Abstract
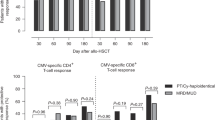
Cytomegalovirus (CMV) infection is a common, potentially life-threatening complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT). We assessed prospectively the safety and efficacy of stem cell-donor- or third-party-donor-derived CMV-specific T cells for the treatment of persistent CMV infections after allo-HSCT in a phase I/IIa trial. Allo-HSCT patients with drug-refractory CMV infection and lacking virus-specific T cells were treated with a single dose of ex vivo major histocompatibility complex-Streptamer-isolated CMV epitope-specific donor T cells. Forty-four allo-HSCT patients receiving a T-cell-replete (D+ repl; n=28) or T-cell-depleted (D+ depl; n=16) graft from a CMV-seropositive donor were screened for CMV-specific T-cell immunity. Eight D+ depl recipients received adoptive T-cell therapy from their stem cell donor. CMV epitope-specific T cells were well supported and became detectable in all treated patients. Complete and partial virological response rates were 62.5% and 25%, respectively. Owing to longsome third-party donor (TPD) identification, only 8 of the 57 CMV patients transplanted from CMV-seronegative donors (D−) received antigen-specific T cells from partially human leukocyte antigen (HLA)-matched TPDs. In all but one, TPD-derived CMV-specific T cells remained undetectable. In summary, adoptive transfer correlated with functional virus-specific T-cell reconstitution in D+ depl patients. Suboptimal HLA match may counteract expansion of TPD-derived virus-specific T cells in D− patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant 2014; 49: 744–750.
Zhou W, Longmate J, Lacey SF, Palmer JM, Gallez-Hawkins G, Thao L et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood 2009; 113: 6465–6476.
Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010; 116: 4360–4367.
Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995; 333: 1038–1044.
Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med 2005; 202: 379–386.
Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Löffler J et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99: 3916–3922.
Stemberger C, Graef P, Odendahl M, Albrecht J, Dössinger G, Anderl F et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood 2014; 124: 628–637.
Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 2011; 52: 49–57.
Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med 2002; 8: 631–637.
Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 2011; 51: 591–599.
Odendahl M, Grigoleit GU, Bönig H, Neuenhahn M, Albrecht J, Anderl F et al. Clinical-scale isolation of ‘minimally manipulated’ cytomegalovirus-specific donor lymphocytes for the treatment of refractory cytomegalovirus disease. Cytotherapy 2014; 16: 1245–1256.
Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity 2007; 27: 985–997.
Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 2014; 41: 116–126.
Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Gräf P et al. Disparate individual fates compose robust CD8+ T cell immunity. Science 2013; 340: 630–635.
Restifo NP . Big bang theory of stem-like T cells confirmed. Blood 2014; 124: 476–477.
Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007; 110: 1123–1131.
Uhlin M, Gertow J, Uzunel M, Okas M, Berglund S, Watz E et al. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis 2012; 55: 1064–1073.
Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010; 116: 5045–5049.
Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013; 121: 5113–5123.
Dössinger G, Bunse M, Bet J, Albrecht J, Paszkiewicz PJ, Weissbrich B et al. MHC multimer-guided and cell culture-independent isolation of functional T cell receptors from single cells facilitates TCR identification for immunotherapy. PLoS ONE 2013; 8: e61384.
Koehne G, Hasan A, Doubrovina E, Prockop S, Tyler E, Wasilewski G et al. Immunotherapy with donor T cells sensitized with overlapping pentadecapeptides for treatment of persistent cytomegalovirus infection or viremia. Biol Blood Marrow Transplant 2015; 21: 1663–1678.
Blyth E, Clancy L, Simms R, Ma CKK, Burgess J, Deo S et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013; 121: 3745–3758.
Peggs KS, Verfuerth S, Pizzey A, Chow S-LC, Thomson K, Mackinnon S . Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis 2009; 49: 1851–1860.
Feucht J, Opherk K, Lang P, Kayser S, Hartl L, Bethge W et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015; 125: 1986–1994.
Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2011; 11: 284–292.
Boeckh M, Geballe AP . Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 2011; 121: 1673–1680.
Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015; 125: 2677–2689.
Stemberger C, Dreher S, Tschulik C, Piossek C, Bet J, Yamamoto TN et al. Novel serial positive enrichment technology enables clinical multiparameter cell sorting. PLoS ONE 2012; 7: e35798.
Eiz-Vesper B, Maecker-Kolhoff B, Blasczyk R . Adoptive T-cell immunotherapy from third-party donors: characterization of donors and set up of a T-cell donor registry. Front Immunol 2012; 3: 410.
Acknowledgements
This work was supported by the Federal Ministry of Education and Research and the SFB (Sonderforschungsbereich/Collaborative Research Centre) TR36 (TP-A10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LG is an employee of and holds shares in Stage Cell Therapeutics, Göttingen, Germany; now Juno Therapeutics GmbH, Munich, Germany. DHB invented the Streptamer technology and holds shares of Juno Cell Therapeutics Inc. The other authors declare no conflict of interest.
Additional information
Author contributions
MN, JA, MO, GD, FS, SL, HB, TT, KM and MS performed purification or monitoring analyses; JA, MN and GUG analyzed the data; UG, DHB, HE and LG conceived the study; MN, MO, JA and DHB planned the monitoring experiments; GUG, DHB, SH, EMW, HM, MV, LU, NK, EW, GK, MS and GH were responsibly involved in patient treatment; DHB, HE, TT, HB and LG performed and supervised the clinical cell selection; MN, JA, HB, HE, GUG and DHB wrote the paper.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Neuenhahn, M., Albrecht, J., Odendahl, M. et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 31, 2161–2171 (2017). https://doi.org/10.1038/leu.2017.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.16
This article is cited by
-
Comparable anti-CMV responses of transplant donor and third-party CMV-specific T cells for treatment of CMV infection after allogeneic stem cell transplantation
Cellular & Molecular Immunology (2022)
-
Features of repertoire diversity and gene expression in human cytotoxic T cells following allogeneic hematopoietic cell transplantation
Communications Biology (2021)
-
In-depth summary over cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation recipients
VirusDisease (2021)
-
Epstein–Barr virus and cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation in patients with non–Hodgkin lymphoma: the prevalence and impacts on outcomes
Annals of Hematology (2021)
-
CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies
Current Treatment Options in Infectious Diseases (2021)