Abstract
Recent advances in the understanding of molecular mechanisms of cancer have led to the development of novel compounds that target specific cancer pathways. These drugs encompass monoclonal antibodies and tyrosine and non-tyrosine kinase inhibitors, and have been approved by the FDA and the European Medicines Agency, among others, for cancer treatment. These agents are associated with several toxic effects including potentially unacceptable gastrointestinal adverse effects. Diarrhea and hepatotoxicity, the most common adverse events experienced with these treatments, can frequently lead to treatment discontinuation and consequently decreased cancer control. We review the incidence and clinical patterns of the gastrointestinal and hepatic toxic effects induced by the main molecular-targeted therapies and propose some hypotheses for the causes of each adverse event.
Key Points
-
Gastrointestinal and hepatic adverse effects are common features of molecular-targeted therapies
-
Limited data are available regarding the pathophysiology of such toxicities
-
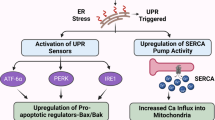
Pathophysiological mechanisms of diarrhea mainly involve secretory diarrhea, and direct blockage of tyrosine kinases present in the intestinal epithelium
-
Pharmacokinetic interactions are often involved in hepatic toxicity
-
Hepatic and gastrointestinal adverse effects might provide insights into the mechanisms of action of molecular-targeted agents and, more widely, into the physiology of the liver and the gastrointestinal tract
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hanahan D and Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70
Shepherd FA et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132
Fukuoka M et al. (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial). J Clin Oncol 21: 2237–2246
Wacker B et al. (2007) Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 13: 3913–3921
Perez-Soler R et al. (2004) Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 22: 3238–3247
Amador ML et al. (2004) An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res 64: 9139–9143
Fujiwara Y et al. (2006) Relationship between epidermal growth factor receptor gene mutations and the severity of adverse events by gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer 52: 99–103
Thomas SK et al. (2006) Asian ethnicity as a predictor of response in patients with non-small-cell lung cancer treated with gefitinib on an expanded access program. Clin Lung Cancer 7: 326–331
Strumberg D et al. (2006) Pooled analysis of BAY 43-92006 (sorafenib) monotherapy in patients with advanced solid tumours: is rash associated with treatment outcome? Eur J Cancer 42: 548–556
Escudier B et al. (2005) Randomized phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell cancer [abstract #4510]. Proc Am Soc Clin Oncol 23: 380
Motzer RJ et al. (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295: 2516–2524
Shah NT et al. (2005) Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol 23: 165–174
Van Glabbeke M et al. (2006) Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG). Eur J Cancer 42: 2277–2278
Demetri GD et al. (2002) Efficacity and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472–480
Liu G et al. (2004) A phase II trial of flavopiridol (NSC #649890) in patients with previously untreated metastatic androgen-independent prostate cancer. Clin Cancer Res 10: 924–928
Senderowicz AM et al. (1998) Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol 16: 2986–2999
Papandreou CN et al. (2004) Phase I of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 22: 2108–2121
Aghajanian C et al. (2002) A phase I of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8: 2505–2511
Wong KK et al. (2006) HKI-272, an irreversible pan erB receptor tyrosine kinase inhibitor: preliminary phase 1 results in patients with solid tumors [abstract #3018]. Proc Am Soc Clin Oncol 24 (Suppl 18S)
Benson AB et al. (2004) Recommended guidelines for the treatment of cancer-treatment-induced diarrhea. J Clin Oncol 22: 2918–2926
Thomas JP et al. (2002). Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol 50: 465–472
Uribe JM et al. (1996) Epidermal growth factor inhibits calcium-dependent chloride secretion in T84 human colonic epithelial cells. Am J Physiol Cell Physiol 271: 914–922
Uribe JM et al. (1996) Phosphatidylinositol 3-kinase mediates the inhibitory effect of epidermal growth factor on calcium-dependent chloride secretion. J Biol Chem 271: 26588–26595
Burrhis HA et al. (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23: 5305–5313
Kahn MES et al. (2001) Possible mechanisms of diarrhea side effects associated with the use of a novel chemotherapeutic agent, flavopiridol. Clin Cancer Res 7: 343–349
Keely SJ and Barett KE (1999) ErbB2 and ErbB3 receptors mediate inhibition of calcium-dependent chloride secretion in colonic epithelial cells. J Biol Chem 274: 33449–33454
Laux I et al. (2006) Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. Br J Cancer 94: 85–92
Lu JF et al. (2006) Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther 80: 136–145
Dias VC et al. (1998) Oral administration of rapamycin and cyclosporin differentially alter intestinal function in rabbits. Dig Dis Sci 43: 2227–2236
Innocenti F et al. (2000) Flavopiridol metabolism in cancer patients is associated with the occurrence of diarrhea. Clin Cancer Res 6: 3400–3405
Lathia C et al. (2006) Lack of effect of ketaconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol 57: 685–692
Sambol EB et al. (2006) Flavopiridol targets c-KIT transcription and induces apoptosis in gastrointestinal stromal tumor cells. Cancer Res 66: 5858–5866
Shafik A et al. (2006) Interstitial cells of cajal in patients with constipation due to total initial inertia. J Invest Surg 19: 147–153
Fanucchi MP et al. (2003) Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol 24: 5025–5033
Kanazawa S et al. (2006) Aspirin reduces adverse effects of gefitinib. Anticancer Drugs 17: 423–427
Suzuki T et al. (2000) Thromboxane A(2)-mediated Cl(-) secretion induced by platelet-activating factor in isolated rat colon. Eur J Pharmacol 400: 297–303
Horie Y et al. (2004) Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 113: 1774–1783
Jager W et al. (2003) Biliary excretion of flavopiridol and its glucuronides in the isolated perfused rat liver: role of the multidrug resistance protein 2 (Mrp2). Life Sci 73: 2841–2854
Burger H et al. (2005) Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther 4: 747–752
Kubitz R et al. (2004) Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology 126: 541–553
Guilhot F (2004) Indications for imatinib mesylate therapy and clinical management. Oncologist 9: 271–281
Pariente A et al. (2006) Imatinib mesylate-induced acute hepatitis in a patient treated for gastrointestinal stromal tumour. Eur J Gastroenterol Hepatol 18: 785–787
Cross TJS et al. (2006) Imatinib mesylate as a cause of acute liver failure. Am J Hematol 81: 189–192
Ohyashiki K et al. (2002) Imatinib mesylate-induced hepato-toxicity in chronic myeloid leukemia demonstrated focal necrosis resembling acute viral hepatitis. Leukemia 16: 2160–2161
Ho C et al. (2005) Side effects related to cancer treatment: CASE 1. Hepatitis following treatment with gefitinib. J Clin Oncol 23: 8531–8533
Rajvanshi P et al. (2002) Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood 99: 2310–2314
Ramanathan RK et al. (2005) Phase I pharmacokinetic-pharmacodynamic study of 17-(Allylamino)-17-demethoxygeldanamycin (17AAG,NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res 11: 3385–3391
Kikuchi S et al. (2004) Severe hepatitis and complete molecular response caused by imatinib mesylate: possible association of its serum concentration with clinical outcomes Leuk Lymphoma 45: 2349–2351
James C et al. (2003) Histological features of acute hepatitis after imatinib mesylate treatment. Leukemia 14: 978–979
Dhalluin-Venier V et al. (2006) Imatinib mesylate-induced acute hepatitis with autoimmune features. Eur J Gastroenterol Hepatol 18: 1235–1237
Lin NU et al. (2003) Fatal hepatic necrosis following imatinib mesylate therapy. Blood 102: 3455–3456
Mc Koy JM et al. (2007) Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res 31: 599–604
Deininger MW et al. (2003) Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol 21: 1637–1647
Peng B et al. (2005) Clinical pharmacokinetics of imatinib. Clin Pharmacokinet 44: 879–894
Swaisland HC et al. (2005) Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 44: 1067–1081
Talpaz M et al. (2002) Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99: 1928–1937
Marin D et al. (2002) The use of imatinib (STI571) in chronic myeloid leukemia: some practical considerations. Haematologica 87: 979–988
Abou-Alfa GK et al. (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 4293–4300
Ferrero D et al. (2006) Corticosteroids can reverse severe imatinib-induced hepatotoxicity. Haematologica 91 (Suppl 6): ECR27
Motzer RJ et al. (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 16–24
Strumberg D et al. (2005) Phase I clinical and pharmacokinetic study of the novel raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23: 965–972
Kantarjian H et al. (2006) Nilotinib in imatinib–resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 24: 2542–2551
Kozloff M et al. (2006) Safety of bevacizumab (BV) among patients (pts) receiving first-line chemotherapy for metastatic colorectal cancer: updated results from a large observational study in the US (BRiTE) [abstract #247]. Gastrointestinal Cancers Symposium.
Lordick F et al. (2006) Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int J Radiat Oncol Biol Phys 64: 1295–1298
Herbst RS et al. (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23: 5892–5899
Herbst RS et al. (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol 22: 785–794
Geyer CE et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733–2743
Acknowledgements
The authors thank Lorna Saint Ange and Professor Ducreux for their help in editing the manuscript. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JC Soria is a Consultant for Roche, sanofi-aventis and Wyeth, and also receives grant funding from Eli Lilly. D Malka is on the speakers bureau for Merck, Pfizer, Roche, sanofi-aventis and Serano, and receives grant or research support from Merck, Roche, sanofi-aventis and Serano. The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Loriot, Y., Perlemuter, G., Malka, D. et al. Drug Insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Rev Clin Oncol 5, 268–278 (2008). https://doi.org/10.1038/ncponc1087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1087
This article is cited by
-
Clinical characteristics and outcomes of tyrosine kinase inhibitor-related lower GI adverse effects
Journal of Cancer Research and Clinical Oncology (2023)
-
Systems biology analysis identifies molecular determinants of chemotherapy-induced diarrhoea
Journal of Molecular Medicine (2020)
-
The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea
Breast Cancer Research and Treatment (2019)
-
Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines
Supportive Care in Cancer (2019)
-
Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes
Nature Reviews Clinical Oncology (2018)