Abstract
Cervical cancer is a major cause of cancer mortality in women worldwide and is initiated by infection with high-risk human papillomaviruses (HPVs). High-risk HPVs, especially HPV-16, are associated with other anogenital cancers and a subgroup of head-and-neck cancers. Indeed, HPV infection could account for the development of head-and-neck cancer in certain individuals that lack the classical risk factors for this disease (tobacco and alcohol abuse). This Review summarizes the main events of the HPV life cycle, the functions of the viral proteins, and the implications of HPV infection on their hosts, with an emphasis on carcinogenic mechanisms and disease outcomes in head-and-neck cancer. The demonstration that HPVs have a role in human carcinogenesis has allowed the development of preventive and therapeutic strategies aimed at reducing the incidence and mortality of HPV-associated cancers.
Key Points
-
High-risk human papillomaviruses encode E6 and E7 oncoproteins that bind and degrade p53 and pRb tumor suppressors, respectively
-
HPV-associated cancers maintain and express the HPV viral genes even in advanced stages of disease, and repression of viral oncogene expression can prevent the growth and survival of cervical cancer cells
-
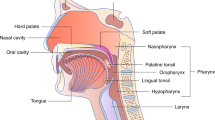
The annual incidence of oropharyngeal cancer in the US increased between 1973 and 2001 in younger individuals, in parallel with an increase in sexual practices that are typically associated with the sexual transmission of HPV
-
HPV-16 is the most prevalent genotype in cervical carcinoma, and is also the most frequently detected HPV type in HNSCC, found in up to 90% of HPV-positive cases
-
HPV-associated oropharyngeal squamous-cell cancers have a better prognosis than HPV-negative tumors
-
Prophylactic vaccination against high-risk HPV types will eventually prevent a significant number of cervical carcinomas and possibly HNSCC, although owing to the prolonged course of carcinogenesis, the reduction in cancer incidence will not be profound for several years
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (1995) Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 64: 1–378
zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342–350
Crum CP et al. (1997) Pathobiology of vulvar squamous neoplasia. Curr Opin Obstet Gynecol 9: 63–69
Kayes O et al. (2007) Molecular and genetic pathways in penile cancer. Lancet Oncol 8: 420–429
Gillison ML et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709–720
Goldie SJ et al. (2003) A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. Int J Cancer 106: 896–904
Munger K et al. (2004) Mechanisms of human papillomavirus-induced oncogenesis. J Virol 78: 11451–11460
Stubenrauch F and Laimins LA (1999) Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol 9: 379–386
Bedell MA et al. (1991) Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J Virol 65: 2254–2260
Cheng S et al. (1995) Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev 9: 2335–2349
Werness BA et al. (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248: 76–79
Scheffner M et al. (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136
Dyson N et al. (1989) The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243: 934–937
Boyer SN et al. (1996) E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin–proteasome pathway. Cancer Res 56: 4620–4624
Flores ER et al. (2000) The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol 74: 6622–6631
McLaughlin-Drubin ME et al. (2005) The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology 338: 61–68
Wu X et al. (1994) Papilloma formation by cottontail rabbit papillomavirus requires E1 and E2 regulatory genes in addition to E6 and E7 transforming genes. J Virol 68: 6097–6102
Yang L et al. (1993) The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA 90: 5086–5090
Hughes FJ and Romanos MA (1993) E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucl Acids Res 21: 5817–5823
Mohr IJ et al. (1990) Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science 250: 1694–1699
Thierry F and Yaniv M (1987) The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J 6: 3391–3397
Skiadopoulos MH and McBride AA (1998) Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J Virol 72: 2079–2088
You J et al. (2004) Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117: 349–360
Scotto J and Bailar JC III (1969) Rigoni-Stern and medical statistics: a nineteenth-century approach to cancer research. J Hist Med Allied Sci 24: 65–75
Boshart M et al. (1984) A new type of papillomavirus DNA is present in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J 3: 1151–1157
Durst M et al. (1983) A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA 80: 3812–3815
Gissmann L et al. (1983) Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci USA 80: 560–563
Walboomers JM et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19
Cullen AP et al. (1991) Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol 65: 606–612
Schlecht NF et al. (2001) Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 286: 3106–3114
Halbert CL et al. (1991) The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol 65: 473–478
Hwang ES et al. (1993) Inhibition of cervical carcinoma cell line proliferation by the introduction of a bovine papillomavirus regulatory gene. J Virol 67: 3720–3729
Desaintes C et al. (1997) Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J 16: 504–514
von Knebel Doeberitz M et al. (1988) Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res 48: 3780–3786
Arbeit JM et al. (1994) Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 68: 4358–4368
Brake T and Lambert PF (2005) Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA 102: 2490–2495
Harper DM et al. (2004) Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364: 1757–1765
Villa LL et al. (2005) Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 6: 271–278
Koutsky LA and Harper DM (2006) Chapter 13: current findings from prophylactic HPV vaccine trials. Vaccine 24 (Suppl 3): S114–S121
Durst M et al. (1987) Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene 1: 251–256
Duensing S and Munger K (2004) Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins. Int J Cancer 109: 157–162
Srivenugopal KS and Ali-Osman F (2002) The DNA repair protein, O(6)-methylguanine-DNA methyltransferase is a proteolytic target for the E6 human papillomavirus oncoprotein. Oncogene 21: 5940–5945
Goodwin EC and DiMaio D (2000) Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci USA 97: 12513–12518
D'Souza G et al. (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1944–1956
Weinberger PM et al. (2006) Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favourable prognosis. J Clin Oncol 24: 736–747
Andl T et al. (1998) Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res 58: 5–13
Frisch M and Biggar RJ (1999) Aetiological parallel between tonsillar and anogenital squamous-cell carcinomas. Lancet 354: 1442–1443
Shiboski CH et al. (2005) Tongue and tonsil carcinoma: increasing trends in the US population ages 20–44 years. Cancer 103: 1843–1849
Frisch M et al. (2000) Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control 11: 489–495
Canto MT and Devesa SS (2002) Oral cavity and pharynx cancer incidence rates in the United States, 1975–1998. Oral Oncol 38: 610–617
Fleming DT et al. (1997) Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 337: 1105–1111
Schwartz SM et al. (1998) Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst 90: 1626–1636
Hemminki K et al. (2000) Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev 9: 433–437
Mork J et al. (2001) Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 344: 1125–1131
Hansson BG et al. (2005) Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol 125: 1337–1344
Herrero R et al. (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772–1783
Smith EM et al. (2004) Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer 108: 766–772
McNeil C (2000) HPV in oropharyngeal cancers: new data inspire hope for vaccines. J Natl Cancer Inst 92: 680–681
Syrjanen K et al. (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12: 418–424
de Villiers EM et al. (1985) Papillomavirus DNA in human tongue carcinomas. Int J Cancer 36: 575–578
Steinberg BM and DiLorenzo TP (1996) A possible role for human papillomaviruses in head and neck cancer. Cancer Metastasis Rev 15: 91–112
Chen Z et al. (1997) Mutations in the long control region of human papillomavirus DNA in oral cancer cells, and their functional consequences. Cancer Res 57: 1614–1619
Maitland NJ et al. (1987) Detection of human papillomavirus DNA in biopsies of human oral tissue. Br J Cancer 56: 245–250
Yeudall WA and Campo MS (1991) Human papillomavirus DNA in biopsies of oral tissues. J Gen Virol 72: 173–176
Mellin H et al. (2002) Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer 102: 152–158
Van Tine BA et al. (2004) Human papillomavirus (HPV) origin-binding protein associates with mitotic spindles to enable viral DNA partitioning. Proc Natl Acad Sci USA 101: 4030–4035
Li Y et al. (1994) Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res 54: 6078–6082
Hara E et al. (1996) Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16: 859–867
Agoff SN et al. (2003) p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol 16: 665–673
Begum S et al. (2003) Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res 9: 6469–6475
Sidransky D (1995) Molecular genetics of head and neck cancer. Curr Opin Oncol 7: 229–233
Gillison ML and Shah KV (2001) Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol 13: 183–188
Schwartz SR et al. (2001) Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg 125: 1–9
Paz IB et al. (1997) Human papillomavirus (HPV) in head and neck cancer: an association of HPV16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer 79: 595–604
Licitra L et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630–5636
Ritchie JM et al. (2003) Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer 104: 336–344
Lindel K et al. (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92: 805–813
Li W et al. (2003) Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer 106: 553–558
Slaughter DP et al. (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6: 963–968
Butz K et al. (1996) Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer 68: 506–513
Wheeler CM et al. (2007) Advances in primary and secondary interventions for cervical cancer: human papillomavirus prophylactic vaccines and testing. Nat Clin Pract Oncol 4: 224–235
FUTURE II Study Group (2007) Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 356: 1915–1927
Pinto LA et al. (2003) Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis 188: 327–338
Feltkamp MC et al. (1993) Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 23: 2242–2249
Borysiewicz LK et al. (1996) A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347: 1523–1527
Acknowledgements
Research in the authors' laboratories was supported by grants from the NIH (CA16038) and the Virginia Alden Wright Fund. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Psyrri, A., DiMaio, D. Human papillomavirus in cervical and head-and-neck cancer. Nat Rev Clin Oncol 5, 24–31 (2008). https://doi.org/10.1038/ncponc0984
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0984
This article is cited by
-
Machine learning-based tissue of origin classification for cancer of unknown primary diagnostics using genome-wide mutation features
Nature Communications (2022)
-
Human Papillomavirus DNA Detection by Droplet Digital PCR in Formalin-Fixed Paraffin-Embedded Tumor Tissue from Oropharyngeal Squamous Cell Carcinoma Patients
Molecular Diagnosis & Therapy (2021)
-
Evaluation of HPV16 E7 expression in head and neck carcinoma cell lines and clinical specimens
Scientific Reports (2020)
-
Comprehensive profiling of extracellular RNA in HPV-induced cancers using an improved pipeline for small RNA-seq analysis
Scientific Reports (2020)
-
LKB1 inhibits HPV-associated cancer progression by targeting cellular metabolism
Oncogene (2017)