberg AD (2005) Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol 45: 872–877
Dean M et al. (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275–284
Goodell MA et al. (2005) Isolation and characterization of side population cells. Methods Mol Biol 290: 343–352
Chiba T et al. (2006) Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology 44: 240–251
Hirschmann-Jax C et al. (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 101: 14228–14233
Patrawala L et al. (2005) Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2– cancer cells are similarly tumorigenic. Cancer Res 65: 6207–6219
Szotek PP et al. (2006) Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA 103: 11154–11159
Comerford KM et al. (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62: 3387–3394
Modok S et al. (2006) Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol 6: 350–354
Pusztai L et al. (2005) Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 104: 682–691
Xu D et al. (2005) Delivery of MDR1 small interfering RNA by self-complementary recombinant adeno-associated virus vector. Mol Ther 11: 523–530
Piccirillo SG et al. (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444: 761–765
Kelly PN et al. (2007) Tumor growth need not be driven by rare cancer stem cells. Science 317: 337
Blair A and Sutherland HJ (2000) Primitive acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo lack surface expression of c-kit (CD117). Exp Hematol 28: 660–671
Jordan CT et al. (2000) The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14: 1777–1784
Cox CV et al. (2004) Characterization of acute lymphoblastic leukemia progenitor cells. Blood 104: 2919–2925
Cox CV et al. (2007) Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukemia. Blood 109: 674–682
Hermann PC et al. (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1: 313–323
Yang ZF et al. (2008) Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13: 153–166
Acknowledgements
The authors would like to thank Dr Christelle Adolphe and Dr Anne Wilson for critical comments on the manuscript and members of the Trumpp laboratory for numerous discussions. This work was supported by grants to AT from the Swiss National Science Foundation, the Swiss Cancer League and the EU-FP6 Program “INTACT” as well as by funds from the Helmholtz association to ODW and to the DKFZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Trumpp, A., Wiestler, O. Mechanisms of Disease: cancer stem cells—targeting the evil twin. Nat Rev Clin Oncol 5, 337–347 (2008). https://doi.org/10.1038/ncponc1110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1110
This article is cited by
-
Radiochemotherapy-induced DNA repair promotes the biogenesis of gastric cancer stem cells
Stem Cell Research & Therapy (2022)
-
MicroRNAs as the critical regulators of tyrosine kinase inhibitors resistance in lung tumor cells
Cell Communication and Signaling (2022)
-
The efficacy of PI3Kγ and EGFR inhibitors on the suppression of the characteristics of cancer stem cells
Scientific Reports (2022)
-
Loss of oral mucosal stem cell markers in oral submucous fibrosis and their reactivation in malignant transformation
International Journal of Oral Science (2020)
-
The great escape: tumour cell plasticity in resistance to targeted therapy
Nature Reviews Drug Discovery (2020)