Abstract
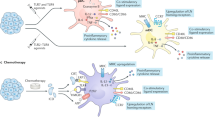
After stimulation, dendritic cells (DCs) mature and migrate to draining lymph nodes to induce immune responses1. As such, autologous DCs generated ex vivo have been pulsed with tumour antigens and injected back into patients as immunotherapy. While DC vaccines have shown limited promise in the treatment of patients with advanced cancers2,3,4 including glioblastoma5,6,7, the factors dictating DC vaccine efficacy remain poorly understood. Here we show that pre-conditioning the vaccine site with a potent recall antigen such as tetanus/diphtheria (Td) toxoid can significantly improve the lymph node homing and efficacy of tumour-antigen-specific DCs. To assess the effect of vaccine site pre-conditioning in humans, we randomized patients with glioblastoma to pre-conditioning with either mature DCs8 or Td unilaterally before bilateral vaccination with DCs pulsed with Cytomegalovirus phosphoprotein 65 (pp65) RNA. We and other laboratories have shown that pp65 is expressed in more than 90% of glioblastoma specimens but not in surrounding normal brain9,10,11,12, providing an unparalleled opportunity to subvert this viral protein as a tumour-specific target. Patients given Td had enhanced DC migration bilaterally and significantly improved survival. In mice, Td pre-conditioning also enhanced bilateral DC migration and suppressed tumour growth in a manner dependent on the chemokine CCL3. Our clinical studies and corroborating investigations in mice suggest that pre-conditioning with a potent recall antigen may represent a viable strategy to improve anti-tumour immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007)
Palucka, A. K. et al. Spontaneous proliferation and type 2 cytokine secretion by CD4+T cells in patients with metastatic melanoma vaccinated with antigen-pulsed dendritic cells. J. Clin. Immunol. 25, 288–295 (2005)
Palucka, A. K. et al. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J. Immunother. 26, 432–439 (2003)
Palucka, A. K. et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J. Immunother. 29, 545–557 (2006)
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nature Rev. Cancer 12, 265–277 (2012)
Liau, L. M. et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 11, 5515–5525 (2005)
Yu, J. S. et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 64, 4973–4979 (2004)
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003)
Dziurzynski, K. et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 14, 246–255 (2012)
Ranganathan, P., Clark, P. A., Kuo, J. S., Salamat, M. S. & Kalejta, R. F. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J. Virol. 86, 854–864 (2012)
Mitchell, D. A. et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 10, 10–18 (2008)
Cobbs, C. S. et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62, 3347–3350 (2002)
Myers, M. G., Beckman, C. W., Vosdingh, R. A. & Hankins, W. A. Primary immunization with tetanus and diphtheria toxoids. Reaction rates and immunogenicity in older children and adults. J. Am. Med. Assoc. 248, 2478–2480 (1982)
Stupp, R. et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 352, 987–996 (2005)
Curran, W. J., Jr et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J. Natl Cancer Inst. 85, 704–710 (1993)
Gorlia, T. et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol. 9, 29–38 (2008)
Nakano, H. & Gunn, M. D. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 166, 361–369 (2001)
Zhang, Y. et al. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1α in mice. J. Natl Cancer Inst. 96, 201–209 (2004)
He, S. et al. MIP-3α and MIP-1α rapidly mobilize dendritic cell precursors into the peripheral blood. J. Leukoc. Biol. 84, 1549–1556 (2008)
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440, 890–895 (2006)
McLendon, R. E. et al. Immunohistochemical detection of the DNA repair enzyme O6-methylguanine-DNA methyltransferase in formalin-fixed, paraffin-embedded astrocytomas. Lab. Invest. 78, 643–644 (1998)
Nair, S., Archer, G. E. & Tedder, T. F. Isolation and generation of human dendritic cells. 99, 7.32:7.32.1–7.32.23 Curr. Protoc. Immunol. (2012)
Thurner, B. et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223, 1–15 (1999)
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000)
Inaba, K., Swiggard, W. J., Steinman, R. M., Romani, N. & Schuler, G. Isolation of dendritic cells. Curr. Protoc. Immunol. 25, 3.7:3.7.1–3.7.15 (2001)
Nakano, H. et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nature Immunol. 10, 394–402 (2009)
Sanchez-Perez, L. et al. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 65, 2009–2017 (2005)
Daniels, G. A. et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nature Biotechnol. 22, 1125–1132 (2004)
Fidler, I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 35, 218–224 (1975)
Acknowledgements
The authors thank the staff who supported this study, including R. Schmittling, P. Norberg, W. Xie, P. Healy, D. Lally-Goss, S. McGehee-Norman, B. Perry, S. Snipes and R. Edward Coleman. This work was supported by grants from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Specialized Program of Research Excellence in brain cancer (P50-CA108786, D.D.B. and J.H.S.) and SRC on Primary and Metastatic Tumors of the CNS (P50-NS20023, D.D.B. and J.H.S.) as well as NIH RO1 (R01-CA177476-01, J.H.S.; R01-NS067037, R01-CA134844, D.A.M.) and P01 (P01-CA154291-01A1, D.D.B. and J.H.S.) funding sources. Additional support is from the National Brain Tumor Society (D.A.M. and J.H.S.), the American Brain Tumor Association (D.A.M. and J.H.S.), Accelerate Brain Cancer Cure Foundation Young Investigator’s Award (D.A.M.), The Kinetics Foundation, (J.H.S.) Ben and Catherine Ivy Foundation (J.H.S.), and in part by Duke University’s Clinical & Translational Science Awards grant 1UL2 RR024128-01 from the National Institutes of Health National Center for Research Resources.
Author information
Authors and Affiliations
Contributions
D.A.M., G.E.A. and J.H.S. jointly conceived and implemented the clinical study; D.A.M. and K.A.B. jointly designed early DC migration studies in mice. K.A.B. conceived and designed the remainder of the mouse research; A.D., A.H.F., H.S.F., R.E.M., D.A.R., J.J.V., D.D.B. and J.H.S. were responsible for provision of clinical study resources, materials and patient access. S.K.N., E.A.R. and G.E.A. prepared human samples and conducted human in vitro experiments; K.A.B. performed all preclinical experiments and patient analyses; M.-N.H. provided additional support for preclinical experiments. D.A.M., K.A.B., M.D.G., K.L.C., E.A.R., G.E.A. and J.H.S. performed data analysis and interpretation; J.E.H. and A.C. provided statistical support for design and analysis of human and mouse studies. D.A.M., D.D.B. and J.H.S. contributed laboratory reagents and tools; and D.A.M., K.A.B., M.D.G., K.L.C. and J.H.S. wrote the paper. All authors gave their final approval to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.A.M., K.A.B. and J.H.S. have filed provisional patents related to the use of Td pre-conditioning as a method to improve immunization efficacy. D.A.M. has served as a paid member of the Schering Plough North American Investigators Advisory Board. S.K.N. is a co-inventor on a patent that describes the use of DCs transfected with tumour antigen encoding RNA that has been licensed by Argos Therapeutics through Duke University. S.K.N. has no financial interests in Argos Therapeutics and is not compensated by Argos Therapeutics. D.A.R. has served as paid speaker for Schering/Merck and Genentech/Roche. The remaining authors declare no competing financial interests.
Extended data figures and tables
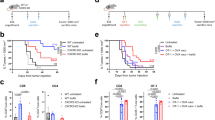
Extended Data Figure 1 Schema of clinical trial.
SPECT/CT, single photon emission computed tomography/computed tomography; TMZ, temozolomide; XRT, external beam radiotherapy.
Extended Data Figure 2 Recall responses induced by other CD4+ T-cell-dependent protein antigens increase DC migration to VDLNs.
Primary immunization and vaccine site pre-conditioning with CD4+ T-cell-dependent protein antigens increase DC migration to VDLNs. Mice were immunized with either Haemophilus b conjugate (Hib) or pneumococcal 13-valent conjugate (PCV) intramuscularly, and 2 weeks later received vaccine site pre-conditioning with the recall antigen (recall) or saline (control). A separate cohort of mice received saline only throughout the immunization schedule (saline). Scatter plot shows biological replicates of individually processed right and left iLN per mouse (4 mice per group). Percentage migration of RFP+ DCs to VDLNs; one-way ANOVA, P < 0.0001; post-hoc Tukey t-test, PCV control versus recall, P < 0.05, Hib control versus recall, P < 0.05. Representative of n = 3 experiments; mean ± s.e.m.
Extended Data Figure 3 Bilateral migration of OVA-DCs after Td pre-conditioning or Td-activated CD4+ T-cell transfer.
Uptake of injected DCs to right and left iLNs 48 h after DC vaccination in Td-immune mice receiving Td pre-conditioning or naive mice administered Td-activated CD4+ T cells. Scatter plot shows biological replicates of individually processed right and left iLN per mouse (5 mice per group). CD4act ipsilateral versus contralateral, paired t-test, P = 0.41. Representative of n = 4 experiments; mean ± s.e.m.
Extended Data Figure 4 Unilateral pre-conditioning with unpulsed DCs or TNF-α results in increased DC homing to ipsilateral draining inguinal lymph nodes.
Td-immune mice pre-conditioned with Td or saline before administration of OVA RNA-pulsed DC vaccine. Separate cohorts of naive mice received either 1 × 106 unpulsed DCs or 30 ng TNF-α on one side of the groin 24 h before the bilateral RFP+ DC vaccine. DC migration was quantified 24 h after vaccination. a, DC migration to ipsilateral lymph nodes (one-way ANOVA, P = 0.0018; post-hoc Tukey t-test, saline i.d. versus Td i.d., P = 0.007; saline i.d. versus TNF-α, P < 0.05; saline i.d. versus DCs, P < 0.05; Td i.d. versus TNF-α, P = 0.042; DCs versus Td i.d. and DCs versus TNF-α, P > 0.05. b, DC migration to contralateral lymph nodes; one-way ANOVA, P = 0.003; post-hoc Tukey t-test, saline i.d. versus DCs or TNF-α, P > 0.05; Td i.d. versus TNF-α, DCs or saline i.d., P < 0.05. n values are biological replicates of individually processed right and left iLNs per mouse (4 mice per group). Representative of n = 3 experiments; mean ± s.e.m.
Extended Data Figure 5 Serum cytokine and chemokine profile after Td pre-conditioning in patients and mice.
a, Serum cytokine panel of patients following vaccine site pre-conditioning with Td or unpulsed DCs; Wilcoxon rank sum, IFN-γ and IL-4, P < 0.05 (n = 6 patients). b, Similar to a in mice; Wilcoxon rank sum, all comparisons, P > 0.05 (Td recall n = 5, non-Td n = 6). c, Patient serum chemokines after vaccine site pre-conditioning. Patient CCL2 and CCL3 in Td recall (Td, n = 6) versus non-Td (unpulsed DC, n = 5); one-way ANOVA and Wilcoxon rank sum, P < 0.05. d, Mouse CCL22, CCL7 and CCL3 in Td recall (Td, n = 8 mice) and non-Td (saline, n = 8 mice); one-way ANOVA and Wilcoxon rank sum, P < 0.05. For a–d, individual values represent biological replicates; mean ± s.e.m.
Extended Data Figure 6 Td vaccine site pre-conditioning results in CCL3 upregulation in Td-immune hosts.
a, CCL3 production in skin site after Td pre-conditioning (Td ipsilateral versus contralateral). Representative of four independent experiments. b, CCL3 production in skin after Td recall response. c, CCL3 induction at skin site is abrogated with previous host depletion of CD4+ T cells. Bars in a–c represent CCL3 protein detected in skin sites from n = 2 mice with n = 2 technical replicates performed per mouse. d, CCL3 remains increased at the Td pre-conditioning site in the skin after DC vaccination (24, 48 and 72 h, one-way ANOVA, P = 0.0001, Td plus Td i.d. and OVA-DC versus Td plus saline i.d. and OVA-DC, and saline plus saline i.d. and OVA-DC, P < 0.05, post-hoc Tukey t-test). Individual values represent biological replicates from n = 4 mice; mean ± s.e.m.
Extended Data Figure 7 Migratory DC subsets in wild-type and Ccl3−/− mice after induction of Td recall responses.
Both wild-type and Ccl3−/− mice were first immunized with Td and then challenged with Td pre-conditioning. Migration of endogenous DC subsets to inguinal lymph nodes contralateral to the site of Td pre-conditioning was assessed at days 4 and 8 after Td administration. a, Gating strategy used to quantify DC subsets in inguinal lymph nodes after skin pre-conditioning with Td. LC, Langerhans cells; MoDC, monocyte-derived DCs. b, Day-8 migration of LC population to non-draining inguinal lymph nodes in Ccl3−/− hosts is reduced in absence of CCL3; two-sample t-test, P = 0.046. Representative of three experiments. Individual values represent biological replicates from n = 4 mice; mean ± s.e.m.
Extended Data Figure 8 Anti-tetanus toxoid memory responses are induced and maintained in wild-type and Ccl3−/− mice throughout Td priming and boosting.
Wild-type and Ccl3−/− mice primed and boosted with Td. Serum from immunized mice was collected 2 weeks after each immunization before the next booster vaccine (for each boosting phase, wild-type versus Ccl3−/−, two-sample t-test, P > 0.05). i.m., intramuscular. Scatter plot showing averaged values from n = 4 mice with n = 2 technical replicates performed per mouse. Representative of three experiments; mean ± s.e.m.
Extended Data Figure 9 CCL21 levels in Td pre-conditioning skin sites and draining lymph nodes of wild-type and Ccl3−/− mice.
a, CCL21 levels in skin site of Ccl3−/− hosts after induction of Td recall response and CCL3 administration. Mixed model accounting for within-mouse correlation of measurements, F-test, P < 0.001; pairwise comparisons, WT Td plus Td i.d. (n = 4) versus Ccl3−/− Td plus Td i.d. (n = 3), P = 0.049; Ccl3−/− Td plus Td i.d. and CCL3 i.v. (n = 4) versus Ccl3−/− Td plus Td i.d. and versus Ccl3−/− plus CCL3 i.v. (n = 4), P = 0.044 and P = 0.0045, respectively. Scatter plot shows averaged values with n = 2 technical replicates performed per mouse. b, Bilateral iLN CCL21 levels in wild-type mice after Td recall with skin site pre-conditioning. Bars represent CCL21 protein within iLN ipsilateral and contralateral to the side of Td pre-conditioning from n = 2 mice with n = 2 technical replicates performed per iLN. Representative of three experiments. c, Increased lymph node CCL21 in Ccl3−/− hosts after CCL3 reconstitution and induction of Td recall response (all two group comparisons). CCL21 levels in bilateral iLNs of Ccl3−/− hosts after induction of Td recall response and CCL3 administration. Mixed model accounting for within-mouse correlation of measurements, F-test, P < 0.001; pairwise comparisons, WT Td plus Td i.d. (n = 4 iLNs) versus Ccl3−/− Td plus Td i.d. (n = 3 iLNs), P = 0.045; Ccl3−/− Td plus Td i.d. and CCL3 i.v. (n = 4 iLNs) versus Ccl3−/− Td plus Td i.d. and versus Ccl3−/− plus CCL3 i.v. (n = 4 iLNs), P = 0.0066 and P = 0.026, respectively. Scatter plot shows averaged values with n = 2 technical replicates performed per lymph node sampled. In a and c, mean ± s.e.m.
Source data
Rights and permissions
About this article
Cite this article
Mitchell, D., Batich, K., Gunn, M. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369 (2015). https://doi.org/10.1038/nature14320
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14320
This article is cited by
-
Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma
Cell Communication and Signaling (2023)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
-
Advancing personalized medicine in brain cancer: exploring the role of mRNA vaccines
Journal of Translational Medicine (2023)
-
Advances in blood–brain barrier-crossing nanomedicine for anti-glioma
Cancer Nanotechnology (2023)
-
Novel Immunotherapeutic Approaches for the Treatment of Glioblastoma
BioDrugs (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.