Abstract
In the phase 3 Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) study, nilotinib resulted in earlier and higher response rates and a lower risk of progression to accelerated phase/blast crisis (AP/BC) than imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). Here, patients’ long-term outcomes in ENESTnd are evaluated after a minimum follow-up of 5 years. By 5 years, more than half of all patients in each nilotinib arm (300 mg twice daily, 54%; 400 mg twice daily, 52%) achieved a molecular response 4.5 (MR4.5; BCR-ABL⩽0.0032% on the International Scale) compared with 31% of patients in the imatinib arm. A benefit of nilotinib was observed across all Sokal risk groups. Overall, safety results remained consistent with those from previous reports. Numerically more cardiovascular events (CVEs) occurred in patients receiving nilotinib vs imatinib, and elevations in blood cholesterol and glucose levels were also more frequent with nilotinib. In contrast to the high mortality rate associated with CML progression, few deaths in any arm were associated with CVEs, infections or pulmonary diseases. These long-term results support the positive benefit-risk profile of frontline nilotinib 300 mg twice daily in patients with CML-CP.
Similar content being viewed by others
Introduction
Nilotinib is a tyrosine kinase inhibitor (TKI) approved for the treatment of adult patients with newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) or imatinib-resistant or imatinib-intolerant Ph+ CML in CP or accelerated phase (AP).1, 2 Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) is a randomized phase 3 study evaluating nilotinib 300 or 400 mg twice daily vs imatinib 400 mg once daily in patients with newly diagnosed CML-CP.2, 3, 4, 5 The primary endpoint of ENESTnd, the rate of major molecular response (MMR; BCR-ABL⩽0.1% on the International Scale (BCR-ABLIS)) at 12 months, was met, with significantly higher rates of MMR at 12 months in the nilotinib 300-mg (44%) and 400-mg (43%) twice-daily arms than in the imatinib arm (22%; P <0.001 vs each nilotinib arm).2
Throughout the first 4 years of ENESTnd, nilotinib resulted in earlier and higher rates of molecular response than imatinib and was associated with a lower risk of progression to AP or blast crisis (BC).2, 3, 4, 5 Nilotinib also resulted in higher rates of early molecular response (EMR; BCR-ABLIS⩽10%) at 3 months,5 an important early indicator of efficacy,6, 7 and fewer treatment-emergent BCR-ABL mutations than imatinib.8
Because CML-CP is a chronic disease and patients receive TKI therapy indefinitely,6, 7 the long-term safety of treatment must be considered. As patients age, concurrent illnesses may develop or preexisting conditions may progress and become clinically apparent;9, 10, 11 in addition, long-term TKI therapy can lead to the development of different types of adverse events (AEs) from those seen soon after initiating therapy.6 Thus, with multiple BCR-ABL TKIs available,6, 7 physicians are called on to choose the best therapy for each individual patient. Such decisions are informed by a detailed understanding of the distinct benefits and risks of each agent, along with careful consideration of patient-specific factors such as age and comorbidities.6
To enable a comprehensive evaluation of the long-term benefits and risks of nilotinib and imatinib for the treatment of patients with newly diagnosed CML-CP, here, we report updated results from ENESTnd based on a minimum follow-up of five calendar years, representing the full follow-up duration designated in the original study protocol. ENESTnd remains ongoing, with a planned follow-up of 10 years.
Patients and methods
Study design
Eligibility criteria and trial design have been described previously.2, 3, 4, 12 Briefly, adults (N=846) within 6 months of CML-CP diagnosis and without previous CML therapy (except hydroxyurea and/or anagrelide or ⩽2 weeks of imatinib) and an Eastern Cooperative Oncology Group performance status of ⩽2 were randomized (stratified by Sokal risk score at baseline) 1:1:1 to receive nilotinib 300 mg twice daily (n=282), nilotinib 400 mg twice daily (n=281) or imatinib 400 mg once daily (n=283). ENESTnd was designed to compare each nilotinib arm with the imatinib arm but was not powered to make comparisons between the two nilotinib arms. ENESTnd was conducted according to the principles of the Declaration of Helsinki and registered at ClinicalTrials.gov (NCT00471497). Written informed consent was obtained from each patient. The study protocol was approved by the appropriate review board or ethics committee for each center. The original trial design called for 5 years of follow-up; however, the study sponsor informed health authorities that the trial would be extended to 10 years to collect additional long-term data. The study remains ongoing, with an active management committee.
Endpoints and assessments
Long-term endpoints included rates of MMR, molecular response 4 (MR4; BCR-ABLIS⩽0.01%) and molecular response 4.5 (MR4.5; BCR-ABLIS⩽0.0032%); progression to accelerated phase/blast crisis (AP/BC); event-free survival (EFS); progression-free survival (PFS); overall survival (OS); and safety. Molecular responses were assessed by BCR-ABL/ABL transcript ratios using real-time quantitative polymerase chain reaction (RQ-PCR) at a central laboratory (MolecularMD, Portland, OR, USA) standardized to the International Scale (IS), as described in the Supplementary Methods. BCR-ABL mutations were assessed as described previously.8
Data on progression to AP/BC and survival were prospectively collected (including after discontinuation of study treatment) every 3 months for up to 5 years and then every 6 months (until up to 10 years after the date when the last patient started study treatment). Time to progression to AP/BC was defined as the time from randomization until progression to AP/BC or death due to advanced CML, whichever occurred first. PFS was defined as the time from randomization until progression to AP/BC or death from any cause. The rates of freedom from progression to AP/BC and of PFS on core treatment and on study were evaluated. Rates on core treatment considered only events that occurred during treatment with the assigned study drug; rates on study considered both events that occurred during study treatment and those that occurred during follow-up after discontinuation of study treatment. EFS was defined as the time from randomization until loss of complete hematologic response, loss of partial cytogenetic response, loss of complete cytogenetic response, progression to AP/BC or death from any cause. EFS on core treatment was evaluated; EFS on study could not be evaluated owing to the lack of access to data regarding the loss of hematologic or cytogenetic responses following discontinuation of treatment. OS was defined as the time from randomization until death due to any cause at any time (including during follow-up after discontinuation of study treatment). Death due to advanced CML was defined as any death (at any time) for which the principal cause was reported by the investigator as ‘study indication’ (that is, due to CML) or, if subsequent to documented progression to AP/BC, any death for which the cause was reported as ‘unknown’ or was not reported.
Additional details regarding assessments, safety analyses and evaluation of patients’ Framingham general cardiovascular risk scores are provided in the Supplementary Methods.
Statistical analysis
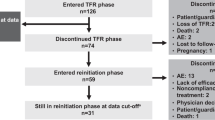
All analyses were conducted on the basis of a data cutoff date of 30 September 2013 (minimum follow-up of 5 years). Efficacy analyses included the intent-to-treat population (all randomized patients; Figure 1). Landmark analyses included only patients with typical BCR-ABL transcripts (b2a2 and/or b3a2)13, 14 at baseline, with evaluable 3-month RQ-PCR assessments, and without the analyzed outcome by 3 months. Safety analyses included all patients who received ⩾1 dose of study treatment (nilotinib 300 mg twice daily, n=279; nilotinib 400 mg twice daily, n=277; imatinib, n=280).
CONSORT diagram for ENESTnd 5-year analysis (data cutoff 30 September 2013). Efficacy analyses, including molecular and cytogenetic response rates, were based on all randomized patients (intent-to-treat population). Safety analyses were based on patients who received ⩾1 dose of study treatment. aReasons for discontinuation: AEs/abnormal laboratory values (n=34), suboptimal response/treatment failure (n=34), withdrawal of consent (n=17), death (n=6), disease progression (n=2), other (n=17). On discontinuation, 24 patients entered the extension study. bReasons for discontinuation: AEs/abnormal laboratory values (n=56), withdrawal of consent (n=16), suboptimal response/treatment failure (n=13), disease progression (n=4), death (n=1), other (n=15). On discontinuation, three patients entered the extension study. cReasons for discontinuation: suboptimal response/treatment failure (n=59), AEs/abnormal laboratory values (n=38), withdrawal of consent (n=17), disease progression (n=12), death (n=1), other (n=12). On discontinuation, 43 patients entered the extension study. dDiscontinued before receiving intervention owing to protocol deviation (n=1), withdrawal of consent (n=2). eDiscontinued before receiving intervention owing to protocol deviation (n=1), withdrawal of consent (n=2), QTc>450 ms at baseline (n=1). fOne patient allocated to nilotinib 400 mg twice daily received imatinib 400 mg once daily for 6 days before discontinuing intervention and was excluded from safety analysis for nilotinib 400 mg twice daily. gDiscontinued before receiving intervention owing to protocol deviation (n=2), withdrawal of consent (n=1), QTc>450 ms at baseline (n=1). hOne patient allocated to nilotinib 400 mg twice daily received imatinib 400 mg once daily for 6 days before discontinuing intervention and was included in safety analysis for imatinib 400 mg once daily. iOwing to atypical transcripts at baseline (n=5), discontinuation prior to the month 3 assessment (n=15), or missing month 3 assessment (n=4). jOwing to atypical transcripts at baseline (n=1), discontinuation prior to the month 3 assessment (n=17), or missing month 3 assessment (n=3). kOwing to atypical transcripts at baseline (n=2), discontinuation prior to the month 3 assessment (n=12), or missing month 3 assessment (n=5).
Time-to-response graphs are presented as cumulative incidence. Patients who achieved a response at or before each time point were considered responders by that time point. Patients with atypical transcripts (that is, transcripts other than b2a2 and/or b3a2) at baseline (nilotinib 300 mg twice daily, n=5; nilotinib 400 mg twice daily, n=1; imatinib, n=2)2 or missing RQ-PCR assessments were considered nonresponders for molecular response rates. Response rates were compared using the Cochran-Mantel-Haenszel test stratified by Sokal risk group. Clopper-Pearson 95% two-sided confidence intervals (CIs) for response rates were also presented. Time-to-event variables were analyzed using the Kaplan–Meier method and were compared between groups using log-rank tests stratified by Sokal risk group. Hazard ratios and 95% two-sided CIs were derived from a Cox model stratified by Sokal risk group; 95% CIs for Kaplan–Meier estimates were derived using the standard error calculated with Greenwood’s formula. Nominal two-sided P values, when provided, are for descriptive purposes only without multiplicity adjustments; therefore, no formal statistical claim can be made and statistical interpretation should be made with caution.
Results
Five-year outcomes
At the data cutoff, 169 (59.9%), 174 (61.9%) and 141 (49.8%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively, remained on core treatment; more than 80% of patients in each arm remained on study (either on treatment or in follow-up after discontinuation of study treatment; Supplementary Table 1).
By 5 years, 217 (77.0%; 95% CI, 71.6–81.7%), 217 (77.2%; 95% CI, 71.9–82.0%) and 171 (60.4%; 95% CI, 54.5–66.2%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively, achieved MMR (Figure 2a); among patients in each arm who achieved MMR at any time before the data cutoff, 13 of 218 (6.0%), 16 of 220 (7.3%) and 17 of 173 (9.8%), respectively, had a confirmed loss of first MMR (7, 9 and 10 of these patients, respectively, later regained MMR). Very few patients without MMR by 5 years remained on core treatment at the data cutoff (5, 9 and 10 patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively, including 3, 0 and 2, respectively, with atypical transcripts at baseline).
The frequency of deep molecular responses by 5 years was higher with nilotinib than with imatinib (Figures 2b and c). In the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, 185 (65.6%; 95% CI, 59.7–71.1%), 177 (63.0; 95% CI, 57.1–68.6%) and 118 (41.7%; 95% CI, 35.9–47.7%) patients achieved MR4 by 5 years, respectively, and 151 (53.5%; 95% CI, 47.5–59.5%), 147 (52.3%; 95% CI, 46.3–58.3%) and 89 (31.4%; 95% CI, 26.1–37.2%) achieved MR4.5, respectively. The differences between the cumulative rates of MR4.5 in patients treated with nilotinib vs imatinib grew with each year of follow-up. Compared with the imatinib arm, the rate of MR4.5 in the nilotinib 300-mg twice-daily arm was 16.7% higher (representing 47 more patients with MR4.5 in the nilotinib 300-mg twice-daily arm vs the imatinib arm) by 3 years, 17.1% (48 patients) higher by 4 years, and 22.1% (62 patients) higher by 5 years.
The number of progressions to AP/BC by the data cutoff was lower in both nilotinib arms vs the imatinib arm when considering either progressions occurring during treatment with the assigned study drug (that is, progressions on core treatment) or all progressions at any time during follow-up, including after discontinuation of study treatment (that is, progressions on study; Table 1). No patient in any arm has progressed to AP/BC on core treatment since the 2-year analysis. Progression to AP/BC after discontinuation of core treatment was reported in three patients between the 4-year and 5-year data cutoffs,5 including one patient in the nilotinib 300-mg twice-daily arm (progression reported ⩽28 days after discontinuation of nilotinib due to treatment failure (treatment-emergent T315I BCR-ABL mutation)) and two patients in the imatinib arm (progression for both patients reported >3 years after discontinuation of imatinib due to AE (one patient) or treatment failure (treatment-emergent D276G BCR-ABL mutation; one patient)). All three patients had high Sokal risk scores at baseline and BCR-ABLIS>10% at 3 months.
Overall, 18, 10 and 22 deaths were reported in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively. CML as a cause of death was more common in the imatinib arm (n=16) than in the nilotinib arms (nilotinib 300-mg twice-daily, n=6; nilotinib 400-mg twice-daily, n=4). Other causes of death are shown in Figure 3. Of 50 patients who died by the data cutoff, cardiovascular events (CVEs) were reported shortly before death in three patients: two in the nilotinib 300-mg twice-daily arm and one in the imatinib arm (death occurred within 3 months of a reported CVE during study treatment in all three patients). No patient in the nilotinib 400-mg twice-daily arm died within 3 months of a reported CVE during study treatment. Considering all causes of death, 5-year PFS and OS rates were highest in the nilotinib 400-mg twice-daily arm (Figure 4).
Summary of deaths on study by treatment arm. aThe presence/absence of cardiovascular events (CVEs) was collected during treatment (core or extension) only. bDeath due to advanced chronic myeloid leukemia (CML) was defined as any death for which the principal cause was reported by the investigator as ‘study indication’ or, if subsequent to documented progression to accelerated phase/blast crisis (AP/BC), any death for which the cause was reported as ‘unknown’ or was not reported. cOne patient randomized to imatinib who died prior to receiving treatment is not shown. CABG, coronary artery bypass grafting.
Treatment-emergent BCR-ABL mutations were detected in 12 (4.3%), 11 (3.9%) and 22 (7.8%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively, including 4 (1.4%), 2 (0.7%) and 3 (1.1%) patients, respectively, with the BCR-ABL T315I mutation. All but two BCR-ABL mutations (one in the nilotinib 300-mg twice-daily arm (T315I) and one in the imatinib arm (F317L)) were detected by the 3-year data cutoff and were previously described in detail;8 both of the patients with a treatment-emergent BCR-ABL mutation detected after the 3-year data cutoff had high Sokal risk scores at baseline.
Nilotinib was associated with better 5-year outcomes than imatinib across all Sokal risk groups (Table 2). Among patients with low, intermediate and high Sokal risk, the rates of MR4.5 by 5 years in the nilotinib arms were 16.9–25.6%, 17.3–27.7% and 19.2–21.8% higher, respectively, than the corresponding rates in the imatinib arm. In all three arms, progression to AP/BC occurred most frequently in the high Sokal risk group; in the low and intermediate Sokal risk groups, no progressions to AP/BC have been reported in any arm since year 2. PFS and OS according to Sokal risk score are shown in Supplementary Figures 1 and 2. As previously reported,8 treatment-emergent BCR-ABL mutations were detected most frequently in patients with high Sokal risk scores.
Rates of EMR and BCR-ABLIS⩽1% at 3 months were higher in the nilotinib arms than in the imatinib arm.5 Within each arm, rates of MR4.5 by 5 years and PFS and OS at 5 years were higher for patients with EMR or BCR-ABLIS⩽1% at 3 months than for patients with BCR-ABLIS>10% at 3 months (Supplementary Table 2). Treatment-emergent BCR-ABL mutations were detected in a larger proportion of patients with BCR-ABLIS>10% at 3 months (3/24 (12.5%), 2/28 (7.1%) and 14/88 (15.9%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively) vs EMR at 3 months (9/234 (3.8%), 9/232 (3.9%) and 8/176 (4.5%), respectively).
Safety
The total frequencies of patients with grade 3/4 AEs, serious AEs and AEs leading to discontinuation of study treatment were comparable in the nilotinib 300-mg twice-daily (169 (60.6%), 72 (25.8%) and 34 (12.2%), respectively) and imatinib (165 (58.9%), 71 (25.4%) and 39 (13.9%), respectively) arms, and slightly higher in the nilotinib 400-mg twice-daily arm (198 (71.5%), 91 (32.9%) and 55 (19.9%), respectively). The most common nonhematologic AEs of any cause in both nilotinib arms were rash and headache; in the imatinib arm, diarrhea and nausea were most common (Table 3). In all three treatment arms, most nonhematologic AEs were grade 1/2.
Medically severe fluid retention (namely, peripheral edema (vast majority of events in all arms), fluid retention, pleural effusion, pericardial effusion, pulmonary edema and cardiac tamponade (not reported in any arm)) was less common with nilotinib (300 mg twice daily, 11.1%; 400 mg twice daily, 14.4%) than with imatinib (23.2%). Second malignancies were reported in 4.7%, 3.2% and 3.2% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively; specific types of second malignancies reported in each arm are listed in Supplementary Table 3. Pancreatitis and symptomatic QT prolongation were infrequent (<3% of patients in each arm). Hypertension was reported in 10.4%, 8.3% and 4.3% of patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily, and imatinib arms, respectively. Very few patients in any arm developed pulmonary hypertension (n=0 (nilotinib 300-mg twice-daily), 2 (nilotinib 400-mg twice-daily) and 0 (imatinib)) or AEs related to venous thrombosis or embolism, including retinal vein occlusion (n=1, 0 and 0, respectively), thrombophlebitis (n=1, 3 and 0, respectively), superficial thrombophlebitis (n=0, 1 and 0, respectively) and deep venous thrombosis (n=0, 1 and 1, respectively). No case of pulmonary embolism was reported in any arm. CVEs, namely, ischemic heart disease, ischemic cerebrovascular events and/or peripheral artery disease were reported in 21 (7.5%), 37 (13.4%) and 6 (2.1%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively. Within each arm, the cumulative frequency of patients with CVEs increased linearly with time on treatment (Figure 5).
Kaplan–Meier estimated time to first cardiovascular event (CVE) on study. The shaded portion of the graph shows censored data and events that occurred after 60 months of treatment and up to the data cutoff date. Because patients had variable follow-up durations beyond the 60-month time point at the data cutoff for this exploratory analysis, the shaded portion of the graph does not fully reflect outcomes after 60 months of treatment exposure.
To probe for the impact of preexisting cardiovascular risk on the development of CVEs during nilotinib therapy, baseline Framingham general cardiovascular risk scores15 were calculated for all evaluable patients (nilotinib 300 mg twice daily, n=259; nilotinib 400 mg twice daily, n=266; imatinib, n=264). The majority of patients had scores placing them in the low-risk category (that is, <10% predicted risk of experiencing a first cardiovascular disease event over 10 years (per Framingham Heart Study definition15); nilotinib 300 mg twice daily, 178 (68.7%); nilotinib 400 mg twice daily, 176 (66.2%); imatinib, 182 (68.9%)); smaller proportions of patients had scores placing them in the intermediate-risk (that is, ⩾10% to <20% predicted risk of experiencing a first cardiovascular disease event over 10 years; 41 (15.8%), 52 (19.5%) and 49 (18.6%), respectively) or high-risk (that is, ⩾20% predicted risk of experiencing a first cardiovascular disease event over 10 years; 40 (15.4%), 38 (14.3%) and 33 (12.5%), respectively) categories. During study treatment, CVEs occurred most frequently among patients in the high-risk (nilotinib 300 mg twice daily, 17.5%; nilotinib 400 mg twice daily, 23.7%; imatinib, 3.0%) and intermediate-risk (12.2%, 25.0% and 4.1%, respectively) categories in each arm, whereas patients in the low-risk category in each arm experienced fewer CVEs by the data cutoff (1.7%, 6.3% and 1.1%, respectively; Supplementary Table 4). Framingham general cardiovascular risk scores were evaluable for two of the three patients with deaths temporally associated with CVEs during study treatment (one patient each in the nilotinib 300-mg twice-daily and imatinib arms); both of these patients had scores in the intermediate-risk range.
Throughout 5 years of follow-up, newly occurring or worsening grade 3/4 elevations in lipase, glucose, alanine aminotransferase and bilirubin were more common in both nilotinib arms than in the imatinib arm, whereas most newly occurring or worsening grade 3/4 hematologic abnormalities (particularly neutropenia and leukopenia) were more common with imatinib than with nilotinib.
Newly occurring or worsening total cholesterol elevations of any grade occurred in 77 (27.6%), 74 (26.7%) and 11 (3.9%) patients in the nilotinib 300-mg twice-daily, nilotinib 400-mg twice-daily and imatinib arms, respectively (0, 3 (1.1%) and 0 patients, respectively, developed grade 3/4 total cholesterol elevations); newly occurring or worsening glucose elevations of any grade occurred in 139 (49.8%), 146 (52.7%) and 86 (30.7%) patients, respectively (20 (7.2%), 19 (6.9%) and 1 (0.4%) patients, respectively, developed grade 3/4 glucose elevations). Elevations in total cholesterol, low-density lipoprotein cholesterol and glycated hemoglobin (HbA1c) above clinically relevant thresholds15, 16, 17, 18 also occurred more frequently in both nilotinib arms than in the imatinib arm (Supplementary Table 5). In the nilotinib arms, cholesterol elevations tended to develop during the first year of treatment, and among evaluable patients who initiated statin therapy after developing cholesterol elevations, median cholesterol levels decreased following initiation of statin therapy (Supplementary Figure 3). The limited number of evaluable patients in all treatment arms prevented a similar analysis about the impact of antidiabetic medication in patients who developed HbA1c elevations on study.
Discussion
With 5 years of follow-up in ENESTnd, the balance of benefits and risks of nilotinib vs imatinib for the treatment of patients with newly diagnosed CML-CP can be evaluated more comprehensively than was previously possible. Throughout the study, nilotinib has demonstrated several benefits over imatinib in surrogate endpoints of therapeutic efficacy, such as higher rates of response and lower rates of disease progression, death due to advanced CML and treatment-emergent BCR-ABL mutations.2, 3, 4, 5 The risk of AEs (regardless of AE type) appears to be similar with nilotinib and imatinib; however, each TKI is associated with different types of AEs, including a higher risk of CVEs with nilotinib vs imatinib.
Overall and within each Sokal risk group, more patients achieved MR4.5 with nilotinib vs imatinib. Moreover, the difference in the rates of MR4.5 with nilotinib vs imatinib increased with longer follow-up, suggesting that the benefits of nilotinib over imatinib may become more marked over time. Achievement of deep molecular responses such as MR4.5 is associated with better clinical outcomes for patients with CML.19, 20 In the German CML-IV study, patients with confirmed MR4.5 at 4 years had a higher rate of 8-year OS than patients with complete cytogenetic response (CCyR) but without MMR at 4 years, and no patient who achieved MR4.5 experienced disease progression.20 In another study, long-term rates of EFS and failure-free survival were significantly higher among patients who achieved undetectable levels of BCR-ABL transcripts (with ⩾4.5-log sensitivity) than among patients who achieved CCyR without deep molecular response.19 Furthermore, data from clinical studies and reports of patients who have attempted treatment-free remission suggest that achievement of a deep molecular response is important for successful cessation of TKI therapy.21 Results from the large ENEST1st study, which evaluated MR4 as the primary endpoint, have confirmed the high rates of deep molecular response achieved with frontline nilotinib.22 The increased rates of deep molecular response achieved with nilotinib vs imatinib may allow more patients to attempt treatment-free remission in clinical trials.
Nilotinib also resulted in a rates of EMR and BCR-ABLIS⩽1% at 3 months than imatinib. Three-month BCR-ABLIS levels are key predictors of long-term outcomes in patients with CML-CP.5, 23, 24, 25 Treatment guidelines for CML include EMR at 3 months as the first landmark for evaluating responses to TKI therapy in patients with CML-CP.6, 7 The higher rate of MR4.5 by 5 years among patients who had BCR-ABLIS⩽1% at 3 months vs those who had BCR-ABLIS>1% to ⩽10% at 3 months suggests that early, deeper levels of molecular response may provide additional long-term benefits. For patients with BCR-ABLIS>10% at 3 months, it is not known whether a change in therapy can improve outcomes; therefore, maximizing patients’ likelihood of achieving EMR at 3 months is an important consideration during the initial management of CML-CP.
Avoiding disease progression is a primary goal of therapy for patients with CML-CP because median survival following progression is poor (≈10.5 months).4 Consistent with previous reports,2, 3, 4, 5 the risk of progression remained lower with nilotinib than with imatinib, and fewer patients treated with nilotinib died because of advanced CML. Three progressions to AP/BC after discontinuation of core treatment were newly reported since the 4-year data cutoff, highlighting the fact that progression can occur at any time. Thus, the lower risk of disease progression with nilotinib vs imatinib remains clinically important for patients receiving long-term TKI therapy, particularly for those with high Sokal risk scores or failure to achieve EMR at 3 months. In contrast to the high mortality rate associated with disease progression, very few patients in ENESTnd died within 3 months of experiencing a CVE during study treatment (two patients in the nilotinib 300-mg twice-daily arm, none in the nilotinib 400-mg twice-daily arm and one in the imatinib arm).
The observed safety results in all three arms of ENESTnd remained consistent with those reported in prior analyses.2, 3, 4 Notably, although both nilotinib arms showed similar efficacy, several types of AEs and laboratory abnormalities were more common with nilotinib 400 mg twice daily than with the approved dose of nilotinib 300 mg twice daily, underscoring the importance of considering dosage when evaluating TKI safety. Overall, the rates of grade 3/4 AEs, serious AEs and AEs leading to discontinuation of study treatment were similar with nilotinib 300 mg twice daily and imatinib.
CVEs occurred more frequently with nilotinib than with imatinib, particularly in the nilotinib 400-mg twice-daily arm. The cumulative incidence of CVEs in each arm has increased with longer follow-up. As expected, baseline Framingham general cardiovascular risk scores were predictive of patients’ risk of developing a CVE during nilotinib therapy. These findings are consistent with previous reports noting the presence of baseline cardiovascular risk factors in patients with CML who developed CVEs.26, 27, 28, 29 Together, our analyses and previously published data suggest that patients at risk of developing CVEs during TKI therapy might be identifiable at baseline.
Cholesterol and glucose elevations occurred more frequently in the nilotinib arms than in the imatinib arm. However, for patients who developed cholesterol elevations while receiving nilotinib, cholesterol levels decreased following initiation of statin therapy, demonstrating the importance of active monitoring and treatment of comorbidities and cardiovascular risk factors in all patients. Lipid-lowering therapies and/or lifestyle interventions may be indicated for some patients with sustained, low-grade cholesterol elevations.16, 17 For patients receiving TKIs who require lipid-lowering therapy, the potential for drug–drug interactions with some statins must be considered.30
Whereas some AE types are more common with nilotinib than with imatinib, the risk of other relevant AE types, including chronic low-grade AEs and potentially serious late-onset AEs, is relatively low with nilotinib. Edema, effusions, pulmonary hypertension and AEs related to venous thrombosis and embolism, all of which are known safety concerns associated with other BCR-ABL TKIs,31, 32, 33 have not been frequently reported in patients treated with nilotinib.
When choosing a frontline TKI for patients with newly diagnosed CML-CP, physicians must consider the entire benefit-risk profile of each available option. Viewed as a whole, the combined efficacy and safety results from ENESTnd demonstrate that nilotinib provided patients with meaningful long-term clinical benefits over imatinib, with a positive balance of benefit and risk, particularly with the 300-mg twice-daily dose, as frontline therapy for patients with CML-CP.
References
Tasigna (nilotinib) [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2015.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011; 12: 841–851.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012; 26: 2197–2203.
Hughes TP, Saglio G, Kantarjian HM, Guilhot F, Niederwieser D, Rosti G et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood 2014; 123: 1353–1360.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. 2013 Blood 2013; 122: 872–884.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia. V1 2016.
Hochhaus A, Saglio G, Larson RA, Kim DW, Etienne G, Rosti G et al. Nilotinib is associated with a reduced incidence of BCR-ABL mutations versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood 2013; 121: 3703–3708.
Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC et al. Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis. Stroke 2014; 45: 48–53.
Kim MS, Kang SJ, Lee CW, Han S, Park DW, Lee SW et al. Prevalence of coronary atherosclerosis in asymptomatic healthy subjects: an intravascular ultrasound study of donor hearts. J Atheroscler Thromb 2013; 20: 465–471.
Wilke T, Mueller S, Groth A, Fuchs A, Seitz L, Kienhöfer J et al. Treatment-dependent and treatment-independent risk factors associated with the risk of diabetes-related events: a retrospective analysis based on 229,042 patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2015; 14: 14.
Hughes TP, Hochhaus A, Kantarjian HM, Cervantes F, Guilhot F, Niederwieser D et al. Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on frontline imatinib or nilotinib 300 mg twice daily. Haematologica 2014; 99: 1204–1211.
Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica 2009; 94: 1362–1367.
Cross NC, Melo JV, Feng L, Goldman JM . An optimized multiplex polymerase chain reaction (PCR) for detection of BCR-ABL fusion mRNAs in haematological disorders. Leukemia 1994; 8: 186–189.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–753.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren M et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33: 1635–1701.
Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S1–S45.
American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011; 34: S11–S61.
Etienne G, Dulucq S, Nicolini FE, Morrisset S, Fort MP, Schmitt A et al. Achieving deeper molecular response is associated with a better clinical outcome in chronic myeloid leukemia patients on imatinib frontline therapy. Haematologica 2014; 99: 458–464.
Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol 2014; 32: 415–423.
Mahon FX, Etienne G . Deep molecular response in chronic myeloid leukemia: the new goal of therapy? Clin Cancer Res 2014; 20: 310–322.
Hochhaus A, Rosti G, Cross NCP, Steegmann JL, le Coutre P, Ossenkoppele G et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia 2016; 30: 57–64.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012; 30: 232–238.
Jain P, Kantarjian H, Nazha A, O'Brien S, Jabbour E, Romo CG et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood 2013; 121: 4867–4874.
Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012; 26: 2096–2102.
Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia 2013; 27: 1310–1315.
Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013; 27: 1316–1321.
Gambacorti-Passerini C, Cortes JE, Lipton JH, Dmoszynska A, Wong RS, Rossiev V et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol 2014; 89: 947–953.
Breccia M, Molica M, Zacheo I, Serrao A, Alimena G . Application of systematic coronary risk evaluation chart to identify chronic myeloid leukemia patients at risk of cardiovascular diseases during nilotinib treatment. Ann Hematol 2015; 94: 393–397.
Haouala A, Widmer N, Duchosal MA, Montemurro M, Buclin T, Decosterd LA . Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011; 117: e75–e87.
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123: 494–500.
Montani D, Bergot E, Günther S, Savale L, Bergeron A, Bourdin A et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 2012; 125: 2128–2137.
Iclusig (ponatinib) [package insert]. ARIAD Pharmaceuticals: Cambridge, MA, USA, 2014.
Acknowledgements
The ENESTnd study and work presented here were sponsored and funded by Novartis Pharmaceuticals Corporation. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Karen Kaluza, PhD, and Staci Heise, PhD (Articulate Science), for medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Authors declare the following relationships with pharmaceutical companies: Novartis—receipt of honoraria (AH, GS, TPH, DWK, PdlC, GE, REC, HN), research funding (all authors), nonfinancial support (GE, HN), employment (BD, WD, DD, HDM) and stock ownership (BD, DD, HDM); Pfizer—receipt of honoraria (AH, RAL, PdlC, GE, REC) and research funding (AH, DWK, REC); Ariad—receipt of honoraria (AH, TPH, GS, PdlC, GE) and research funding (AH, TPH); Bristol-Myers Squibb—receipt of honoraria (AH, GS, TPH, DWK, PdlC), research funding (AH, TPH, REC, IWF) and nonfinancial support (GE); Sanofi—receipt of honoraria (REC) and research funding (REC); Ilyang—receipt of honoraria (DWK).
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hochhaus, A., Saglio, G., Hughes, T. et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 30, 1044–1054 (2016). https://doi.org/10.1038/leu.2016.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.5
This article is cited by
-
Chronic myeloid leukemia diagnosed in pregnancy: management and outcome of 87 patients reported to the European LeukemiaNet international registry
Leukemia (2024)
-
BCR/ABL-Positive Chronic Myeloid Leukemia in Children: Current Treatment Approach
Current Oncology Reports (2024)
-
Spotlight on the real-world treatment of CML pts in Germany: a retrospective survey in private oncology practices
Annals of Hematology (2024)
-
Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib – interim results from the DAstop2 trial
Leukemia (2024)
-
Endothelial function measured by peripheral arterial tonometry in patients with chronic myeloid leukemia on tyrosine kinase inhibitor therapy: a pilot study
Cardio-Oncology (2023)