Abstract
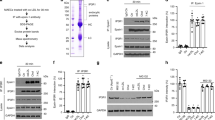
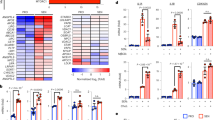
Excess cellular cholesterol induces apoptosis in macrophages, an event likely to promote progression of atherosclerosis. The cellular mechanism of cholesterol-induced apoptosis is unknown but had previously been thought to involve the plasma membrane. Here we report that the unfolded protein response (UPR) in the endoplasmic reticulum is activated in cholesterol-loaded macrophages, resulting in expression of the cell death effector CHOP. Cholesterol loading depletes endoplasmic reticulum calcium stores, an event known to induce the UPR. Furthermore, endoplasmic reticulum calcium depletion, the UPR, caspase-3 activation and apoptosis are markedly inhibited by selective inhibition of cholesterol trafficking to the endoplasmic reticulum, and Chop−/− macrophages are protected from cholesterol-induced apoptosis. We propose that cholesterol trafficking to endoplasmic reticulum membranes, resulting in activation of the CHOP arm of the UPR, is the key signalling step in cholesterol-induced apoptosis in macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shio, H., Haley, N.J. & Fowler, S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab. Invest. 41, 160–167 (1979).
Rapp, J.H., Connor, W.E., Lin, D.S., Inahara, T. & Porter, J.M. Lipids of human atherosclerotic plaques and xanthomas: clues to the mechanism of plaque progression. J. Lipid Res. 24, 1329–1335 (1983).
Small, D.M., Bond, M.G., Waugh, D., Prack, M. & Sawyer, J.K. Physicochemical and histological changes in the arterial wall of non-human primates during progression and regression of atherosclerosis. J. Clin. Invest. 73, 1590–1605 (1984).
Kruth, H.S. & Fry, D.L. Histochemical detection and differentiation of free and esterified cholesterol in swine atherosclerosis using filipin. Exp. Mol. Pathol. 40, 288–294 (1984).
Libby, P. & Clinton, S.K. The role of macrophages in atherogenesis. Curr. Opin. Lipidol. 4, 355–363 (1993).
Ball, R.Y. et al. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis 114, 45–54 (1995).
Fazio, S. et al. Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J. Clin. Invest. 107, 163–171 (2001).
Tabas, I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J. Clin. Invest. 110, 905–911 (2002).
Kellner-Weibel, G. et al. Effects of intracellular free cholesterol accumulation on macrophage viability: a model for foam cell death. Arterioscler. Thromb. Vasc. Biol. 18, 423–431 (1998).
Yao, P.M. & Tabas, I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J. Biol. Chem. 275, 23807–23813 (2000).
Yao, P.M. & Tabas, I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J. Biol. Chem. 276, 42468–42476 (2001).
Kellner-Weibel, G., Geng, Y.J. & Rothblat, G.H. Cytotoxic cholesterol is generated by the hydrolysis of cytoplasmic cholesteryl ester and transported to the plasma membrane. Atherosclerosis 146, 309–319 (1999).
Yeagle, P.L. Modulation of membrane function by cholesterol. Biochimie 73, 1303–1310 (1991).
Bretscher, M.S. & Munro, S. Cholesterol and the Golgi apparatus. Science 261, 1280–1281 (1993).
Patil, C. & Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–355 (2001).
Travers, K.J. et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 (2000).
Zhang, D. et al. Macrophages deficient in CTP:Phosphocholine cytidylyltransferase-α are viable under normal culture conditions but are highly susceptible to free cholesterol-induced death. Molecular genetic evidence that the induction of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response. J. Biol. Chem. 275, 35368–35376 (2000).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Nishitoh, H. et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Underwood, K.W., Andemariam, B., McWilliams, G.L. & Liscum, L. Quantitative analysis of hydrophobic amine inhibition of intracellular cholesterol transport. J. Lipid Res. 37, 1556–1568 (1996).
Shiratori, Y., Okwu, A.K. & Tabas, I. Free cholesterol loading of macrophages stimulates phosphatidylcholine biosynthesis and up-regulation of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 269, 11337–11348 (1994).
Aikawa, K., Furuchi, T., Fujimoto,Y., Arai, H. & Inoue, K. Structure-specific inhibition of lysosomal cholesterol transport in macrophages by various steroids. Biochim. Biophys. Acta 1213, 127–134 (1994).
Liscum, L. & Klansek, J.J. Niemann-Pick disease type C. Curr. Opin. Lipidol. 9, 131–135 (1998).
Feng, B. & Tabas, I. ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full NPC1 activity. J. Biol. Chem. 277, 43271–43280 (2002).
Yancey, P.G. et al. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271, 16026–16034 (1996).
Christian, A.E., Haynes, M.P., Phillips, M.C. & Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38, 2264–2272 (1997).
Khan, N. et al. Plasma membrane cholesterol: A possible barrier to intracellular oxygen in normal and mutant CHO cells defective in cholesterol metabolism. Biochemistry 42, 23–29 (2003).
Wang, X.Z. & Ron, D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 272, 1347–1349 (1996).
Harding, H.P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Bertolotti, A., Zhang, Y., Hendershot, L.M., Harding, H.P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Harding, H.P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Harding, H.P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Plump, A.S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992).
Treiman, M. Regulation of the endoplasmic reticulum calcium storage during the unfolded protein response — significance in tissue ischemia? Trends Cardiovasc. Med. 12, 57–62 (2002).
Harding, H.P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Zhang, P. et al. The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell Biol. 22, 3864–3874 (2002).
McCullough, K.D., Martindale, J.L., Klotz, L.O., Aw, T.Y. & Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 21, 1249–1259 (2001).
Lange, Y. Tracking cell cholesterol with cholesterol oxidase. J. Lipid Res. 33, 315–321 (1992).
Lange, Y., Ye, J., Rigney, M. & Steck, T.L. Cholesterol movement in Niemann-Pick Type C cells and in cells treated with amphiphiles. J. Biol. Chem. 275, 17468–17475 (2000).
Lange, Y. & Steck, T.L. Quantitation of the pool of cholesterol associated with acyl-CoA:cholesterol acyltransferase in human fibroblasts. J. Biol. Chem. 272, 13103–13108 (1997).
Graham, J.M. & Green, C. The properties of mitochondria enriched in vitro with cholesterol. Eur. J. Biochem. 12, 58–66 (1970).
Ross, A.C., Go, K.J., Heider, J.G. & Rothblat, G.H. Selective inhibition of acyl coenzyme A:cholesterol acyltransferase by compound 58–035. J. Biol. Chem. 259, 815–819 (1984).
Basu, S.K., Goldstein, J.L., Anderson, R.G.W. & Brown, M.S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl Acad. Sci. USA 73, 3178–3182 (1976).
Tabas, I., Boykow, G. & Tall, A. Rabbit liver microsomal ACAT: Smooth ER enzyme associated with a lipid ACAT inhibitor. Arteriosclerosis 8, 559A (1988).
Lowry, O.H., Rosenbrough, N.J., Farr, A.L. & Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Trogan, E. et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 99, 2234–2239 (2002).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants HL54591, HL57560 and HL56984 to I.T, DK47119 and ES08681 to D.R. and HL61814 to E.A.F. We gratefully acknowledge R. Soccio and F. Wang for assistance with the CHOP Taqman and in-situ hybridization assays, respectively. We also thank Y. Zhang for technical assistance and R. Jungreis for help with the Perk−/− and Chop−/− mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Feng, B., Yao, P., Li, Y. et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5, 781–792 (2003). https://doi.org/10.1038/ncb1035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1035
This article is cited by
-
Diallyl disulfide alleviates hypercholesterolemia induced by a western diet by suppressing endoplasmic reticulum stress in apolipoprotein E-deficient mice
BMC Complementary Medicine and Therapies (2023)
-
Anti-inflammation-based treatment of atherosclerosis using Gliclazide-loaded biomimetic nanoghosts
Scientific Reports (2023)
-
A Lipid-Structured Model of Atherosclerotic Plaque Macrophages with Lipid-Dependent Kinetics
Bulletin of Mathematical Biology (2023)
-
ORP5 promotes migration and invasion of cervical cancer cells by inhibiting endoplasmic reticulum stress
Cell Stress and Chaperones (2023)
-
Metabolism of tissue macrophages in homeostasis and pathology
Cellular & Molecular Immunology (2022)