Abstract
Background Mitochondria of circulating white blood cells (WBC) and platelets sense oxidative stress during capillary passage and react by producing reactive oxygen species (ROS). Although evidence indicates that congestive heart failure (CHF) is associated with oxidative stress, the role of WBC and platelets as mediators in CHF has not been investigated.
Methods Patients with CHF (n = 15) and healthy volunteers (n = 9) were enrolled between 2006 and 2007 into this observational study. Arterial and venous blood samples from participants were incubated with probes to detect cytosolic and mitochondrial ROS. Fluorescence-activated cell sorting was used to measure the degree of fluorescence in WBC and platelets.
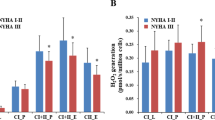
Results Patients with CHF had a higher proportion of ROS-positive arterial WBC and platelets than did controls (67% ± 47% versus 16% ± 9%; P <0.005), as well as venous WBC and platelets (77% ± 43% versus 38% ± 13%; P <0.01). In the control group, the proportion of cytosolic ROS-positive arterial WBC and platelets was lower than that for ROS-positive venous WBC and platelets (16% ± 9% versus 38% ± 13%; P <0.005). CHF patients had a higher proportion of mitochondrial ROS-positive arterial and venous WBC and platelets than did controls.
Conclusion In CHF, the proportion of WBC and platelets that are ROS-positive is raised, possibly because cytosolic ROS-positive WBC and platelets are normally cleared in the lungs; this function is deficient in CHF while mitochondrial ROS production is increased. The raised numbers of circulating ROS-positive WBC and platelets amplify oxidative stress in CHF.
Key Points
-
In CHF, the proportion of WBC and platelets that are ROS-positive is raised in the cytosol
-
In CHF, WBC and platelets have more ROS-producing mitochondria
-
Oxidative stress is amplified in CHF because of mitochondrial production of ROS in WBC and platelets
-
Oxidative stress is amplified in CHF because of absent pulmonary clearance of ROS-loaded WBC and platelets
-
Pulmonary removal of ROS and transfer of redox active molecules to the mitochondria are new potential targets for CHF therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Givertz MM and Colucci WS (1998) New targets for heart-failure therapy: endothelin, inflammatory cytokines, and oxidative stress. Lancet 352 (Suppl 1): SI34–SI38
Grieve DJ and Shah AM (2003) Oxidative stress in heart failure: more than just damage. Eur Heart J 24: 2161–2163
Linke A (2005) Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770
Ellis GR et al. (2000) Neutrophil superoxide anion-generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J Am Coll Cardiol 36: 1474–1482
Kijima Y et al. (2001) Transcardiac 8-iso-prostaglandin F2α generation from acute myocardial infarction heart: insight into abrupt reperfusion and oxidant stress. Prostaglandins Leukot Essent Fatty Acids 64: 161–166
Diaz-Velez CR et al. (1996) Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J 131: 146–152
White M et al. (2006) Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin Sci (Lond) 110: 483–489
Leu HB et al. (2006) Circulating mononuclear superoxide production and inflammatory markers for long-term prognosis in patients with cardiac syndrome X. Free Radic Biol Med 40: 983–991
Hofmann MA et al. (1999) Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia 42: 222–232
Kong C et al. (2001) Leukocyte mitochondria depolarization and apoptosis in advanced heart failure: clinical correlations and effect of therapy. J Am Coll Cardiol 38: 1693–1700
van Heerebeek et al. (2008). Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117: 43–51
CellQuestPro vs 5.2, BD CellQuest (BD Biosciences, San Jose, CA, USA)
Thomas ML and Brown EJ (1999). Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today 20: 406–411
Simunek T et al. (2005) SIH—a novel lipophilic iron chelator—protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. J Mol Cell Cardiol 39: 345–354
Slidebook software, version 4.0 (Intelligent Imaging Innovations, Denver, CO, USA)
Prism Software, Inc., version 4.0 (GraphPAD, La Jolla, CA, USA)
SPSS® version 14.0 (SPSS@Inc Software for Windows, Chicago, IL, USA)
Araneda OF et al. (2005) Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: a possible predictor of acute mountain sickness. Eur J Appl Physiol 95: 383–390
Takahashi M et al. (2008) Angiotensin II and tumor necrosis factor-α synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-κB, p38, and reactive oxygen species. Am J Physiol 294: H2879–H2888
van Overveld FJ et al. (2005) New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteine. J Physiol Pharmacol 56: 135–142
Vanderheyden M et al. (1998) Pro-inflammatory cytokines and endothelium-dependent vasodilation in the forearm. Serial assessment in patients with congestive heart failure. Eur Heart J 19: 747–752
Kuebler WM et al. (1994) Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol 76: 65–71
Parthasarathi K et al. (2002) Mitochondrial reactive oxygen species regulate spatial profile of proinflammatory responses in lung venular capillaries. J Immunol 169: 7078–7086
Chang HJ et al. (2003) The origin of proinflammatory cytokines in patients with idiopathic dilated cardiomyopathy. J Kor Med Sci 18: 791–796
Heymes C et al. (2003) Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171
Takimoto E et al. (2005) Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115: 1221–1231
Redout EM et al. (2007) Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 75: 770–781
Saliaris AP et al. (2007) Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and beta-adrenergic hyporesponsiveness in heart failure. Am J Physiol Heart Circ Physiol 292: 1328–1335
Cheng W et al. (1995) Stretch-induced programmed myocyte cell death. J Clin Invest 96: 2247–2259
Johar S et al. (2006) Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548
Opie LH et al. (2006) Controversies in ventricular remodelling. Lancet 367: 356–367
Zorov DB et al. (2006) Mitochondrial ROS-induced ROS release: An update and review. Biochim Biophys Acta 1757: 509–517
Griendling KK and Fitzgerald GA (2003) Oxidative stress and cardiovascular injury, part II: animal and human studies. Circulation 108: 2034–2040
Takeda T et al. (2006) Serofendic acid, a novel substance extracted from fetal calf serum, protects against oxidative stress in neonatal rat cardiac myocytes. J Am Coll Cardiol 47: 1882–1890
Higashi Y et al. (2005) Angiotensin II type I receptor blocker and endothelial function in humans: role of nitric oxide and oxidative stress. Curr Med Chem Cardiovasc Hematol Agents 3: 133–148
Kukin ML et al. (1999) Prospective, randomized comparison on the effect of long-term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction, and oxidative stress in heart failure. Circulation 99: 2645–2651
Kurian KC et al. (2006) The effect of statins in heart failure: beyond its cholesterol-lowering effect. J Card Fail 12: 473–478
Rudolph V et al. (2007) Activation of polymorphonuclear neutrophils in patients with impaired left ventricular function. Free Radic Biol Med 43: 1189–1196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
IJsselmuiden, A., Musters, R., de Ruiter, G. et al. Circulating white blood cells and platelets amplify oxidative stress in heart failure. Nat Rev Cardiol 5, 811–820 (2008). https://doi.org/10.1038/ncpcardio1364
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1364
This article is cited by
-
Atrial natriuretic peptide stimulates autophagy/mitophagy and improves mitochondrial function in chronic heart failure
Cellular and Molecular Life Sciences (2023)
-
Alterations of sodium-hydrogen exchanger 1 function in response to SGLT2 inhibitors: what is the evidence?
Heart Failure Reviews (2022)
-
Inflammation and its association with oxidative stress in dogs with heart failure
BMC Veterinary Research (2021)
-
Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in heart failure patients
Scientific Reports (2021)
-
Evaluation the relationship of left ventricular global longitudinal strain and laboratory parameters in discharged patients with COVID-19: a follow-up study
The International Journal of Cardiovascular Imaging (2021)